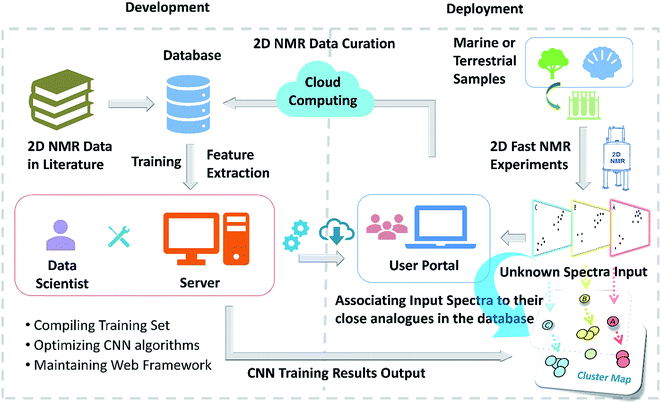
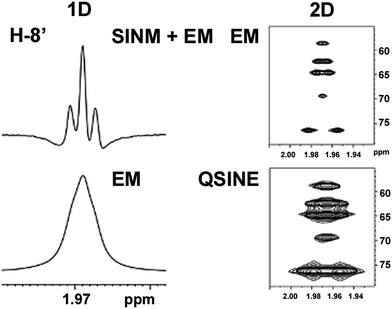
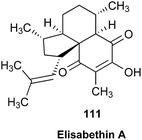
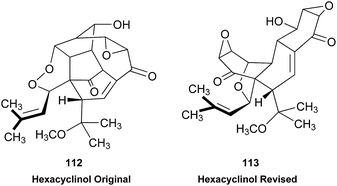
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The value of universally available raw NMR data for transparency, reproducibility, and integrity in natural product research†
James B.
McAlpine
 *a,
Shao-Nong
Chen
*a,
Shao-Nong
Chen
 a,
Andrei
Kutateladze
a,
Andrei
Kutateladze
 b,
John B.
MacMillan
b,
John B.
MacMillan
 c,
Giovanni
Appendino
c,
Giovanni
Appendino
 d,
Andersson
Barison
d,
Andersson
Barison
 e,
Mehdi A.
Beniddir
e,
Mehdi A.
Beniddir
 f,
Maique W.
Biavatti
f,
Maique W.
Biavatti
 g,
Stefan
Bluml
g,
Stefan
Bluml
 h,
Asmaa
Boufridi
h,
Asmaa
Boufridi
 i,
Mark S.
Butler
i,
Mark S.
Butler
 j,
Robert J.
Capon
j,
Robert J.
Capon
 j,
Young H.
Choi
j,
Young H.
Choi
 k,
David
Coppage
c,
Phillip
Crews
k,
David
Coppage
c,
Phillip
Crews
 c,
Michael T.
Crimmins
c,
Michael T.
Crimmins
 l,
Marie
Csete
l,
Marie
Csete
 m,
Pradeep
Dewapriya
m,
Pradeep
Dewapriya
 j,
Joseph M.
Egan
j,
Joseph M.
Egan
 n,
Mary J.
Garson
n,
Mary J.
Garson
 o,
Grégory
Genta-Jouve
o,
Grégory
Genta-Jouve
 p,
William H.
Gerwick
p,
William H.
Gerwick
 qr,
Harald
Gross
qr,
Harald
Gross
 s,
Mary Kay
Harper
t,
Precilia
Hermanto
u,
James M.
Hook
s,
Mary Kay
Harper
t,
Precilia
Hermanto
u,
James M.
Hook
 u,
Luke
Hunter
u,
Luke
Hunter
 u,
Damien
Jeannerat
u,
Damien
Jeannerat
 v,
Nai-Yun
Ji
v,
Nai-Yun
Ji
 w,
Tyler A.
Johnson
c,
David G. I.
Kingston
w,
Tyler A.
Johnson
c,
David G. I.
Kingston
 x,
Hiroyuki
Koshino
x,
Hiroyuki
Koshino
 y,
Hsiau-Wei
Lee
c,
Guy
Lewin
f,
Jie
Li
y,
Hsiau-Wei
Lee
c,
Guy
Lewin
f,
Jie
Li
 r,
Roger G.
Linington
r,
Roger G.
Linington
 n,
Miaomiao
Liu
i,
Kerry L.
McPhail
n,
Miaomiao
Liu
i,
Kerry L.
McPhail
 z,
Tadeusz F.
Molinski
z,
Tadeusz F.
Molinski
 aa,
Bradley S.
Moore
aa,
Bradley S.
Moore
 qr,
Joo-Won
Nam
qr,
Joo-Won
Nam
 ab,
Ram P.
Neupane
ac,
Matthias
Niemitz
ab,
Ram P.
Neupane
ac,
Matthias
Niemitz
 ad,
Jean-Marc
Nuzillard
ad,
Jean-Marc
Nuzillard
 ae,
Nicholas H.
Oberlies
ae,
Nicholas H.
Oberlies
 af,
Fernanda M. M.
Ocampos
af,
Fernanda M. M.
Ocampos
 e,
Guohui
Pan
e,
Guohui
Pan
 ag,
Ronald J.
Quinn
ag,
Ronald J.
Quinn
 i,
D. Sai
Reddy
b,
Jean-Hugues
Renault
i,
D. Sai
Reddy
b,
Jean-Hugues
Renault
 ae,
José
Rivera-Chávez
ah,
Wolfgang
Robien
ae,
José
Rivera-Chávez
ah,
Wolfgang
Robien
 ai,
Carla M.
Saunders
ai,
Carla M.
Saunders
 aj,
Thomas J.
Schmidt
aj,
Thomas J.
Schmidt
 ak,
Christoph
Seger
ak,
Christoph
Seger
 al,
Ben
Shen
al,
Ben
Shen
 ag,
Christoph
Steinbeck
ag,
Christoph
Steinbeck
 am,
Hermann
Stuppner
am,
Hermann
Stuppner
 al,
Sonja
Sturm
al,
Orazio
Taglialatela-Scafati
al,
Sonja
Sturm
al,
Orazio
Taglialatela-Scafati
 an,
Dean J.
Tantillo
an,
Dean J.
Tantillo
 aj,
Robert
Verpoorte
aj,
Robert
Verpoorte
 k,
Bin-Gui
Wang
k,
Bin-Gui
Wang
 wao,
Craig M.
Williams
wao,
Craig M.
Williams
 o,
Philip G.
Williams
o,
Philip G.
Williams
 ac,
Julien
Wist
ac,
Julien
Wist
 ap,
Jian-Min
Yue
ap,
Jian-Min
Yue
 aq,
Chen
Zhang
ar,
Zhengren
Xu
aq,
Chen
Zhang
ar,
Zhengren
Xu
 ag,
Charlotte
Simmler
ag,
Charlotte
Simmler
 a,
David C.
Lankin
a,
David C.
Lankin
 a,
Jonathan
Bisson
a,
Jonathan
Bisson
 a and
Guido F.
Pauli
a and
Guido F.
Pauli
 *a
*a
aCenter for Natural Product Technologies (CENAPT), Program for Collaborative Research in the Pharmaceutical Sciences (PCRPS), Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, University of Illinois at Chicago, 833 S. Wood St., Chicago, IL 60612, USA. E-mail: gfp@uic.edu, mcalpine@uic.edu
bDepartment of Chemistry and Biochemistry, University of Denver, Denver, CO 80210, USA
cDepartment of Chemistry and Biochemistry, University of California, Santa Cruz, CA 95064, USA
dDipartimento di Scienze Chimiche, Alimentari, Farmaceutiche e Farmacologiche, Universita` del Piemonte Orientale, Via Bovio 6, 28100 Novara, Italy
eNMR Center, Federal University of Paraná, Curitiba, Brazil
fÉquipe “Pharmacognosie-Chimie des Substances Naturelles” BioCIS, Univ. Paris-Sud, CNRS, Université Paris-Saclay, 5 rue J.-B. Clément, 92290 Châtenay-Malabry, France
gDepartment of Pharmaceutical Sciences, Federal University of Santa Catarina, Florianópolis, Brazil
hUniversity of Southern California, Keck School of Medicine, Los Angeles, CA 90089, USA
iGriffith Institute for Drug Discovery, Griffith University, Brisbane, QLD 4111, Australia
jInstitute for Molecular Bioscience, The University of Queensland, St. Lucia, QLD 4072, Australia
kDivision of Pharmacognosy, Section Metabolomics, Institute of Biology, Leiden University, P.O. Box 9502, 2300 RA Leiden, The Netherlands
lKenan and Caudill Laboratories of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
mUniversity of Southern California, Huntington Medical Research Institutes, 99 N. El Molino Ave., Pasadena, CA 91101, USA
nDepartment of Chemistry, Simon Fraser University, Burnaby, BC V5A 1S6, Canada
oSchool of Chemistry and Molecular Sciences, University of Queensland, St. Lucia, QLD 4072, Australia
pC-TAC, UMR 8638 CNRS, Faculté de Pharmacie de Paris, Paris-Descartes University, Sorbonne, Paris Cité, 4, Aveue de l’Observatoire, 75006 Paris, France
qSkaggs School of Pharmacy and Pharmaceutical Sciences, University of California, La Jolla, San Diego, CA 92093, USA
rCenter for Marine Biotechnology and Biomedicine, Scripps Institution of Oceanography, La Jolla, CA 92093, USA
sPharmaceutical Institute, Department of Pharmaceutical Biology, Eberhard Karls University of Tübingen, Auf der Morgenstelle 8, 72076 Tübingen, Germany
tDepartment of Medicinal Chemistry, University of Utah, Salt Lake City, UT 84112, USA
uNMR Facility, Mark Wainwright Analytical Centre, University of New South Wales, Sydney, NSW 2052, Australia
vUniversity of Geneva, Department of Organic Chemistry, 30 quai E. Ansermet, CH 1211 Geneva 4, Switzerland
wYantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Chunhui Road 17, Yantai 264003, People's Republic of China
xDepartment of Chemistry, M/C 0212, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061, USA
yRIKEN Center for Sustainable Resource Science, Wako, Saitama, 351-0198, Japan
zDepartment of Pharmaceutical Sciences, College of Pharmacy, Oregon State University, Corvallis, OR 97331, USA
aaDepartment of Chemistry and Biochemistry and Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California, San Diego, 9500 Gilman Drive MC-0358, La Jolla, CA 92093, USA
abCollege of Pharmacy, Yeungnam University, 280 Daehak-ro, Gyeongsan, Gyeongbuk 38541, Republic of Korea
acDepartment of Chemistry, University of Hawaii at Manoa, 2545 McCarthy Mall, Honolulu, HI 96822, USA
adNMR Solutions Limited, Puijonkatu 24B5, 70110, Kuopio, Finland
aeFRE CNRS 2715, IFR 53, Université de Reims Champagne-Ardenne, Bât. 18, Moulin de la Housse, BP 1039, 51687 Reims, Cedex 2, France
afDepartment of Chemistry and Biochemistry, University of North Carolina at Greensboro, Greensboro, NC 27402, USA
agDepartment of Chemistry, Department of Molecular Medicine, and Natural Products Library Initiative at the Scripps Research Institute, Jupiter, FL 33458, USA
ahInstituto de Química, Universidad Nacional Autónoma de México, Ciudad de México 04510, Mexico
aiUniversity of Vienna, Department of Organic Chemistry, Währingerstrasse 38, A-1090 Vienna, Austria
ajDepartment of Chemistry, University of California, Davis, One Shields Avenue, Davis, CA 95616, USA
akInstitute of Pharmaceutical Biology and Phytochemistry (IPBP), University of Münster, Pharma Campus, Corrensstrasse 48, D-48149 Münster, Germany
alInstitute of Pharmacy, Pharmacognosy, Member of CMBI, University of Innsbruck, Innrain 80-82, 6020 Innsbruck, Austria
amInstitute of Inorganic and Analytical Chemistry, Friedrich-Schiller-University, D-07743 Jena, Germany
anDipartimento di Farmacia, Università; di Napoli Federico II, Via Montesano 49, 80131 Napoli, Italy
aoLaboratory of Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, Nanhai Road 7, Qingdao 266071, People's Republic of China
apDepartamento de Química, Universidad del Valle, AA 25360, Cali, Colombia
aqState Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Zhangjiang Hi-Tech Park, Shanghai 201203, People's Republic of China
arDepartment of Nanoengineering, University of California, La Jolla, San Diego, CA 92093, USA
First published on 13th July 2018
Abstract
Covering: up to 2018
With contributions from the global natural product (NP) research community, and continuing the Raw Data Initiative, this review collects a comprehensive demonstration of the immense scientific value of disseminating raw nuclear magnetic resonance (NMR) data, independently of, and in parallel with, classical publishing outlets. A comprehensive compilation of historic to present-day cases as well as contemporary and future applications show that addressing the urgent need for a repository of publicly accessible raw NMR data has the potential to transform natural products (NPs) and associated fields of chemical and biomedical research. The call for advancing open sharing mechanisms for raw data is intended to enhance the transparency of experimental protocols, augment the reproducibility of reported outcomes, including biological studies, become a regular component of responsible research, and thereby enrich the integrity of NP research and related fields.
1 Introduction
1.1 Preamble
Throughout organic chemistry, and especially in natural products (NPs), where new bioactive metabolites are frequently isolated in minute, often sub-milligram quantities, nuclear magnetic resonance (NMR) has become the primary tool for structure determination. Typically, practitioners “extract” the structural information from NMR spectra that were generated via Fourier Transformation (FT) of free induction decays (FIDs), which represent the actual (raw) spectroscopic data from the excited nuclear spins in the NMR experiment (“spin choreography”). The deduction of structural information entails not only human interpretation and viewpoints (Fig. 1), but commonly also involves a significant loss of information (e.g., signal phase, peak shape, and signal multiplicity in tabulated representations), which leads to the inability to reprocess the spectra ab initio and/or employ computational tools to derive additional information from the same experimental data. For example, extracting the complete information contained in the FID of the most basic and sensitive NMR experiment, 1D 1H NMR, can avoid the ubiquitous nondescript designation of “multiplet” and exemplifies the concept of exploiting raw NMR data for additional information (e.g., Section 3 Structure Revision). The importance of extracting all of the information contained in an experimental data set is exemplified by the simple analogy presented in (Section 1.2 Dimensionality and Completeness).This community-driven review calls for a re-examination of NMR-based structural analysis of NPs and represents the logical next step in the NMR Raw Data Initiative that commenced in 2016.1 The seven major rationales used to organize this text evolve from the urgent need for raw NMR data dissemination and are explained in Section 2 Introduction to the Organization of this Review. This led to the separation of the material into sections that cover chemical structure (Sections 3–5), analytical methodology (Sections 4–7), followed by applications and future perspectives (Sections 8–10) of raw NMR data. Located at the heart of the intent to promote the free dissemination of raw NMR data, Section 10 Conclusions & Outlook should be of particular interest to scientists increasing the use of NMR in NP research.
1.2 Dimensionality and completeness
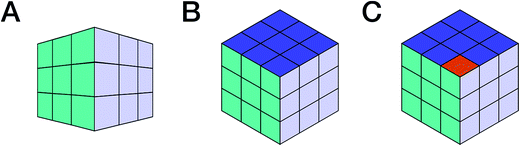
Consider a picture of a Rubik's cube: the full 3D object cannot be captured by a single 2D picture, as it only provides a projection of the original object. The reduced dimensionality makes the representation incomplete, as observed in Fig. 1, and the incompleteness may lead to false conclusions. E.g., projection A (Fig. 1) does not permit conclusions on solving the puzzle. No faithful conclusion is possible until at least five faces have been examined, which requires at least two projections since no more than three faces may be observed at once. A single projection may lead to an erroneous conclusion. Further projections increase the amount of available information, which may either confirm the original hypothesis or refute it (B vs. C, respectively, in Fig. 1).Now consider a molecule. Each NMR experiment can be seen as a projection of the original spin system. The structural elucidation may require several projections/experiments to reconstruct the full picture, i.e., approach the complete Hamiltonian as closely as possible. Note that, for the Rubik's cube, five of the total of six faces is sufficient for absolute certainty. In chemistry, however, structures are sometimes postulated on the basis of a single 1H NMR spectrum, often erroneously. Moreover, it is not possible to predict how many experiments will be required. Instead, the researcher will perform experiments based on budget, time, and the possibly the expectation that the analysis is complete once the first possible solution that matches all the available constraints (e.g., chemical shifts, multiplicity, and correlations) has been found. Often, solutions are proposed based on previous results obtained for similar molecules; yet other solutions may exist and further experiments be required to single out the correct structure. Thus, an“elucidated” structure can be viewed as a possible solution that fits the available experimental data.
While other factors may contribute to erroneous structural assignments, the urge to stop after an apparent solution and failure to recognize that more than one structure can be equally or more consistent with the experimental data is likely the root cause of the errors. Computer-Aided Structure Elucidation (CASE) software2 is invaluable for overcoming this limitation by finding all structures which are consistent with the available data. Moreover, CASE tools are capable of ranking candidate structures by comparison of experimental and empirically predicted 1H and 13C chemical shifts, and remaining ambiguities can be resolved by inclusion of DFT calculations.3
Once an incorrect structure has been detected, the correct structure may still not be obvious, particularly if the structure is unusual.4 In such cases, CASE software can be valuable by providing probable structures for further consideration. While this can potentially be done using the tabulated correlation data, access to the raw NMR data it is valuable or even essential for this process. Collectively, the uncertainty inherent to structure elucidation is significant. Moreover, new structures are published daily without their corresponding experimental support, or with the compressed molecular formula strings (e.g., Simplified Molecular Input Line Entry System [SMILES]), making peer-review a difficult or an almost impossible task. In this context it is safe to assume that the literature may contain erroneous structures and that a strategy is needed to deal with this issue.
1.3 Human and machine processing of NMR data
Progress in cheminformatics permitted the building of tools to help validate assignments and, thus, unveil incorrect structures.5–8 Indeed, computers may calculate all the solutions allowed by a potentially incomplete set of constraints. Software already exists that can handle all aspects of interpretation of NMR spectra, from peak-picking and chemical shift prediction9,10 to assignment and elucidation.6,7,11–14 The last two heavily rely on the accuracy of the chemical shift prediction, which in turn heavily relies on the quality and amount of known structure assignments available for training algorithms. As a consequence, most automatic spectral interpretation programs rely on large databases of previously assigned spectra; tools such as LSD [http://www.univ-reims.fr/LSD] or CCASA15 developed by Nuzillard et al. are notable exceptions. Ensuring that these data are correctly assigned is essential to avoid continual propagation of structural errors. Therefore, even with the assistance of cheminformatics, the challenge of peer-reviewing published spectral interpretations still remains. But there may be another approach.Acknowledging the fact that several signals can be assigned from integration and correlation constraints alone11,12 paves the way for unsupervised self-learning procedures that interpret spectra completely from scratch.13 During the first iteration, the procedure tries to assign as many atoms’-signal pairs as possible without the help of chemical shift constraints. In other words, assignment is performed based on signal area, multiplicity and correlations, and only unambiguous assignments are stored. These assignments link the observed chemical shifts to the assigned substructures, providing new knowledge to the chemical shift predictor. In a second iteration, the algorithm will reassign the same data, but this time using chemical shift constraints inferred from the knowledge just acquired. Iterations continue until a steady state is reached, i.e., no new atom-NMR signal pairs can be assigned. When new data is submitted, the system assigns it and may run a new iteration. Hence, the algorithm builds its own database of assigned spectra without any human intervention.
Peak-picking should be implemented as part of this self-learning loop also. Indeed, modified data must be considered a representation of the original. A missing signal because of low signal to noise ratio or an additional signal from a poorly identified impurity are common errors that affect the outcomes of such a system. Although assignment is performed on peak-picked data, automatic peak-picking itself should be seen and implemented as an iterative process that ends when a successful assignment is found. Having brought assignment, prediction and peak-picking into a self-learning loop allowed the demonstration that a program may be conceived to avoid any human assumptions and faithfully generate all the solutions to the assignment problem. A similar approach can be implemented that applies CASE2 strategies and DFT calculations3 to generate all possible solutions to the elucidation problem and verify them. Such a program would see all possibilities allowed by the visible faces of the cube and allow thorough review of published assignments. That is, as long as the full, raw, unprocessed and unassigned data are published.
Hence, artificial intelligence may be applied to automatic structure elucidation. However, any operation performed on the truly raw, original NMR data (FID and associated information), as saved initially by the NMR spectrometer, can alter the final representation of the spectrum and may introduce errors. Consequently, any modification of the raw data should be considered part of the elucidation procedure and regarded as a process that can be improved. For this reason, only raw data must be input into the learning procedure of the automatic structure elucidator. Thus, developing new tools to assist researchers in their daily task requires large sets of high quality data stored in a correct manner. This goal can only be reached if the dissemination of original data becomes a standard component, if not a requirement, of established publication mechanisms.
1.4 Molecular transparency
Traceability and reliability of analytical results (detailed knowledge of total error and method specificity) as well as analytical data comparability are of utmost importance to make science transparent on a global level. This holds especially true if such results are key in decision making, as in medical diagnosis, food and feed safety, environmental pollution tracking, and many more areas. Even in the 21st century, the scientific base of such undertakings is often not transparent, albeit that peer reviewed publications are daily business in applied and basic science. Lacking or incomplete information on the technologies used, or unclear declaration of utilized reference materials, hampers not only scientific progress, but also complicates the transfer from science to routine applications. Once an analytical strategy is applied in, and validated for, routine use, vagueness in the basic cornerstones of an assay, including (1) lack of information on identity and purity of reference materials, (2) a poorly documented chain of traceability in calibrator materials, and (3) missing clear-cut communicated measurement conditions, can all lead to unnecessary platform bias and an overall increase in inter-laboratory data scattering and inconsistency. As many scientists are involved with the establishment and execution of LC-/MS-driven assays for routine analysis, the importance of NMR in the total analytical process is unclear or unknown. However, NMR specialists are already aware of the power of “their” methodology.Aside from X-ray crystallography, NMR spectroscopy is still the only spectroscopic method accepted for an unambiguous structure elucidation (not only for identification) of a molecular scaffold, especially in the realm of organic compounds. Today, high-resolution 1H and 13C NMR spectra become more widely recognized as being “molecular fingerprints”, which can even be predicted computationally. While two-dimensional 1H-detected experiments allow the transformation of 1H and 13C NMR resonances into molecular scaffolds, contemporary technologies still do not automate this process. Finally, while carbon–carbon connectivity mapping would complete NMR based molecular cartography, and despite recent progress with these experiments,16–18 this approach is limited by sensitivity and not used widely.
1.5 Molecular topography
By analogy, it is well known that modern terrestrial cartography has changed dramatically recently. Traditionally, the painstaking work started with planes doing analogue aerial photography and technicians deriving a (finally digital) terrain model thereof. This model still is a framework for detailed and accurate maps filled by information derived from the photographs or from terrestrial reconnaissance, often by foot. Such maps, used by almost everyone moving through the environment, have been replaced by highly automated processes relying on space technology based surveying by the “shuttle radar topography mission“ (SRTM) data gathered by the space shuttle Endeavour in 2000. Users who lack detailed knowledge of the involved technologies rely on the assumption that the “maps” involved are reliable. It is assumed that they are comparable and demand that the presented information is representing “the true” environment. However, in reality these claims are quite often not met. Traveling distances do vary, road conditions encountered are discrepant to mapped ones, and hiking maps are too often lacking detailed terrain visualization. Whenever “maps” are involved in legal processes, e.g., when we use cadastral maps as planning tool, it is assumed that certain mapping products are accurate and precise two-dimensional presentations of the three-dimensional open space. It must not be overlooked, that these assumptions are made because the production of such maps is traceable to an agreed digital terrain model, the technological process of the 3D to 2D transformation is well described and its error margins are understood and communicated.NMR spectroscopy is also a “mapping tool”, just on a molecular scale level. It is based on scientific inventions and breakthrough processes made 50+ years ago; its modern digital version, the FT NMR technology, has been on the market for more than four decades. Due to its technological complexity and costs, access to NMR spectroscopy has been limited to a very small number of practitioners. The latest “soft revolution” in the application of NMR spectroscopy reached the public about twenty years ago, meanwhile very successful first attempts have been made to transfer the NMR data interpretation from UNIX or Linux operated work station environments to desktop computers integrating NMR data into the everyday office. Now, for this type of software the Gardner hype cycle “trough of disillusionment” (which was very shallow) has been successfully transversed and a stable, productive working environment has been achieved.
Parallel to the development of NMR technologies, the interpretation of the NMR data is also experiencing constant change. Beginning from reporting selected NMR signals with molecular position annotations based on increment rules and similar estimation tools relying on conclusion by analogy, the introduction of high-resolution cryogenic magnets and the Nobel prize winning innovation of FT-NMR based 2D NMR spectra, changed the situation remarkably. Complete correlation of NMR signals and molecular positions became a must in describing a novel compound. Especially in NP science, comprehensive data representation was understood as mandatory whenever new NPs were claimed. In organic synthesis, standards were kept lower for significant periods of time, some prominent and well-ranked journals did not even request molecular position assignments of any of the NMR signals in spectral data. About a decade ago, Nicolaou and Synder19 showed in a comprehensive study that, in the process of NMR-based structure elucidation, erroneous structures resulted with noticeable frequency and ultimately reflected inadequate structure elucidation efforts.
Very recently, Wolfgang Robien affirmed this postulate by running the 13C NMR database CSEARCH against recently published structures. He again was able to show that erroneous assumptions in the structure elucidation process (e.g., lacking spectral evidence, no 2D methods performed) were leading to incorrect structures.20
2 Introduction to the organization of this review
The numerous scientific rationales that support the urgency of public dissemination of raw NMR data fall into the following groups:2.1 Rationale 1 – structure revisions
This represents the largest group and many cases can be grouped into sub-categories, the largest comprises structures originally proposed with an incorrect ring closure. Another, somewhat embarrassing subgroup, consists of structures which are blatantly incorrect or where, even with a cursory examination, of available data never should have been proposed. In these cases, the raw data would have allowed a reviewer to recommend changes and/or detect issues. A final set involves other types of revisions.2.2 Rationale 2 – impurity detection and quantification
For several decades, the majority of NP research has been fueled by the search for bioactive compounds, drugs (human and veterinary), herbicides and other pesticides. This quest was focused on the use of bioactivity-guided fractionation. Here, a purity assessment of the final product assigned the bioactivity is critical, as high potency minor impurities invalidate the conclusions. Hence, both quantification and identity of impurities are critical.2.3 Rationale 3 – dereplication
The bane of most NP chemists' endeavors is the “rediscovery” of a known compound. The schemes and protocols developed to avoid, or at least minimize, this occurrence have often been complex and varied. They have aimed at detecting known compounds as early in the discovery process as possible. However, none has ever had claims of sterling success. The fact that 1D 1H NMR and 13C spectra can serve as unique fingerprints of a given compound (for 1H methodology, see Sections 3.3 and 5.1) makes NMR a highly specific tool for dereplication, and whenever this can be applied early during fractionation (see Section 5.3), it provides a quantum leap in discovery.2.4 Rationale 4 – enabling new methodology
Science advances with the development and use of new approaches and methods. This section features recently developed and utilized methods, which can provide the scientist with valuable tools to interpret spectra from raw data.2.5 Rationale 5 – other nuclei
This section adds the perspective of 19F, 15N, and 31P NMR spectroscopy. Although fluorine occurs rarely in NPs, it is frequently introduced into derivatives to improve drug pharmacokinetics. Its high magnetic moment, broad chemical shift dispersion, and extensive coupling make 19F NMR spectroscopy almost a sub-specialty. Similar considerations apply to phosphorus, and the raw data from these spectra are every bit as data-intensive as those from a 1H NMR spectrum. Nitrogen is an important heteronucleus in many NPs, but 15N sensitivity has restrained a more widespread application to date. Raw data can play an important role to overcome this limitation by expanding the utility of valuable existing 15N NMR data with regard to structural interpretation.2.6 Rationale 6 – data repositories
Raw NMR data only reaches its maximum potential if it is universally accessible. Unfortunately, chemists have fallen behind the geneticists in the establishment and general acceptance of a universal database. Although, several laudatory efforts have assembled databases, with some described here, the amount of NMR data generated around the world makes the compiling of a single database for each nucleus a growing, and already gargantuan, task, discussed further in the conclusions.2.7 Rationale 7 – clinical applications
Most readers of this review will probably find this section alien to their everyday interests. However, those who have had need to take advantage of this foray of physics into the medical field will surely appreciate its capabilities and enjoy reading of how the raw data has its role here also, and the optimistic view anticipating quantum leaps forward in medicine from progress in this area.3 Structure revision
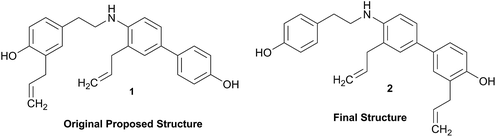
Structural revision can occur at three points of scientific discovery, preferably prior to publication, either in the originating laboratory or at the manuscript review process, or less ideally post publication. One example which was only published after an initial misassignment was discovered in house is represented by the neolignan from Magnolia grandiflora L.21 This is an excellent example of the Rubik's cube philosophy discussed above. The structure, 1, originally proposed on the basis of HRMS, 1H NMR and 13C NMR was questioned on the basis of biosynthetic considerations. A further examination of 2D NMR, specifically one-bond and long-range correlations from HMBC and HSQC experiments, respectively led to a revision to structure 2,21 but this revision would not have been possible from the 1D data alone. In most cases of Structure Revision that see the light of day, the initial incorrect structure is not corrected in-house but published as such, and correction comes when another group isolates and/or studies the same compound. While one can only speculate about the likelihood of a published structure being incorrect, recent systematic studies employing relatively fast parametric/DFT hybrid computational methods have found substantial mismatches between predicted and published data.22–24 For a series of nearly 100 sesquiterpenes, discrepancies occurred for as many as 14% of the published structures and indicated the need for substantial structural revision.Moreover, concerns were expressed as early as in the mid/late 1970s by Zimmerman and co-workers (see footnotes 12 in ref. 25 4 and in ref. 26) regarding the exclusive use of spectroscopic structure elucidation methods while not including more classical approaches involving chemical synthesis and/or chemical degradation together with bulk analytical methods such as elemental analysis for a more thorough approach to structure elucidation. Similar concerns regarding the integration of chemical and spectroscopic structural analysis were expressed by Faulkner (page 1433 in ref. 27) and Robinson (in a letter to Chavrarti, as referred to in ref. 28). Following some (undocumented) statistical analyses, Zimmerman raised the potential apprehension that relying on spectroscopic evidence alone carried with it a substantial probability of structural misassignment. While a classical approach involving total synthesis may not be feasible within a reasonable time frame in NP research, it is of interest to compare Zimmerman's predicted probabilities of erroneous structures of 10–22% with the ca. 14% incidence rate found very recently by Kutateladze and co-workers.22–24 These findings confirm the validity of the cautionary notes raised 40+ years ago,25,26 and demonstrate the importance of purity and residual complexity29 in both analytical and NP chemistry: classical bulk analysis methods such as microanalytical and (mixed) melting point determinations are more sensitive to minor impurities than many of the contemporary spectroscopic methods. Notably, the demand for purity of bioactive NPs and other chemicals is essential for rigor and reproducibility of research outcomes.
Here, raw NMR data plays important roles in documentation by enabling the retrospective determination of the purity of previously investigated materials. Notably, the need for re-assignment of NMR spectra and/or achievement of a complete assignment of at least the full chemical shifts and coupling constants of the 1H and 13C framework, can be estimated to be much greater. Reflecting on the general gap in the assignment of the relatively complex 1H NMR signal patterns, this consideration affects the scientific context of structural correctness, the resulting reproducibility of downstream research, intellectual property issues, and their collective economic impact. The role of (raw) NMR data in the structural revision of NPs has been highlighted prominently in a recent review by Kubanek and co-workers.30
3.1 Incorrect ring closures: furan vs. pyrone ring systems
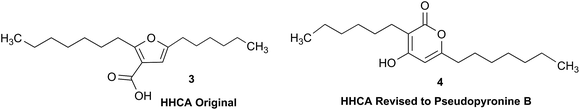
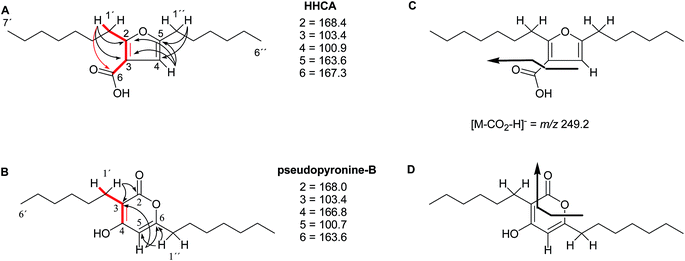
The putative new compound 2-heptyl-5-hexylfuran-3-carboxylic acid (HHCA; CAS 1256499-01-0, compound 3 in Fig. 2A) is produced by the rhizosphere bacterium Pseudomonas sp. strain SJT25.31 HHCA exhibits broad antifungal activity against several phytopathogens and was considered a new promising biopesticide. This led to further fermentation studies32 and a patent being filed and granted in 2012.33 However, biosynthetic considerations raised doubts about the structure. With 18 carbon atoms it was assumed that HHCA was generated by nine acetate units but these units could not be lined up, by a single, or a two chain-mechanism to give upon cyclization HHCA. A database search using the molecular sum formula pointed to pseudopyronine B, an α-pyrone-based compound with an identical NMR data set, that is produced also by several Pseudomonas species.34–37 Indeed, the UV-absorption (208 and 290 nm) spectrum and the 13C NMR data of pseudopyronine B (4) were nearly the same as those for other 3,6-disubstituted 4-hydroxy-2H-pyran-2-one-based compounds.38–42 Thus, the structure of HHCA has to be revised to that of 4 (Fig. 2A/B).Unfortunately, the authors assigned the carbon atom C-6, resonating at 167.3 ppm in the 13C NMR spectrum together with a broad singlet signal at 10.31 ppm in the 1H NMR spectrum to a putative free carboxylic acid moiety, bound to a disubstituted furan ring. This conclusion was thought to be corroborated by IR absorption at 1635 cm−1 and a loss of m/z 44 (loss of the COOH group by decarboxylation, in the MS spectrum (Fig. 2C)). However, actually, the carbon atom C-6 of HHCA (δ 167.3 ppm) corresponds to C-4 of pseudopyronine B; and the OH group of the COOH of HHCA (δ 10.31 ppm) equals the OH group bonded to C-4 of pseudopyronine B. Furthermore, the observed broad IR absorptions at 1635 cm−1 represents an overlapping signal which is generated by the stretching frequencies of the tautomeric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond13 and C5
O bond13 and C5![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C6 of the α-pyrone ring.43,44 In the MS spectrum, the loss a CO2 group is commonly observed from the pyrone ring system (Fig. 2D).45,46
C6 of the α-pyrone ring.43,44 In the MS spectrum, the loss a CO2 group is commonly observed from the pyrone ring system (Fig. 2D).45,46
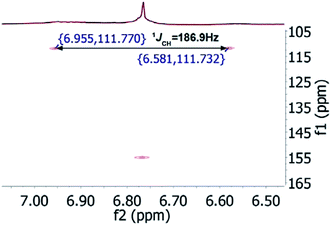
In the original report of HHCA, the tri-substituted furan ring was deduced on the basis of 13C NMR shift values and HMBC correlations observed between H-4 and C-2, C-3, C-5 and C-1″, while the linkages of the alkyl chains were deduced from HMBC correlations from H2-1′ with C-2, C-3 and C-6 and from H2-1″ with C-4 and C-5. Regarding the 1H–13C HMBC correlations, the pair H2-1′–C-6 suggests a questionable 4JC,H coupling, which indicated already that the original core was wrongly determined, because the HMBC experiment is in a standard setup optimized for 2–3 bonds. The observation of long-range coupling over four bonds is not impossible (e.g., foremost in aromatic systems or as a W-coupling in planar aliphatic systems) but commonly presents a weak signal. In the case of a strong signal, it could be an indicator for a misassigned structure. The authors presented in the ESI† the HMBC map, however only a section from 0–120 ppm in the f1 dimension is shown, and the decisive range (150–170 ppm) is regrettably not visible. The availability of NMR raw data could have clarified this issue. During the course of the study of the biosynthetic origin of pseudopyronines, the Gross group re-isolated congener B (4) and observed no correlation between H2-1′ (δ 2.44 ppm) and C-6 (δ 167 ppm) from the 1H–13C HMBC NMR map (Fig. 3). It should be noted that a variety of more recent 2D NMR experiments improve the detection and/or distinction of 2/3/4JC,H couplings, such as H2BC, LR-HSQMBC,47–49 and HSQMBC-COSY/TOCSY50 experiments (see also the review by Breton and Reynolds51).
Nevertheless, such a correlation can be much better rationalized by the pyrone than a furan ring structure. Finally, Gross and coworkers conducted labeling experiments employing doubly 13C-labeled acetate and confirmed in this way the structure by the determination and localization of intact acetate units via measurement of JC,C.37 Similarly, Reibarkh et al. have emphasized the utility of uniform 13C labeling of microbial NPs, which becomes feasible via the availability of uniformly 13C labeled glucose.52
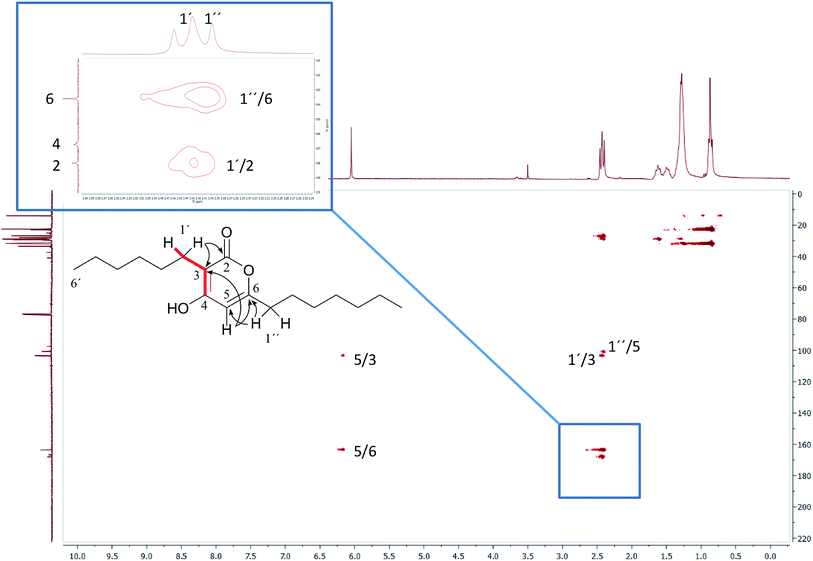
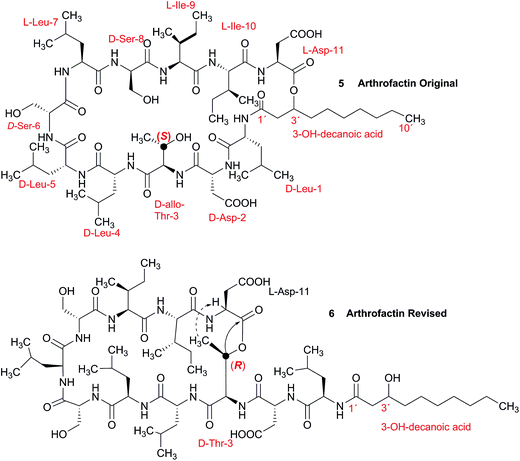
3.2 Incorrect ring closures: the lipopeptide arthrofactin
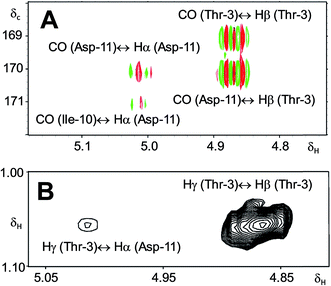
In 1993, Imanaka and co-workers reported the isolation of the cyclic lipo-undecapeptide, arthrofactin from the bacterium Arthrobacter sp. MIS38. This compound possesses a high surface activity and was assigned the structure 5.53 Later, the corresponding biosynthetic gene cluster was characterized.54 The gene cluster (arfABC) coded for the expected 11 NRPS modules, required for the assembly of the linear lipo-undecapeptide portion and a terminal tandem thioesterase (TE-I/TE-II). Particularly, the TE-I enzyme system is responsible for the hydrolysis and cyclization of the linear lipopeptide precursor. Nowadays, it is possible to predict the cyclization process by bioinformatics because the TE's reveal clades of enzymes that reflect the cyclization step. Bioinformatic analyses with the TE-I of ArfC led to the hypothesis that the ring closure occurred between Asp11 and Thr3 to give structure 6 instead of a lactone ring between Asp11 and the 3-hydroxy group of the fatty decanoic acid side chain as originally suggested.55A re-analysis of the 1H–13C HMBC correlation map and the 1H–1H NOESY correlations, enabled by the availability of the raw data, would have revealed problems with the first interpretation. The closure of the cyclic peptide between Thr3 and Asp11 was demonstrated using the following evidence: the carbonyl carbon of Asp11 shows a HMBC correlation with the Asp11 Hα and Thr3 Hβ hydrogens (Fig. 4A). Furthermore, the Thr3 Hγ shows a NOESY correlation with the Asp11 Hα (Fig. 4B). Therefore, the closure of the ring must be situated between the Asp11 carbonyl group and the Thr3 hydroxyl group.
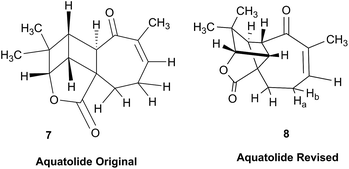
3.3 Incorrect ring closures: the case of aquatolide
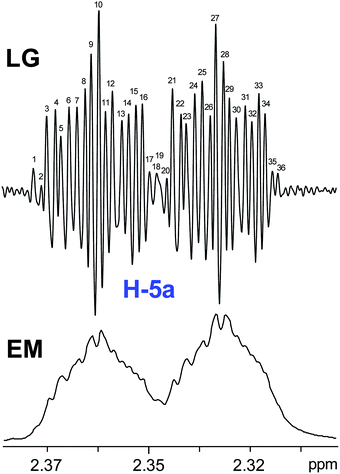
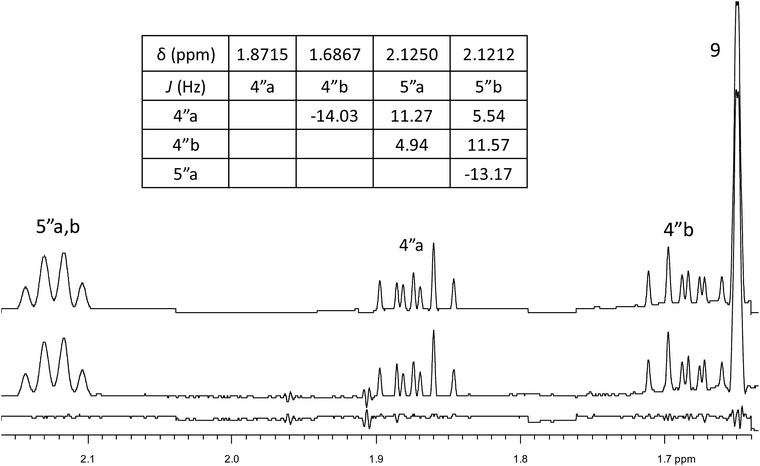
The initial structure for the sesquiterpene aquatolide (7) described in Asteriscus aquaticus,56 contained an unusual bicyclo-hexane ring structure. This was revised recently to 8 by additional NMR experiments, X-ray diffraction analysis and quantum chemical computations,4 as well as by independent total synthesis.57,58 However, a thorough analysis of just the 1H NMR spectrum, enabled by the availability of the raw data, would have revealed problems with the first interpretation. The feasibility of this approach was demonstrated via HiFSA (1H iterative Full Spin Analysis) from the FIDs of the original 1D 1H NMR spectra,59 obtained with both the re-isolated natural4 and synthetic57 material. Using the PERCH software tool and an established HiFSA workflow,60–62 it was possible to extract no less than seven coupling constants from signals that had only been described as “multiplets” in the original work (see example of H-5a in Fig. 5). Some of these are surprising from either the original or the revised structure. E.g., aquatolide shows a 4J coupling of 7.2 Hz through saturated carbons, but this is fully consistent with the quantum mechanical calculations from the revised structure. While being unexpectedly large and not leading to a “hidden” signal splitting, the 7.2 Hz coupling could be fully explained as being due to the spin–spin interaction between two bicyclic bridgehead hydrogens via two routes. It is important to note that the tabulated NMR data were/are not an adequate tool for the reader to verify the assignments, whereas the digital 1H NMR data provided this opportunity. NOESY and 13C NMR spectra were also important for differentiating between the initial and revised structures.Evolving from the aquatolide study, was also the introduction of Quantum Interaction and Linkage Tables (QuILTs),59 which provide a checkerboard presentation rather than a classical table as a means of rapidly viewing the relationship between coupling constants and bonding proximity. The combination of available digital data and a more intuitive representation of the interpreted data, such as in QuILTs, would have pointed out the inconsistencies in the original structure that were in fact expressed in the J-coupling patterns and signal multiplicities. It should be noted that HiFSA profiles enable the calculation of NMR spectra at any desired resonance frequency, meaning that the NMR information extracted from a given spectrum becomes independent of the magnetic field strength. This is particularly useful for 1H NMR based dereplication, when reported data has used a different magnetic field. Compiling HiFSA data in the form of QuILTs has the added advantage of being a more intuitive representation for human interpretation and providing a tabular format that is closely related to the data matrices of spin simulation tools.
Although QuILTs provide a good check on the structure elucidation and a more comprehensive description of the 1H NMR spectra, they do have to be considered together with configurational arrangements. Chemical synthesis and X-ray crystallography will remain the final arbiter of structure determination. However, the former in particular will be greatly simplified by starting with the correct structure, and the initial structure is almost invariable the outcome of spectral analysis. The aquatolide case exemplifies the need for thorough and complete analysis of NMR spectra, and the need to go beyond first order visual analysis of a processed 1H NMR spectrum. It also reminds researchers of the illustrious quote the astronomer, Carl Sagan, whereby “extraordinary claims require extraordinary evidence”, which is widely considered a variation of the principle by the Bayesian statistician, Pierre-Simon Laplace, according to which “the weight of evidence for an extraordinary claim must be proportioned to its strangeness”.63 Finally, the case highlights the power of advanced post-acquisition processing in structure elucidation.
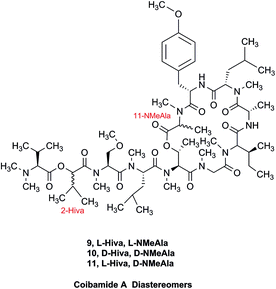
3.4 The case of coibamide A
The cyanobacterial coibamide A (9) is a highly N,O-methylated depsipeptide (1287 Da), comprising 11 residues with 13 stereogenic centers, that was originally proposed as the “all-L” diastereomer (10) in 2008.64 Ensuing attempts at total synthesis were initially plagued by inefficient coupling of the sterically hindered N-methyl amino acids, which promotes racemization and diketopiperazine formation,65 and requires tedious residue-specific optimization of coupling reagents and conditions. Ultimately, Yao et al.66 reported the configurational revision of coibamide A (9) in 2015, with inverted configuration of both the [Hiva] and [MeAla] residues compared to the originally assigned structure. The published 1H NMR spectra for this [D-Hiva2], [D-MeAla11]-coibamide A (9) and the NP were very similar (Fig. 6), while the 13C NMR spectra matched perfectly. The McPhail group collected and fully assigned comprehensive 2D NMR data for this synthetic product, confirming the match with the NP.67 However, the complexity of the 1H NMR spectrum for coibamide A, and their experience with 1H NMR analyses of synthesized methylated oligopeptides, highlighted the potential difficulty in discerning differences between the crowded 1H NMR spectra for closely related diastereomers of a NP with the size and number of stereocenters of coibamide A. Consideration of the potential for multiple N-methyl conformers (rotamers), and/or diastereomers arising from sluggish coupling reactions, as well as the presence of impurities, was critical in evaluating synthetic products and moving ahead with SAR studies. Before the configurational revision of coibamide A was reported, He et al.68 achieved the total synthesis in 2014 of the proposed “all-L” diastereomer 10, which yielded 1H and 13C NMR data that clearly did not match those for the NP (Fig. 7), and was 1000-fold less cytotoxic. Notably, structure 10 also appeared to be more flexible than the NP (in CDCl3), as indicated by apparent N-methyl conformer signals, as judged by the chemical shift pattern and signal areas. Concurrently, while investigating the synthesis and SAR of coibamide A, Fujii and coworkers produced [D-MeAla]-epimer 11,69 as well as several unpublished diastereomers. The latter diastereomers vary by single stereocenters and are under investigation for their variable biological activity, with potential uncoupling of cytotoxicity from their primary mechanism of action as inhibitors of cellular protein secretion70 involving the Sec61 translocon.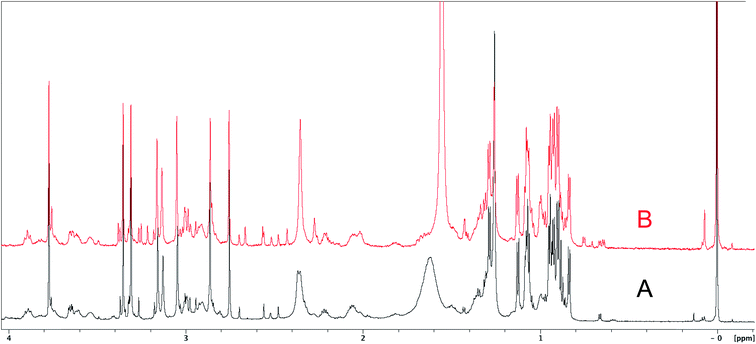 | ||
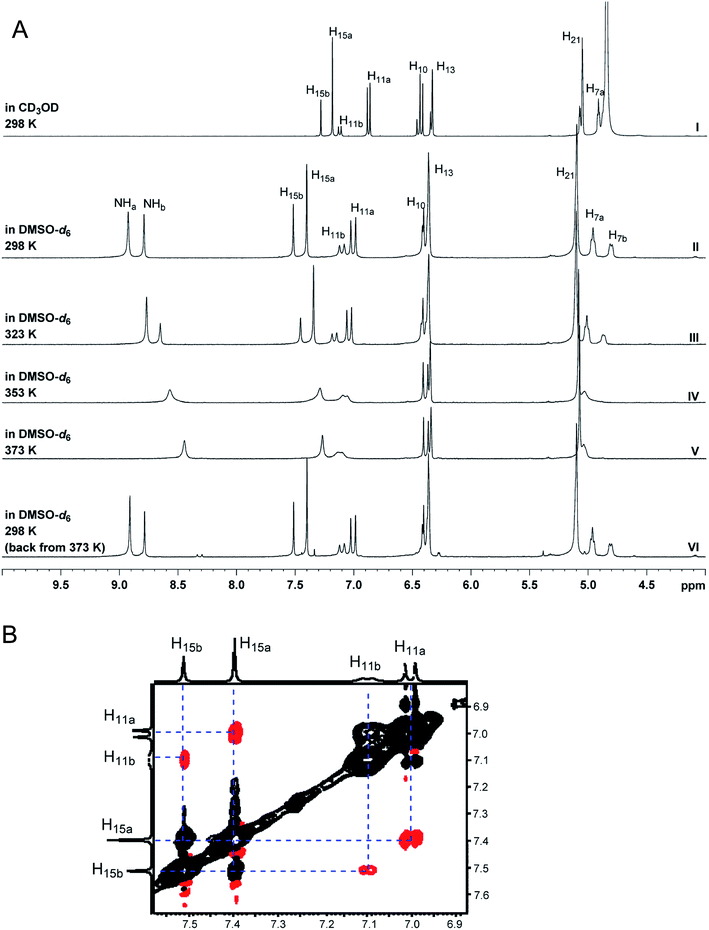
| Fig. 6 Partial 1H NMR spectra of the authentic natural product64 (A) and synthetic [D-Hiva2], [D-MeAla11]-coibamide66 (B). | ||
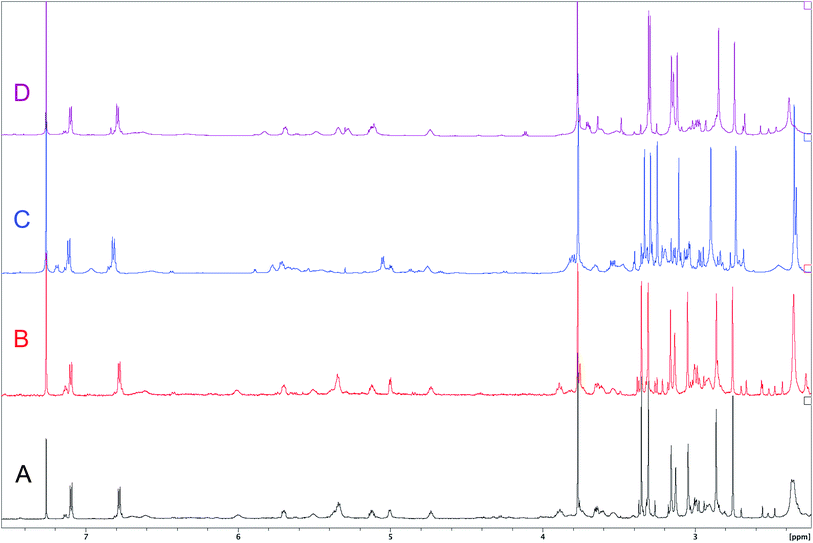 | ||
| Fig. 7 Downfield portion of the 1H NMR spectra of the authentic natural product (A),64 synthetic [D-Hiva2], [D-MeAla11]-coibamide (B),153 all-L-coibamide (C),68 and [D-MeAla11]-all-L-coibamide (D).69 | ||
Accurate verification of the absolute structure of each synthetic product is, thus, critical. Thus far, the 1H NMR data for published diastereomers do show discernible differences and consistencies relevant to configuration (Fig. 7), especially when raw data is processed consistently and directly overlaid for comparison to detect slight chemical shift discrepancies and changes in signal shape of overlapped resonances. Access to raw NMR data for synthetic products has also allowed specific integration of minor and/or major signals for quantitative evaluation of the contribution of N-methyl conformers, diastereomers and impurities, which substantially affect the biological activity of coibamide compounds.
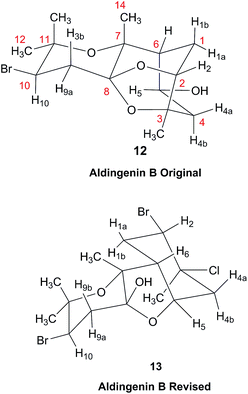
3.5 The structure of aldingenin B
The initially reported structure of aldingenin B (12), containing a highly unusual intramolecular ketal, was assigned based on extensive analysis of NMR spectral data (COSY, HMQC, HMBC).71 The reported structure was recently determined to be incorrect by total synthesis of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72 An alternate five-membered hemiacetal structure (13), was proposed based on computational simulations of the 1H NMR spectrum of both the originally reported structure and the revised proposed structure with comparison to the experimental NMR data for the synthetic material corresponding to the reported structure and the original NMR spectrum of aldingenin B.73
72 An alternate five-membered hemiacetal structure (13), was proposed based on computational simulations of the 1H NMR spectrum of both the originally reported structure and the revised proposed structure with comparison to the experimental NMR data for the synthetic material corresponding to the reported structure and the original NMR spectrum of aldingenin B.73Inspection of models of the reported structure reveals the H-6–H-5 dihedral angle to be 90° (±2°); the expected coupling of such vicinally orthogonal hydrogens is <2 Hz. The natural sample displayed an 8.4 Hz coupling between these nuclei, while there was no detected coupling between H-5–H-6 in the synthetic sample. Furthermore, the reported coupling constants for the “bridgehead” hydrogens H-6 and H-2 in the natural sample were reported as 9.0, 8.4 and 9.6, 6.3 Hz respectively. The expected value of coupling constants of such bridgehead hydrogens is <4 Hz, as observed in the couplings of H-2 (J = 3.6, 1.8 Hz) and H-6 (br.s) in the synthetic sample and similar structures reported by Dudley.74 Additionally, the HMBC correlation map of the natural sample did not display an H-2–C-8 correlation, whereas this vital HMBC signal was observed in the synthetic sample.
A major complicating factor with analysis of the NMR data for aldingenin B was interpretation of the coupling constants for the H-1 and H-2 hydrogen signals. The H-1 signal was reported as a multiplet and the H-2 signal J values were misinterpreted due to their non-first-order nature. Computation of the spin–spin coupling constants for the reported structures and the proposed structure (Table 1) reveal a tight correlation of the proposed structure with the calculated values.72 The originally reported H-2 apparent J's, 9.6 and 6.3 Hz, which are significantly different from those obtained by calculation (11.3 and 4.4 Hz), are more in line with the original bridged acetal structure, while the calculated values fit well with the proposed structure where the six membered carbocycle is more chair-like. It is noteworthy that the sum of the apparent J's, 9.6 + 6.3 = 15.9 Hz, is very close to the sum of the constants obtained from the multiplet simulation (Fig. 8), 11.2 + 4.8 = 16 Hz, and that of calculated J's for the proposed hemiacetal structure (11.3 + 4.4 = 15.7 Hz; Table 1; Fig. 9).
| Match | Match | |||
|---|---|---|---|---|
| Exp. J's (ref. 54), natural aldingenin B | DU8-calcd J's hemiacetal 13 | DU8-calcd J's aldingenin B | Exp. J'sb synthetic aldingenin B | |
| a Calculated J's are listed in descending order with a cutoff value of 2 Hz. b For consistency, an experimental 1H NMR spectrum of aldingenin B in CDCl3 was used. c Second order multiplet, simulation gives 11.2, 4.8 Hz with these simulated constants, calculated J's for hemiacetal 13 match the experimental with rmsd = 0.46 Hz. d It seems that this ddd (pseudo-quartet) was misreported as dd in ref. 71. | ||||
| 1 | m (overlap) | 14.8, 8.8, 4.4 | 14.2, 2.5, 2.4 | 14.5, 2.4, 2.2 |
| 14.8, 11.3, 8.5 | 14.2, 3.7, 2.0 | 14.5, 3.8, 2.1 | ||
| 2 | dd (9.6, 6.3)c | 11.3, 4.4 | 2.5, 2.0 | 2.5, 2.0 |
| 11.2, 4.8 | ||||
| 4 | dd 14.5, 9.6 | 14.6, 9.6 | 14.1, 8.1 | 13.7, 7.9 |
| dd 14.5, 4.7 | 14.6, 5.2 | 14.1, 7.2 | 13.7, 7.5 | |
| 5 | ddd 9.6, 8.4, 4.7 | 9.6, 9.0, 5.2 | 8.1, 7.2 | 8.1, 7.5 |
| 6 | dd 9.0, 8.4d | 9.0, 8.8, 8.5 | 3.7, 2.4 | br.s. |
| 9 | t 13.5 | 13.4, 12.9 | 13.1, 12.8 | 13.0, 12.6 |
| dd 13.5, 3.6 | 13.4, 4.6 | 12.8, 4.9 | 12.6, 4.6 | |
| 10 | dd 13.5, 3.6 | 12.9, 4.6 | 13.1, 4.9 | 13.0, 4.6 |
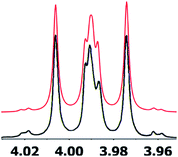 | ||
| Fig. 8 Simulation of the H2 multiplet (3.99 ppm) of aldingenin B with J1a,2 = 11.2 Hz and J1b2 = 4.8 Hz (apparent constants: 9.6 and 6.3 Hz, reported by Crimmins et al.96). | ||
Had the raw electronic FID been available, once the original structure was in question, a reanalysis could have revealed the incorrect interpretation of the H-1, H-2 coupling constants and significantly simplified the structural revision. This case further exemplifies the clear need for thorough and careful analysis of NMR spectra when assigning structure and highlights the need to look past first order analysis of 1H NMR data. This example demonstrates the continued need for synthetic (or X-ray crystallographic) verification of structure and illustrates the power of computational methods in structural assignment.
A major part of the theme of this review is the need to be able to extract all of the data pertaining to a proposed structure, especially from 1H NMR spectra. However, in the context of the structures discussed here, it is critical to emphasize that NMR-centric elucidation work does not exclude the need to examine other data, in particular data related to the molecular formula. It is obvious that the initial investigators71 did not critically consider the mass spectrum, by quoting an HR-EIMS of 346.0748 and not considering the challenges associated with the EIMS of highly halogenated compounds.
3.6 Clearing the literature of blatantly incorrect natural product structures
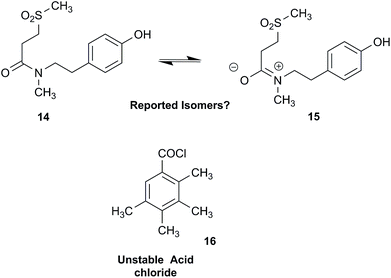
NPs present a colorful palette of functional groups, and it is indeed difficult to find totally “abiotic” combinations of atoms, at least between those unreactive with water, the milieu of life. Phosphines and azides are among the most remarkable examples, but unusual functional groups that are unprecedented or very rarely documented in synthetic compounds can occur as NPs. One such case is that of β-lactam antibiotics: at the time of their original structure elucidation, it took long to dispel the proposal of considering them being oxazole derivatives.75 While it is, in principle, possible that NPs could “anticipate” the existence of some functional groups or combination of functional groups overlooked by synthesis or by the known biosynthetic pathways,76 formulas that are chemically impossible or too unstable for isolation are still reported as NPs, despite continuous and significant advances in spectroscopic techniques.Correction sometimes requires only basic knowledge of organic chemistry. For example, the doubling of NMR resonances in the spectra of the amide 14 was ascribed to equilibration with its “isomer”, 15.77 The latter is actually a resonance form of 14, and the equilibration process detected in the NMR is what has to be expected for the rotameric interconversion of E‐and Z‐amide stereoisomers. Also doubtful is the isolation of the acyl chloride 16, since this functional group is unstable in water and unlikely to exist in Nature.78
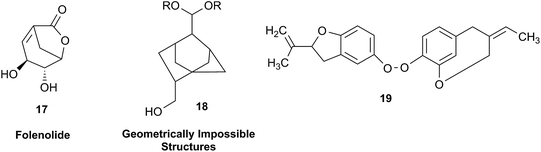
In other cases, correction can be achieved via re‐analysis of the NMR data, which typically requires the raw NMR data to be available. Several examples exist, such as folenolide (17)79 which violates Bredt's rule; the “isoprenoid” core of the antifungal 18,80 which is geometrically impossible in any isomeric form; or the trans‐cycloheptene structure assigned to the peroxide, 19.81 A re‐evaluation based on the tabulated data of chemical shifts, coupling constants, and 2D correlations can lead to a successful revision.82 However, this kind of re-evaluation is generally difficult as documented spectroscopic assignments can be biased, as “problematic” signals might have been overlooked originally, or entire sets of signal have been misassigned. As a result, even with the availability of a synthetic version of the alleged formula, comparison of tabulated NMR spectroscopic data alone is insufficient for a structural revision, leaving the issue unsettled. The availability of the original FIDs would make such revisions possible without the need of synthesizing a non-existent NP.19 This would accelerate correction of wrong structures and minimize their appearance via peer review by making the NMR data fully transparent to peers, reviewers, editorial teams, and subsequently to readers.
3.7 Bredt's rule as a check on structure correctness
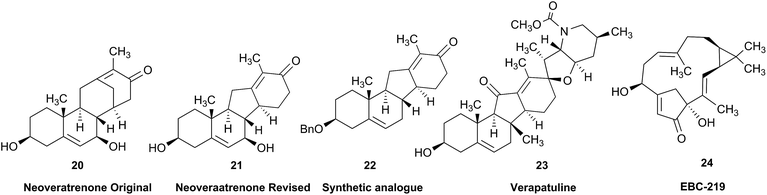
Research into the structure determination of monoterpenes by Julius Bredt in the late 1800's, early 1900's, gave rise to the term Bredt's rule. This rule states that the terminus of a double bond can not exist at bridgehead positions (i.e., branching position) of a bridged bicyclic system (Fig. 9).83–85 Interestingly, however, it was the physical organic chemistry community that laid out empirical guidelines for anti-Bredt systems,86–91 which became the holy grail of synthetic chemists for decades. Meanwhile, the area became somewhat foreign to the NP community. Classification of NPs with a bridgehead double bond as anti-Bredt or not was difficult, because the underlying aspect of Bredt's rule was stability and the large majority of NPs are stable.92 The Williams group became intrigued with a report by Cong et al., reporting the isolation of neoveratrenone (20).93 The structure presented caught their attention because it contained a bicyclo[3.3.1] moiety with a bridgehead double bond. Although, the parent bicyclo[3.3.1] anti-Bredt system had been previously synthesized it was reported to be unstable. It was possible that physical properties of the entire NP skeleton enhanced stability, or the structure had been misassigned. Williams and Savchenko82 turned to the elucidation data, however, only 1H, 13C, HMBC and NOESY NMR data were presented in the article, with no ESI† available (i.e., no additional 1D and 2D NMR data). Without the full gamut of 1D and 2D digital data, considerable detective work was required to interrogate the proposed structure. Nevertheless, they were able to reassign the structure of neoveratrenone, as 21, based on a combination of the available data, comparison with related synthetic analogues (e.g., 22, and the co-isolation of verapatuline (23)) by Cong et al.82 The latter lending substantial biosynthetic support to the proposed reassigned structure 21.By serendipity they later isolated EBC-219 (24), containing a bridgehead double bond, but in a larger macrocycle.94 This led Krenske and Williams to develop in silico parameters based on olefin strain (OS) energies that now enable the NP community to cross check the validity of NPs that are proposed with bridgehead double bonds.95
3.8 Correct analysis of coupling constants
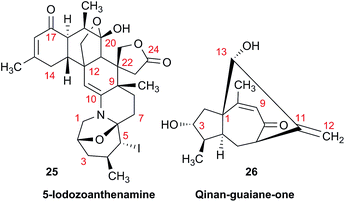
As fast and accurate computational methods become available to organic and NP chemists, there is an increasing demand for high quality NMR data available for examination and processing in different ways. The need for raw FID data is most pressing in 1D 1H NMR spectra, where signal overlap and second order effects often present challenges in transcribing the complexity of the spectra into neat tables. Often complex multiplets are interpreted with oversimplification. Few research groups report nuclear spin–spin coupling constants (SSCCs) with due diligence and high precision. It is common to encounter a doublet of doublets with two SSCCs differing by as much as 2 Hz or more described as a triplet with an average coupling constant reported. The accuracy of computational predictions of SSCCs has reached 0.3–0.5 Hz.96–98 Often, one faces a situation where a difference in 1–2 Hz is the only criterion for differentiating between two candidate structures. Computations may provide the answer but, without experimental data reported with appropriate accuracy, this becomes a moot point. In addition to this, typos and other errors made in the process of transcribing spectra into publication tables are inevitable, while the low quality images of these spectra in the ESI† section do not help, and serve mostly as a quality/purity control.An example is the zoanthamine-type alkaloid 5α-iodozoanthenamine (25), from Zoanthus kuroshio.99 DU8+ computations22,23 of its NMR spectra identified irreconcilable differences between the computed and the experimentally reported 1H SSCCs, implying a misassignment. However, the predicted 13C NMR chemical shifts satisfactorily matched the experimental values. Closer examination of the SSCCs from a 600 MHz experiment revealed that many of them deviate from the calculated values by a factor of 1.5. For example, the constants for H-1 through H-14a needed multiplication by 1.5 to reconcile them with the computed values; H-14b did not need such correction, while most of the remaining SSCCs needed it again. As the 1H NMR spectra for several alkaloids reported in this paper were run at either 600 or 400 MHz, it was hypothesized that a “clerical” error had been introduced by measuring the line spacing on a hard copy spectrum and multiplying it by the wrong working frequency of the spectrometer. Revisiting the raw FID data with NMR processing software would have alleviated all problems.
Qinan-guaiane-one, (26) a guaiane sesquiterpene isolated from Aquilaria sinensis,100 is another representative example where raw NMR data would have helped alleviate confusion with structure assignment. The reported geminal spin–spin coupling constant J6a–6b = 10.3 Hz differs from the calculated value by almost 2.5 Hz (Jcalc = 12.7 Hz). This error is probably not a typo, but rather it is due to the fact that the multiplets are not first order and therefore more sophisticated line fitting of the multiplets is needed to extract the actual SSCCs here. Qinan-guaiane-one is also an instructive example of the importance of accurate determination of small constants. The signal for H-13 is accurately described as a 2.3 Hz triplet. It does not have vicinal neighbors and therefore the configuration of the C-13–OH group is more difficult to assess. Luckily, the calculated allylic H-13–H-22 SSCCs for the correct (shown) stereoisomer, 2.4 and 2.1 Hz, are much closer to the reported experimental value of 2.3 Hz than the calculated allylic constants for the alternative epimer at C-13, 0.51 and 0.54 Hz. The combined evidence, together with a good match of 13C NMR chemical shifts (rmsd = 1.44 ppm) indicate that the originally reported qinan-guaiane-one structure is correctly assigned, but the discrepancy in the calculated and experimental values for geminal J6a–6b is most likely due to second-order effects which are not accounted for in the authors' reporting the apparent value for this constant.
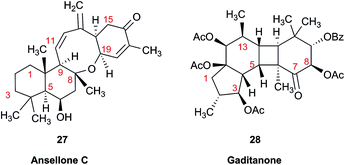
Another common problem is misinterpretation of multiplet shape in 1H NMR spectra. The terpene metabolite, ansellone C (27) was isolated from the marine sponge Clathria gombawuiensis.101 A multiplet belonging to H-19, critical for the determination of the configuration at the fusion of rings C and D, was reported as a dd 8.5 and 4.6 Hz, while the calculated values were 4.7 and 4.3 Hz. In the copy of the spectrum in the ESI,† this multiplet does not look like a dd 8.5 and 4.6 Hz, but it is virtually impossible to extract any useful information from the picture. In summary, the configuration of ansellone C (28) is either misassigned or the H-19 multiplet is interpreted and reported incorrectly. Raw FID data would have helped to resolve this issue.
In general, 13C NMR spectra are less prone to the problems outlined above, but even there one sees occasional misinterpretation of an impurity signal and typos in transcribed tables of chemical shifts are plentiful. For example, a complex diterpenoid, gaditanone, (28) possessing an unprecedented 5/6/4/6-fused tetracyclic ring skeleton, was recently isolated and characterized by solution NMR,102 with its only carbonyl carbon, C-7, assigned the chemical shift value of 206.6 ppm. The DU8+ calculated value for this carbonyl carbon is 213.8 ppm, indicative of misassignment. However, a cursory look at the copy of the spectrum in the ESI† revealed an unannotated extra signal at 29–30 ppm, implying that acetone is an impurity in the sample. It is plausible that the actual carbonyl signal belonging to 28 was overlooked as it was too small. Exclusion of the carbonyl signal from the statistics improves the match of the experimental and computed 13C NMR chemical shifts to rmsd = 1.23 ppm. This excellent accuracy leaves no doubt that the structure of the diterpenoid is correctly assigned. It also suggests that the authors should examine the vicinity of 212–214 ppm for the actual carbonyl signal belonging to gaditanone (28).
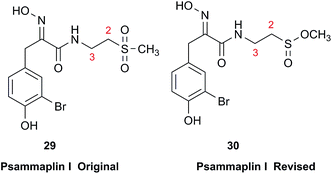
3.9 Sulfones vs. sulfinates
Chemical investigation of an Australian sponge, Aplysinella rhax, led to the isolation of psammaplins A, I, and J.103 Psammaplin I (29) was first isolated from Pseudoceratina purpurea and formulated to contain a sulfone moiety, from IR data.104 The metabolite was later reported from a Jaspis/Poecillastra sponge association without additional comment on its structure.105 The first published NMR data reported the H-2 signals at 2.96 ppm (m) and 3.75 (s), and H-3 as a triplet of doublets (td) centered at 3.62 ppm with J values of 6.5 and 2.0 Hz. Sulfones are not normally chiral since two of the substituents attached to sulfur are oxygen, therefore each set of the methylene hydrogens at C-2 and C-3 should have been equivalent. Data acquired at 500 MHz in CD3OD by the Garson group revealed diastereotopic 1H multiplets at 2.91 and 3.01 ppm assigned to the H-2 hydrogens, and a complex two hydrogen signal centered at 3.62 ppm for the methylene hydrogens at C-3; these data supported a methyl sulfinate, as in 30.Even despite the incorrect chemical shift value originally reported for one of the H-2 signals, their data were inconsistent with a sulfone functionality. Although the (H-3)2 signal superficially resembled the triplet of doublets as reported, it showed ten lines on close inspection, and was best described as an AB system (3.63 and 3.61 ppm) in which each line is split into a triplet by two vicinal couplings of ∼6 Hz. Owing to signal overlap, only ten of the predicted twelve lines were resolved. Repeated acquisition of the 1H NMR data at 900 MHz confirmed the complexity of the H-3 and H-2 signals. At 500 MHz, the two chemical shifts for H-3 were calculated as 3.630 and 3.614 ppm with 2J = 14.8 Hz, and at 900 MHz as 3.631 and 3.615 ppm with 2J = 14.9 Hz. Detailed modeling of the H-2 and H-3 spin systems was carried out on the 900 MHz spectrum of psammaplin I (29). The signal at 3.75 ppm for the OMe group of the methyl sulfinate had been incorrectly assigned to H-2; however, the signal integrated for 1.8H owing to partial transesterification by the NMR solvent.
Concurrently with the above NMR study, the Ireland group independently prepared two methyl sulfinate ester derivatives of psammaplin A, one of which had spectroscopic data identical to psammaplin I.106 However, their 1H NMR data were run at 500 MHz, as were the original data,104 so the nonequivalence of the H-3 hydrogens that resulted from the presence of the chiral sulfur atom in psammaplin I may not have been evident.
This case study highlights the valuable role of very high field NMR in the dereplication of marine NPs. When chemical shifts and coupling constants are reported accurately, the values can be compared for a sample run at any field strength.
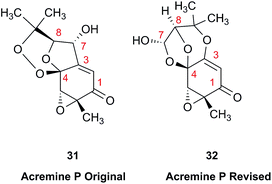
The prediction of chemical shift values by quantum chemical methods has provided valuable insights into NP structures, including the correction of published structures. The Garson group recently revised their published structure for acremine P, a metabolite of Acremonium persicinum, following a comparison of calculated and experimental NMR chemical shift data.107 When the originally published structure, 31,108 was examined using a combination of computational approaches that provide 13C NMR shifts with mean absolute error (MAE) of ∼1.6 ppm, there were deviations of 20.4 ppm for the alkene carbon (C-2) and −23.0 ppm for the hydroxymethine carbon (C-7). Re-evaluation suggested the signal at 95.0 ppm (C-7) had been incorrectly assigned to a secondary alcohol instead of an acetal or lactol. Furthermore, the alkene carbon signals (102.4 and 162.5 ppm) indicated a polarized double bond, likely enolised given the number of oxygen atoms in the molecule. HMBC correlations of both the lactol hydrogen at 5.83 ppm (d) and the signal at 4.15 ppm (s) for the hydroxymethine hydrogen H-8 to the acetal carbon at 99.0 ppm supported the revised planar structure, 32.
DFT computations did not safely distinguish between four proposed diastereomers of acremine P owing to the close similarity of the calculated 13C NMR shift values. The calculated chemical shifts were further examined using the DP4+ computational approach developed by Sarotti et al.109 to assign the most probable diastereomer.109 Using the 13C NMR data alone, the probability was 99.7% that 32 was the correct diastereomer. Coupling information, notably the zero coupling between the vicinal lactol and hydroxymethine hydrogens, as well as JH7–H8 couplings calculated for each stereoisomer using the methods of Kutateladze et al.,98 together with NOE data further supported the relative configuration shown.
Garson et al. had earlier reported that hydrogenation of acremine P yielded acremine A as the sole product;108 clearly structure 31 could not be correct as the dioxolane ring of the revised structure was incompatible with the tetrahydrofuran ring previously ascribed to acremine P. The revision of the structure of acremine P highlights the valuable role of computational studies in evaluating the structures and configuration of complex NPs. In each of these cases, the original FIDs of both the 1H and 13C spectra can provide a basis for quantum mechanical analysis and a rapid resolution of the structural assignment problems.
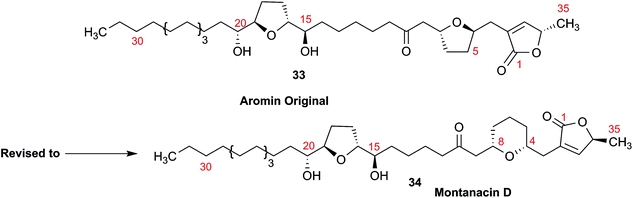
3.10 Methylene signal assignments in the structural revision of aromin to montanacin D
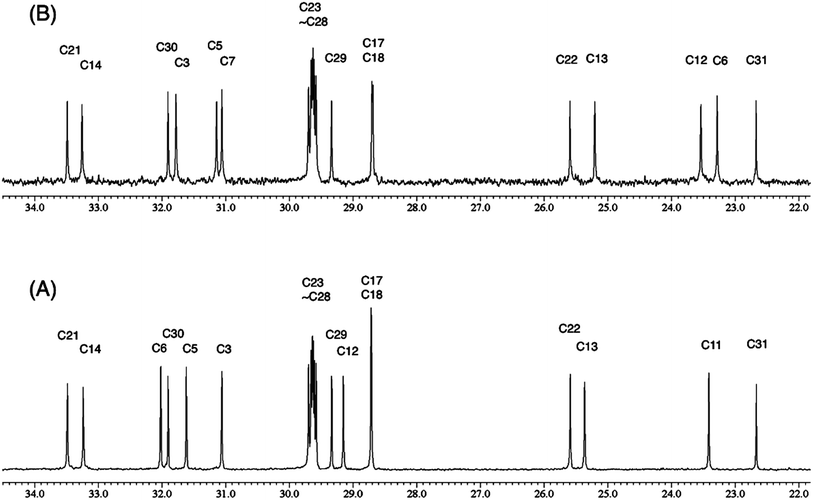
The originally proposed structure of aromin (33)110 an Annonaceous acetogenin,111,112 was revised recently to be montanacin D (34) by total synthesis of the proposed structure of aromin,113 and re-examination of NMR data of synthetic montancin D114 and other related isomers,115 especially 13C NMR data using CAST/C NMR Structure Elucidator,116 and MS fragmentation analysis of TMS derivatives of 33 and 34.113When comparing originally reported NMR data and synthetic compounds, 13C NMR data with a tabulated 13C NMR chemical shifts of aromin and montanacins were insufficient because exchangeable methylene signals were lumped together in the region of 31.1–31.9 ppm for C-3, C-5, and C-6 of aromin,110 and in the wide range chemical shifts such as 23.4–31.9 ppm for thirteen carbon signals in the case of montanacins D and E.117 Complete assignments of severely overlapped methylene signals in 1H and also 13C NMR were difficult or impossible in some cases, but relevant information of exact chemical shift values, number of signals, and intensities of them are very important for comparison of NMR spectra directly among NPs and synthetic compounds. Fig. 10 shows the 13C NMR spectra for methylene regions of synthetic montanacin D and the proposed structure of aromin are shown. From the viewpoint of structural revision of aromin, the assignment of 23.28 ppm for C-6 of 34 is critical as the methylene signal at the γ-positions from the ether oxygen in the tetrahydropyran ring, which is absent in the spectra of the synthetic 33. In comparison between 13C NMR data of 33 and 34, assignment of 29.15 ppm for C-12 at the γ-position from the carbonyl group at C-9 and the δ-position from the hydroxyl group on C-15 will be important to determine the methylene chain length between C-9 carbonyl and C-15 hydroxyl groups. Together with these assignments, signal assignments of C-3, C-5, and C-6 for 33, and C-3, C-5, and C-7 for 34 are important to characterize the partial structure of the tetrahydrofuran or tetrahydropyran ring system, respectively. Exact 13C chemical shift values could be obtained from raw NMR data readily and, e.g., are useful for direct comparison and to create database queries for the CAST/C NMR system. For acetogenins, 1H NMR raw data of intact compounds are also useful, but also raw data of MTPA ester derivatives are very important. These are required to determine the absolute configuration of hydroxyl groups and relative configurations of separated chiral centers.114,115
3.11 The case of aglalactone
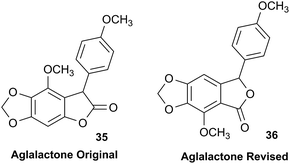
The NMR based elucidation process generally follows two major strategies, or a combination thereof. One strategy involves spin–spin interactions utilized to generate different spin networks, the other is focused on chemical shifts and relies on increment rules. The number of experimental data-points (chemical shifts, J-couplings, dipolar couplings) outnumbers the atoms present in a molecule by far. This results in a high number of degrees of freedom, and successful structure assignment requires a combination of two major thought processes in parallel: intuitive interpretation of all available data, and knowledge of NP biosynthesis. Also, wherever expert reasoning is part of a research strategy, errors are well within the realm of possibility.When investigating the genus Aglaia in the late 1990s, Hofer and colleagues came across a molecular scaffold that was unusual for the Meliaceae: a benzofuranone lactone congener named aglalactone. It was determined to bear a lactone moiety and appeared to fit well into the biogenetic reasoning for far more complex compound classes such as the panellins or flavaglins.118,119 Integrated analysis of HR-MS, IR, and NMR data was straightforward and led to the assignment of structure, 35. “Missing” NOE contacts were explained by configurational and spatial considerations. However, when re-investigating the aglalactone 13C NMR data by means of the CSEARCH database (see also Section 8.2),120,121 it became evident that a single 13C NMR shift value (a CH element resonating at about 81 ppm) showed a significant mismatch relative to the calculated value. Hence, a reinvestigation of the structure elucidation process was commenced. An alternative hypothesis was generated and a set of possible regional isomers formulated. Independent acquisition of additional spectroscopic evidence on a re-isolated analyte was key for this strategy. After time consuming procurement of the analyte, the generation of a complete NMR data set including HMBC and NOE spectra as well as a lanthanide induced shift (LIS) NMR data sets were recorded. The new data strongly supported a new structural hypothesis, 36, which was based on “inversion” of the lactone moiety. Subsequently, the structure and the scaffold ring system were revised from a 2,3-dihydrobenzofuran-2-one to a 3H-isobenzofuranone.122 Within the past decade, the isolation of aglalactone from several sources and the discovery of an additional congener123–125 represent an independent and strong confirmation of the scaffold correction undertaken by Seger and colleagues.
Although NMR data from the original investigation were available at the time of the aglalactone revision, the data set was deemed incomplete, as HMBC data was unavailable. While it was possible to re-isolate the compound, the group of Hofer and Greger experienced further difficulties. In one instance, a collaborative effort was necessary,126 in another case, only total synthesis was able127 to correct a structure.
Almost two decades after the structural revision, the correct structure of aglalactone is still not disseminated properly to the scientific community, including in major resources and databases. Notably, if NMR raw data sets would be available and become a routine part of deposited data, it would be straightforward to correlate the structures and (different) structural proposals via their fingerprint NMR spectra, independent of the limitations of spectral figures in publications and their ESI.† Furthermore, such raw data would enhance the traceability of any novel congener claims relative to the first reported congener of a given compound class. In such instances, a series of NMR data signals would typically show close matches between the congeners, thereby proving unequivocally the relationship of the compounds via spectral similarity. This kind of “similarity feature” can be transferred from the analogue world of expert reasoning to computer based similarity searches. The approach is already very well-known from other research fields such as the LC-MSn or GC-MS/MS based general unknown screening (GUS) in toxicology128 or the spectral feature comparison approaches, followed by IR/NIR based applications in clinical chemistry or forensics.129
3.12 Diastereoisomers and rotamers
1H NMR spectra provide not only two-dimensional structural information but also very detailed configurational data on NPs, e.g., epimers or rotamers, which have been many times neglected, as they were considered as signals due to impurities and are thus not reported in the final discussion on the structure elucidation. Some flavanone glycosides and flavone-8-C-glycosides exemplify this problem.In the past, flavanones were believed to occur in nature as levo-rotatory (2S)-isomers because the enzyme catalyzing the conversion of chalcones to flavanones is highly stereospecific.130 However, flavanones and their glycosides are present as enantiomeric and diastereomeric mixtures, respectively. Among others due to ring-opening of flavanones under basic conditions131 or instability and rapidly recyclization to flavanones in a non-stereospecific manner.132 In the case of the aglycone of a flavanone, naringin, the presence of stereoisomers cannot be observed in the 1H NMR spectra because naringenin has only one chiral center (C-2), so the two enantiomers have identical spectra. The attachment of a sugar yields various glycosides and these represent the most abundant form of naringin in nature. However, the introduction of one or more other enantiomeric centers results in a mixture of different diastereoisomers with different chemical properties and thus also different NMR spectra. Similar to the naringin case, the 1H NMR spectra of other flavanone glycosides, like hesperidin and neohesperidin are characterized by the clear presence of signals of two diastereoisomers.133 In the 1H NMR spectrum the ratio between diastereoisomers is easily calculated from the raw 1H NMR data. For example, in the case of neohesperidin the 1H NMR spectrum shows a bigger difference in the ratio of the two stereoisomers of the molecule (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 between two isomers), as compared to naringin (2
4 between two isomers), as compared to naringin (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 between two isomers).
3 between two isomers).
Another group of isomers with different chemical properties, are the rotamers which are generated from conformational isomerism, in which the rotamers cannot easily be interconverted by rotation around a single bond. In nature, many 8-C-glycosides of flavonoids are often found to have rotamers due to steric hindrance at the C–C glycosyl flavone linkage.134 In the case of vitexin, the chemically equivalent H-2′ and H-6′ hydrogens show two broad signals due to rotamers. In the 1H NMR spectrum of orientin, another flavonoid 8-C-glycoside, signal broadening is detected around 7.5 ppm (H-2′) because of the presence of rotamers. However, the isomers isovitexin and isoorientin with C-glycosidic sugars at C-6 do not show the presence of rotamers.
It is generally accepted that plant metabolites are produced in a stereospecific way because of the involvement of enzymes in many biosynthetic steps. However, different stereoisomers of the same compound may exist in nature, either as side-products of an enzymatic reaction or after a chemical conversion. By neglecting minor signals in the NMR spectra of NPs, by marking them as impurities important information is lost. Not reporting the full raw data, means that later colleagues might have problems in purifying compounds as they are not aware of the extra signals due to these situations. Therefore, any paper on structure elucidation and identification of NPs, should give the full raw NMR data.
3.13 Data ambiguity
The marine NP, gallinamide A (37) was first isolated from a cyanobacterial Schizothrix species collected from a reef near Piedras Gallinas in 2009.135 The structural assignment used classical 1D and 2D NMR and mass spectrometry methods. The absolute configuration required a combination of chemical degradation and chiral chromatographic analyses. Although this was successful for most chiral centers, the absolute configuration of the terminal (N,N-dimethyl isoleucine) residue was not determined due to a lack of material, but was postulated as possessing the L configuration based on biogenetic arguments.136Shortly after this initial publication, a second compound with the same planar structure was published.136 This new compound, symplostatin 4 (38), possessed the same relative configuration as gallinamide A at all the assigned chiral centers (see chemical drawings). In addition, the absolute configuration for the N,N-dimethyl isoleucine residue was determined, and reported as L. A footnote in the manuscript describing the discovery of symplostatin 4 stated that the NMR data between symplostatin 4 and gallinamide A differed significantly in the N,N-dimethyl isoleucine region, and suggested that the two compounds were therefore, logically, diastereomeric.
Subsequently, several groups have pursued total syntheses of these structures.137–140 The first, published in 2010 reported the synthesis of symplostatin 4 and presented NMR data that differed significantly from those reported for gallinamide A, particularly in the N,N-dimethyl isoleucine region (Fig. 11, highlighted in red).139 Subsequently, this same group synthesized all four possible diastereomers of gallinamide A in an attempt to resolve the outstanding uncertainty about the structure of this metabolite. In collaboration with the author who originally isolated gallinamide A, all four of these compounds were subjected to full de novo structure elucidation, with the structures blinded to the chemist performing the structure elucidation to eliminate bias in the assignments. Surprisingly, when the resulting hydrogen and carbon chemical shift values were compared to those for the NP, the values for the L-isoleucine derivative were the only ones that matched the data from the original gallinamide A data.138 Although initially reported to have significant variations in the N,N-dimethyl isoleucine region, subsequent comparisons of the 1D NMR spectra in CDCl3 show that the variations between the spectra are minimal.138 Perhaps confounding the issue, the original isolation of gallinamide A was tabulated in CD3CN in text, but additionally provided the unannotated CDCl3 spectra in the ESI.† Submission of the 1D FID files would have enabled more accurate comparisons between the two spectra directly, helping to minimize ambiguity of the data (Fig. 11).
 | ||
| Fig. 11 NMR profiles of (A) gallinamide A (37), as reported and adopted from;135 (B) synthetic gallinamide A (37) as reported and adopted from;138 (C) symplostatin 4 (38) as isolated and adopted from;136 and (D) synthetic symplostatin 4 (38) as reported and adopted from.139 Variations in the spectra signals in the isoleucine region (1.0 to 3.0) led to speculation that the compounds were diastereomers. Further studies showed this was not the case after investigation and direct comparison of the region (highlighted red) by Conroy et al.139 Variations in pH and/or concentration give rise to other spectral differences, such as those seen in the NH region (highlighted green). The construction of this figure demonstrates the challenge of reporting high quality, scalable comparison data without access to the original files. | ||
As can be seen between the spectra of symplostatin 4 from the initial and the later gallinamide A synthesis reports, and studied thoroughly in (acyclic) peptides, effects of concentration and pH have substantial impact on the spectral characteristics of compounds, even in the same solvent (Fig. 11, highlighted in green).141–143 While beyond the scope of the initial studies, providing spectra of compounds in several solvent systems and under different conditions would still enable more detailed studies into the effects of pH and concentration on the spectra of a metabolite, and provide additional tools for investigators to more accurately dereplicate compounds under a variety of conditions. Additionally, as time progresses and data processing techniques are refined, tools such as deconvolution algorithms and non-FT processing techniques could be profitably applied to retroactive analysis of existing data sets.144,145
This vignette highlights the challenges associated with determining relationships between structures from tabulated data. Had all of the original data files been available, it would have been possible to directly compare the NP samples, and relate these to the synthetic materials. Instead, exhaustive synthetic efforts demonstrated that gallinamide A possesses a structure identical to symplostatin 4 (38).
3.14 The importance of details
Detailed analysis of the chemical shifts and coupling constants can not only elucidate the fine structures of complex natural compounds, but also provide useful information to probe the formation of the different intramolecular H-bonds in very similar analogs. For instance, phainanoids B (39) and F (40), possessed similar structures except for the different substituents at C-25 (Fig. 12). However, the chemical shifts and coupling constants of the OH-24 hydrogen showed major differences (Fig. 13), which were believed to be caused by the formation of different intramolecular H-bonds with the OR-25 moieties.146,147 For phainanoid B (39), the O![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) -24 resonated upfield with a large coupling constant (δ 2.26 ppm, d, J24,OH = 10.1 Hz), suggesting that the H-bond was formed between O
-24 resonated upfield with a large coupling constant (δ 2.26 ppm, d, J24,OH = 10.1 Hz), suggesting that the H-bond was formed between O![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) -24 and the oxygen atom of
-24 and the oxygen atom of ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif) H-25 in a five-membered cyclic interconnection (Fig. 13A), in which the H-bond angle and length simulated for OH-25 were ∼69° and ∼3.9 Å, respectively,148–150 and the dihedral angle between H-24 and O
H-25 in a five-membered cyclic interconnection (Fig. 13A), in which the H-bond angle and length simulated for OH-25 were ∼69° and ∼3.9 Å, respectively,148–150 and the dihedral angle between H-24 and O![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) -24 were 50° as generated by Hartree–Fock/3-21G.
-24 were 50° as generated by Hartree–Fock/3-21G.In contrast, phainanoid (40) showed a stronger H-bond, formed between O![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) -24 and the O-atom of the acyl carbonyl furnishing a seven-membered ring (Fig. 13B), with a more favorable H-bond angle and length of ∼148° and ∼1.7 Å, respectively.148–150 This resulted in a downfield chemical shift and a smaller coupling constant for the O
-24 and the O-atom of the acyl carbonyl furnishing a seven-membered ring (Fig. 13B), with a more favorable H-bond angle and length of ∼148° and ∼1.7 Å, respectively.148–150 This resulted in a downfield chemical shift and a smaller coupling constant for the O![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) -24 signal (δ 3.44 ppm, d, J24,OH = 4.8 Hz) compared with those of phainanoid B (39), owing to the deshielding effects of acyl group and the increased dihedral angle (∼69°). The coupling constants of H-24/O
-24 signal (δ 3.44 ppm, d, J24,OH = 4.8 Hz) compared with those of phainanoid B (39), owing to the deshielding effects of acyl group and the increased dihedral angle (∼69°). The coupling constants of H-24/O![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) -24 and the dihedral angles in the simulated conformers of 39 and 40 satisfied the Karplus equation.151,152 The other reported compounds of two subclasses with different substitution patterns at C-25 were also consistent with this interpretation.153 These insights became possible only via a full analysis of the NMR data and highlight the importance of careful analysis, especially of chemical shifts and coupling constants that together provided a useful tool for insight into the fine structures and conformations of complex NPs in solution.
-24 and the dihedral angles in the simulated conformers of 39 and 40 satisfied the Karplus equation.151,152 The other reported compounds of two subclasses with different substitution patterns at C-25 were also consistent with this interpretation.153 These insights became possible only via a full analysis of the NMR data and highlight the importance of careful analysis, especially of chemical shifts and coupling constants that together provided a useful tool for insight into the fine structures and conformations of complex NPs in solution.
3.15 Structural instability leads to dynamic complexity
In 2014, the Williams group disclosed the isolation of EBC-329 (41) and EBC-324 (42) from a plant collected in an Australian rainforest, together with collaborators from EcoBiotics Ltd and the QIMR Berghofer Medical Research Institute.154 EBC-324 (42) contained an unusually oxidized casbane (43) ring system, whereas EBC-329 (41) was the first example of a seco-casbane reported. Some years after a number of additional examples of both the seco-casbane [EBC-328 (44) and EBC-363 (45)],155 and casbane [EBC-304 (46) and EBC-320 (47)] series,156 were discovered.Approximately one year after reporting EBC-329 (41), Thombal and Jadhav described the synthesis of racemic 41 in 13 steps and 10% overall yield. However, the 1H NMR spectra data was inconsistent with that reported for the NP, although, the 13C NMR appeared to match.157 Unfortunately, the raw digital data was not available to analyze additional expansions that would have facilitated further understanding.
Only by chance, the Williams group was also working on the total synthesis of this molecule (i.e., 41), but lagged behind the Jadhav team by two years. However their route was superior in step count (7 steps), and was chiral, allowing the absolute configuration to be determined.158 It was, however, a serendipitous flaw in this route that revealed why the Jadhav et al.1H NMR spectra did not match that reported in 2014 for 41. The deployment of the Horner–Wadsworth–Emmons olefination protocol here did not provide a high level of E/Z stereocontrol, which led to a mixture of 41 and 48. The 1H NMR spectra of this mixture was a match to the Jadhav spectrum, although the ratio of 41 and 48 was different (i.e., Jadhav obtained a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture).
1 mixture).
The Williams group were able to purify the target (i.e., 41) by HPLC, and discovered that the purified material photoisomerized on exposure to laboratory light, giving an isomer that matched an impurity in their spectra of an isolated sample of 41 from 2014. Although it was not possible to unambiguously determine the structure of the major impurity, it was most likely either 49 or 50.
3.16 Acetogenins-the difficulty of configurational determination
Halogenated C15-acetogenins, containing at least 180 members, are widely present in the marine red algae of the genus Laurencia, and often feature one or more ether rings of different sizes.159 Among them, the structures of elatenyne (51) and its congeners, such as laurendecumenyne B (52), were originally assigned with a pyrano[3,2-b]pyran unit,160,161 but were ultimately corrected to possess a 2,2′-bifuranyl core, which contain the carbon and hydrogen connectivity of a pyrano[3,2-b]pyran unit.162 The overlapping signals in 1D and 2D NMR spectra made the structure and configuration elucidation difficult.Elatenyne was initially isolated from L. elata by Hall and Reiss in 1986 and originally identified as a pyrano[3,2-b]pyran structure (51) from its NMR data.160 In 2007, Wang and co-workers re-isolated elatenyne as a mixture with a structurally related congener, laurendecumenyne B (52), from the marine red alga L. decumbens, and the structures and relative configurations of these two compounds were established as pyrano[3,2-b]pyran derivatives by referring to the original structure and NMR data of elatenyne.161 Later in 2010, the structures were revised to 53 and 54, respectively, as being 2,2′-bifuranyl derivatives by Wang and co-workers,162 based on the total synthesis and the 13C NMR calculations reported by Burton and co-workers.163,164 However, the dibrominated 2,2′-bifuranyl structure, was assigned as a diastereomer of elatenyne, because the 1H NMR data recorded in CDCl3 appeared different.162 Later in 2011, Dias and Urban obtained elatenyne from L. elata and recorded its 1H and 13C NMR spectra in both CDCl3 and C6D6, which indicated that the originally reported 1H NMR signals of elatenyne in CDCl3 were incorrect and confirmed that the dibrominated 2,2′-bifuranyl metabolite obtained by Wang and co-workers was indeed elatenyne.165
The most likely structure (53) for elatenyne produced by DFT calculations of GIAO 13C NMR and its enantiomer were totally synthesized by the Burton and Kim groups in 2012, and their NMR spectra were compared with the raw spectra of the isolated elatenyne, despite the unmatched specific optical rotation values.166 Simultaneously, the relative configurations of the revised laurendecumenyne B (54) and (E)-elatenyne (55) were also confirmed by total syntheses,166,167 and the former was further evidenced to be a stereoisomer of notoryne (56) that was determined by NMR, EIMS, and chemical degradation methods.168 The 13C NMR signals of synthetic elatenyne, laurendecumenyne B, and (E)-elatenyne (55) were usually in good accordance with those of corresponding isolates. However this was not always the case for the 1H NMR data when the reported data was carefully rechecked.160,161,165–167 The splitting patterns and coupling constants of H-9 or H-10 are key to elucidate the relative configuration between the two tetrahydrofuran rings, and they should be the same or similar in view of the identical configurations around these two positions in 53–56. However, most of the isolates and synthetics (53–56) were reported to possess incongruous splitting patterns and coupling constants of H-9 or H-10, as summarized in Table 2. Thus, it is possible that either the coupling constants were calculated inaccurately or the relative configuration between the two tetrahydrofuran rings was assigned incorrectly. This is difficult to clarify with only printed 1H NMR data, and would be achievable with raw or at least digital shared data.
| Compound | Solvent | Frequency [MHz] | δ H-9 (J in Hz) | δ H-10 (J in Hz) | Ref. |
|---|---|---|---|---|---|
| a Overlapping signals with H-13. b Overlapping signals with H-6, H-7, H-12, and H-13. | |||||
| Elatenyne (51) | C6D6 | 199.5 | 3.84, m | 3.84, m | 160 |
| 51 | CDCl3 | 500 | 4.15, m | 4.15, m | 161 |
| 51 | C6D6 | 500 | 3.86, m | 3.86, m | 165 |
| 51 | CDCl3 | 500 | 4.15, ddd (12.0, 7.0, 5.5) | 4.15, ddd (12.0, 7.0, 5.5) | 165 |
| Elatenyne (53) | C6D6 | 500 | 3.84–3.93a, m | 3.84–3.93a, m | 166 |
| 53 | C6D6 | 200 | 3.79–3.97a, m | 3.79–3.97a, m | 166 |
| 53 | CDCl3 | 500 | 4.17, ddd (12.0, 6.8, 5.5) | 4.17, ddd (12.0, 6.8, 5.5) | 166 |
| 53 | CDCl3 | 200 | 3.91–4.29b, m | 3.91–4.29b, m | 166 |
| Laurendecumenyne B (52/54) | CDCl3 | 500 | 4.15, m | 4.15, m | 161 |
| ent-54 | CDCl3 | 500 | 4.15, m | 4.15, m | 166 |
| (E)-Elatenyne (55) | C6D6 | 400 | 3.75, dddd (7.0, 6.9, 6.8, 0.6) | 3.79, dddd (7.1, 7.0, 6.8, 0.6) | 167 |
| 55 | C6D6 | 500 | 3.73–3.83, m | 3.73–3.83, m | 166 |
| 55 | C6D6 | 400 | 3.73–3.83, m | 3.73–3.83, m | 166 |
| ent-55 | C6D6 | 500 | 3.82, dddd (12.9, 12.9, 6.4, 6.4) | 3.82, dddd (12.9, 12.9, 6.4, 6.4) | 166 |
| Notoryne (56) | CDCl3 | 400 | 4.26, ddd (7.3, 7.3, 5.5) | 3.98, ddd (8.3, 6.8, 5.5) | 168 |
The splitting patterns of H-9 and H-10 in the 1H NMR spectrum of the mixture of elatenyne (53) and laurendecumenyne B (54) were originally reported as multiplets by Wang and co-workers,161 but when re-processing the FIDs, a distinct multiplicity was observed (Fig. 14). Even if the signals of H-9 and H-10 of 53 and 54 are completely overlapped, they should still feature the same doublet of triplets (dt) multiplicity, with coupling constants of ∼11.8 (t) and ∼5.9 (d) Hz. However, when the raw FIDs were processed with Reference Deconvolution and Lorentzian–Gaussian multiplication (LG) rather than the typical exponential multiplication (EM; Fig. 14) as window function, the multiplicities of the signal patterns were found to be more complex than one or two overlapping dt signals and appeared to be slightly asymmetric. After closer inspection, the resonances for H-9 and H-10 were recognized as being partially overlapped, resulting from A,B spin particles, and assigned to qdd (J = 6.8, 3.3, 1.4 Hz) and qd (J = 5.0, 2.8 Hz) splitting patterns, respectively. This interpretation was supported by the expanded HMBC correlations (Fig. 15). The overlap of the signals of H-9 and H-10 have also been observed by Kim and co-workers, but were assigned to identical chemical shifts by others.160,161,165–167 Notably, all the above splitting patterns exclude the structures of 51 and 52, although it remains difficult to deduce the relative configuration between the two tetrahydrofuran rings unambiguously when relying on the re-processing and visual analysis of FIDs. Quantum mechanical full spin analysis (see Sections 3.3 and 5.1) will be required for unambiguous assignments. This also requires the availability of the raw data. On a more general note, the case of 53/54 provides another example, of why the ubiquitous use of the EM window function with LB = 0.3 is not a universally suitable post-acquisition processing method for 1H NMR spectra. The use of individually adjusted LG processing schemes typically yields additional structural information. This again speaks for the need to disseminate raw NMR data.
3.17 Second order coupling patterns with first order look vs. “multiplets”
Two prenylated chalcone antibiotics, 5′-O-methyl-3-hydroxyflemingin A (57) and 5′-O-methylflemingin C (58) occurring as enantiomeric mixtures, were isolated from the Sarawak rainforest plant, Desmodium congestum.169 The structures of 57 and 58 were determined using a combination of NMR (1D 1H/13C and appropriate 2D experiments) and HRMS.During the 1H NMR assignment exercise, it was noted that the non-equivalent methylene hydrogens H-4″a + b displayed non-first order coupling patterns (Fig. 16). Although the chemical shift difference between H-4″a and H-4″b was only 0.18 ppm (∼108 Hz), one side of the multiplet for each methylene resonance “appeared” as a dd (J = 7.2, 10.2 Hz), while the other side “appeared” as a t (J = 8.7 Hz). This clearly indicated that the methylene signals for H-4″a and H-4″b exhibit 2nd or higher order effects, and that the measured line distances (from the spectrum) are not reflective of the true J values. Given the relatively large difference between the methylene resonances, this second/higher order coupling pattern was unexpected and difficult to describe in terms of conventional NMR data table format. Designation of the signals as “multiplets” is common practice but not descriptive in the sense that it fails to provide any reproducible information. Retrieving raw NMR data from a repository allows for reprocessing and data analysis (spin simulation, full spin analysis) leading to a precise evaluation of J couplings in a second or higher order context (Fig. 16).
4 Impurity detection and quantification
Notwithstanding the above case of “multiplets”, and in addition to important but relatively straightforward structural revision, raw NMR data (FIDs) also plays an important role by enabling unambiguous reproducibility, as shown in the following cases (Fig. 17). | ||
| Fig. 17 Comparison of the 1H NMR spectra of the target molecules to be isolated (59–63), the impurities contained, 67 and 71 and the mixture initially isolated (A). | ||
4.1 Purification of thiotetronates
A major advantage to accessing raw NMR data lies in the detection, identification, and quantitation of impurities. Impurities can be variable depending on the isolation procedures used as well as their physicochemical properties compared to the target molecules. Occasionally, certain impurity signals appear to be constantly observed in the NMR spectra of a class of compounds isolated. The impurities in these cases are often structurally and/or chemically closely related to the target molecules, likely derived from persistent co‐elution or chemical transformation. Thus, having access to the raw NMR data, in combination with the increasing availability of advanced NMR processing and analysis software, can provide beneficial information about the amount and identity of this type of impurity. Such information can be used to optimize purification procedures and prevent chemical changes during the isolation of the target molecules or analogues. The impurities encountered during the isolation of thiotetronate antibiotics fall into this type, and reflect both scenarios for generating relevant impurities, i.e., co‐elution of structurally similar compounds hard to separate, as well as chemical changes of the target molecules during purification.Thiotetrnate antibiotics are potent fatty acid synthase inhibitors bearing a thiolactone core structure. The isolation and structure identification of several thiotetronate antibiotics have been published.170,171 In comparison with the truncated 1H NMR spectra (0–9.0 ppm selected) in the ESI,†170,171 shown in Fig. 17B–F are the full‐scale 1H NMR spectra (−1.0 to 11.0 ppm) of five thiotetronates (59–63) regenerated from the raw NMR FID files. Similar impurity profiles are observed in the range 2.5–4.5 ppm of 59–63.
Whereas the chemical shifts, integrations, and splitting patterns of these impurities are not readily recognizable in the original publications, the availability of the raw FIDs enabled a flexible, interactive, and facilitated analysis of the quantities and identities of these impurities. Taking the NMR spectra of 63 as an example: the expanded range of 2.5–4.5 ppm in the 1H NMR spectra (Fig. 17F) and analysis of the corresponding 2D NMR spectra (Fig. 18) pointed to the γ‐butyrolactone class (e.g., 64–67) as the source of the impurity signals.
 | ||
| Fig. 18 Expanded 2D NMR spectra of the thiotetronate (63) showing the focused region of the impurity. | ||
The γ‐butyrolactones, common signaling molecules of the genus Streptomyces, share structural similarity with the thiotetronates (59–63) and lack obvious UV absorption. Thus, the isolation of a single symmetric signal from HPLC resulted in an initial 1H NMR spectrum that contained a mixture of compounds (Fig. 17A). Actually, a thorough analysis of the 1D and 2D NMR correlation map of this mixture led, not only to the identification of γ‐butyrolactone as an impurity but also to the further optimization of isolation conditions. Through this optimization, the target molecules 59–63 in improved purity (Fig. 17B–F) were obtained, and a representative γ‐butyrolactone 67 was also isolated for verification (Fig. 18G). This success, combined with the identification of γ‐butyrolactone from the NMR spectra of 63 discussed above, exemplifies that some information about the impurities is often only accessible from the raw NMR FID files.
Furthermore, a characteristic aldehyde signal is observed around 9.5 ppm in the 1H NMR spectra of 59–62, which was not included in the truncated spectra in the ESI† of the original publications.170,171 A thorough and complete analysis of the 1D and 2D NMR spectra of 59–62 containing this aldehyde impurity suggested 68–71 as candidate compounds responsible for this aldehyde signal and an associated singlet at 6.6 ppm. This hypothesis was confirmed by the isolation of a representative impurity 71 (Fig. 17H) from 62.172
An association of the intensity of the aldehyde signal with the temperature and acidity used during the purification process suggested that they were likely the oxidation artifacts of the corresponding thiotetronates (59–62). Despite the unclear mechanism underlying this process, the interpretation of the aldehyde‐containing impurity provided additional information about the chemical stability of the target molecules and helped to optimize the isolation procedure at the early stage of this study. Thus, access to the raw FIDs of these molecules might likewise enable others to gain more information for developing suitable purification procedures.
To sum up, the availability of raw NMR FIDs not only accurately indicates the purity of the target molecules isolated, but also provides otherwise inaccessible information about the identity of relevant impurities co‐eluted with, or chemically transformed from, the target molecules.
4.2 Dynamic equilibria between isomers
Another example dates back more than 20 years to work on sesquiterpenoid lactones (STLs) in North American Star Anise (Illicum) species. From leaves and fruits of I. floridanum (Florida Star Anise), a variety of lactones of the seco-prezizaane (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) seco-allo-cedrane) type were isolated.173–176 In addition to several new related STLs, the known pseudoanisatin (72a) was found, representing one of the major constituents in both the leaves and fruits. Its structure elucidation was based on NMR, whereas, the X-ray crystallographic data had been published previously by Kouno et al.177,178 The NMR data (pyridine-d5) of the isolated constituent were identical with those published, but the isolates (irrespective of the plant part from which they came) always contained some 10% of an impurity whose signals clearly indicated a structural relationship with the main component. However, it was not possible to solve the structure of the minor component on the basis of the available spectra. A number of attempts were made to further purify 72a, especially since bioassays were planned. However, none of these attempts led to a diminution of the impurity but only to a loss of yield.
seco-allo-cedrane) type were isolated.173–176 In addition to several new related STLs, the known pseudoanisatin (72a) was found, representing one of the major constituents in both the leaves and fruits. Its structure elucidation was based on NMR, whereas, the X-ray crystallographic data had been published previously by Kouno et al.177,178 The NMR data (pyridine-d5) of the isolated constituent were identical with those published, but the isolates (irrespective of the plant part from which they came) always contained some 10% of an impurity whose signals clearly indicated a structural relationship with the main component. However, it was not possible to solve the structure of the minor component on the basis of the available spectra. A number of attempts were made to further purify 72a, especially since bioassays were planned. However, none of these attempts led to a diminution of the impurity but only to a loss of yield.
Later, the study on North American Illicium species was extended to the leaves of I. parviflorum from which a new lactone with an unusual and unprecedented cyclic hemiketal structure containing an oxygen bridge between C-4 and C-7 was isolated and named cycloparviflorolide 73b.175 This compound was found to contain some 20% of an isomeric compound which could be identified as 73a (parviflorolide) lacking the hemiketal ring and bearing the oxo and hydroxyl functions at C-7 and C-4 respectively, thus representing a direct analogue of 72a. It became clear that the compound actually exists as an equilibrium mixture between the two forms, which are hence also inseparable from each other.
Given the almost identical structures of 73a and 72a it was straightforward to expect that this type of equilibrium would exist also in the case of pseudoanisatin 72a and a cyclic form 72b, which should then represent the 10% “impurity”. Re-analyzing the NMR spectra of pseudoanisatin showed that the signals of the minor constituent indeed correspond to the cyclic hemiketal form, i.e., cyclopseudoanisatin 72b, and that this is actually the reason for the inseparable “impurity”. While in case of 73a/b, the 4,7-cyclo-form is the major isomer (80%, spectra recorded in acetone-d6), in case of 72a/b the 7-oxo-form was found to be predominant (with a ratio of approximately 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 in this solvent (Fig. 19)). It was subsequently demonstrated, based on theoretical considerations, that the respective oxo-isomers of both compounds are very likely the bioactive forms responsible for the binding to insect GABAA receptors.179
20 in this solvent (Fig. 19)). It was subsequently demonstrated, based on theoretical considerations, that the respective oxo-isomers of both compounds are very likely the bioactive forms responsible for the binding to insect GABAA receptors.179
Had the original spectra (a good copy of the 1D 1H and 13C NMR spectra would certainly have been sufficient) of pseudoanisatin been available, it would have been clear from the beginning, that the “impurity” must also have been present in the previous authors' isolate obtained from a different species, I. anisatum. This could have given a hint that it was not just some other STL present in minor amount but that it actually represents another form of the pseudoanisatin molecule. Much futile purification work could probably have been saved. It is simply not possible to obtain NMR spectra of more than 90% “pure” pseudoanisatin in the solvents used (pyridine-d5, acetone-d6, D2O) due to this equilibrium in solution. In fact it was shown later that the equilibrium composition in both cases is dependent on the solvent. It was found that water stabilizes the cyclic hemiketal isomers and shifts the equilibrium composition in this direction, leading to an approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture in the case of 72a and 72b (Fig. 19).
1 mixture in the case of 72a and 72b (Fig. 19).
4.3 Detection of rotamers
Guangnanmycin A (74a/b), a new member of the leinamycin family of NPs, was isolated from Streptomyces sp. CB01883 by the Shen group's efforts to target Nature's combinatorial biosynthetic potential for the discovery of novel NPs.180 Unlike the other members of this family, as exemplified by leinamycin181 or leinamycin E1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 182 that displayed a single set of signals upon NMR analysis, 74a/b afforded two sets of signals, in a ratio of ∼2
182 that displayed a single set of signals upon NMR analysis, 74a/b afforded two sets of signals, in a ratio of ∼2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in its 1H NMR spectrum recorded in DMSO-d6 at 298 K (Fig. 20, panel A-II). Initially, it was not apparent if the complication of the spectrum resulted from the presence of impurities or two equilibrating rotamers, 74a and 74b.
1, in its 1H NMR spectrum recorded in DMSO-d6 at 298 K (Fig. 20, panel A-II). Initially, it was not apparent if the complication of the spectrum resulted from the presence of impurities or two equilibrating rotamers, 74a and 74b.We thus analyzed the 1H NMR spectrum of guangnanmycin A in CD3OD at 298 K (Fig. 20, panel A-I), revealing that the ratio of the two sets of signals changed to ∼3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, hence suggesting the presence of two rotamers rather than impurities. Other NMR technologies were employed to support the attribution of the two sets of signals to the presence of two rotamers of guangnanmycin A (Fig. 20), as exemplified by the variable-temperature NMR experiment, in which the signals of two rotamers tend to merge at elevated temperature and finally fuse to one set at 393 K (Fig. 20, panel A II–VI), and the ROESY experiment, in which the exchange cross-signals between the resonances of rotameric forms, e.g., H-11 (at 7.00 and 7.10 ppm) or H-15 (at 7.40 and 7.51 ppm), appear in the opposite phase (shown in black), to that of normal NOE correlations between H-11 (at 7.00 and 7.10 ppm) and H-15 (at 7.40 and 7.51 ppm) (shown in red) (Fig. 20 panel B). While the varying NMR experiments afford ultimate confidence to the final structural assignments, analyzing the raw data of 1H NMR obtained in different solvents at ambient temperature requires less time, thereby highlighting its simplicity and usefulness in structure elucidation of NPs that occur as rotamers.
1, hence suggesting the presence of two rotamers rather than impurities. Other NMR technologies were employed to support the attribution of the two sets of signals to the presence of two rotamers of guangnanmycin A (Fig. 20), as exemplified by the variable-temperature NMR experiment, in which the signals of two rotamers tend to merge at elevated temperature and finally fuse to one set at 393 K (Fig. 20, panel A II–VI), and the ROESY experiment, in which the exchange cross-signals between the resonances of rotameric forms, e.g., H-11 (at 7.00 and 7.10 ppm) or H-15 (at 7.40 and 7.51 ppm), appear in the opposite phase (shown in black), to that of normal NOE correlations between H-11 (at 7.00 and 7.10 ppm) and H-15 (at 7.40 and 7.51 ppm) (shown in red) (Fig. 20 panel B). While the varying NMR experiments afford ultimate confidence to the final structural assignments, analyzing the raw data of 1H NMR obtained in different solvents at ambient temperature requires less time, thereby highlighting its simplicity and usefulness in structure elucidation of NPs that occur as rotamers.
5 Dereplication
5.1 Structural dereplication of proanthocyanidin A1 with higher order spin systems
Comparison of basic 1H NMR parameters (chemical shifts, coupling constants, line widths, and signal integrals) of isolated compounds with those of already reported structures is a standard method for a rapid structural dereplication. Proanthocyanidin A1 (PCA1, 75), is one of the most common dimeric proanthocyanidins which has one A-type doubly linked interflavanyl bond (C–C and C–O–C). In a previous study, PCA1 was isolated from the bark of Pinus massoniana, and the structure was identified by interpretation of 1D and 2D NMR spectroscopic data in combination with an electronic circular dichroism (ECD) experiment.183,184 The initial attempt at structural dereplication by comparison of reported 1H NMR data failed due to inconsistency of the reported data caused by difficulties of interpretation of higher order spin systems as shown in Table 3. In the literature, chemical shifts, coupling constants, and multiplicities continue to be described using the tabulated (depicted) method, and this can cause confusion. Two strongly coupled hydrogens (E-H-5′ and E-H-6′; split into d and dd, respectively, following first order analysis) were described inconsistently in summaries. Even if NMR data are collected in the same solvent and at the same temperature, the errors between experimental and reported data, and between the references are outside the acceptable range to be considered as the same compound. Subtle differences considered to be negligible could be from near identical but different structures.60,183–185| a The δH (ppm) and J (Hz) values were determined by 1H iterative full spin analysis (HiFSA). b Very small couplings were detected by HiFSA and required for the overall fit. Depictions in grey colored box are inconstancy due to difficulties of interpretation of higher order spin systems, E-H-5′ and E-H-6'. Signals in this region were described as crange, dmultiplet, and eoverlapped. This can lead to ambiguity. |
|---|
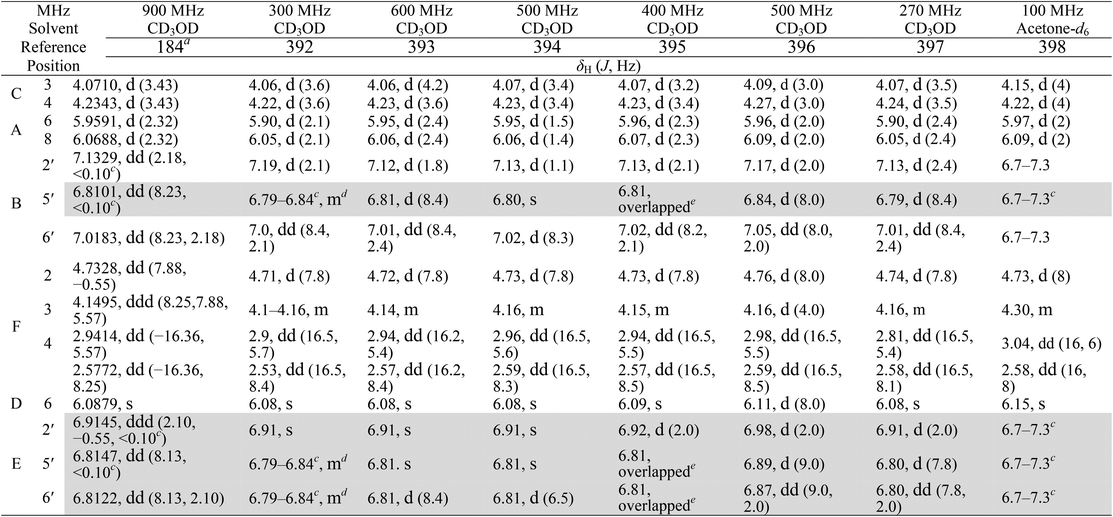
|
These subtle differences can be easily overlooked when the chemical shifts and coupling constants are calculated by a conventional manual measurement. In order to reduce the errors, HiFSA (1H iterative Full Spin Analysis) was applied to calculate the spectral parameters with high precision (δH, 0.1 ppb; J, 10 mHz).60 HiFSA from the FID data can produce accurate NMR parameters (chemical shifts, coupling constants) for even in higher order spin systems (Table 3, Fig. 21). Fig. 22 illustrates the higher order effects as a function of the various distances between the coupled hydrogens (E-H-5′ and E-H-6′), which shows the disappearance of d and dd multiplicities upon decreasing Δδ between these two hydrogens. This case study clearly emphasizes the fact that tabulated summaries can lead to repetitive spectral misinterpretation; therefore, it is necessary to provide access to raw FID data for rapid and accurate structural dereplication of previously identified compounds.
5.2 HSQC as a dereplication tool
A critical aspect of modern NP research is the rapid, efficient and accurate dereplication of known compounds.186 With some 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 NPs reported in AntiMarin187 and 139
000 NPs reported in AntiMarin187 and 139![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in the Dictionary of Natural Products,188 there exists a significant likelihood that a newly isolated substance may be identical or related to a known NP. Unless re-isolation or verification is the ultimate goal, it is typically a poor utilization of laboratory and human resources to spend significant amounts of time in the isolation and complete structure determination of an NP only to find that it was previously reported. A caveat to this, however, is that isolation of a compound of novel biology can be a significant scientific contribution even if the structure is known; nevertheless, one wants to establish this as quickly as possible. A variety of techniques have been utilized effectively for this, including biological assay profiles, variations of LC-DAD-MS analysis,189,190 and NMR metabolomics.191 It was with this goal in mind that a method to categorize the similarities in NMR spectra between different NPs as sought, as an additional basis of automatic dereplication of known NPs and their analogues.
000 in the Dictionary of Natural Products,188 there exists a significant likelihood that a newly isolated substance may be identical or related to a known NP. Unless re-isolation or verification is the ultimate goal, it is typically a poor utilization of laboratory and human resources to spend significant amounts of time in the isolation and complete structure determination of an NP only to find that it was previously reported. A caveat to this, however, is that isolation of a compound of novel biology can be a significant scientific contribution even if the structure is known; nevertheless, one wants to establish this as quickly as possible. A variety of techniques have been utilized effectively for this, including biological assay profiles, variations of LC-DAD-MS analysis,189,190 and NMR metabolomics.191 It was with this goal in mind that a method to categorize the similarities in NMR spectra between different NPs as sought, as an additional basis of automatic dereplication of known NPs and their analogues.
This contribution focuses on the 1H–13C HSQC spectrum as the critical NMR data set as the most robust yet cleanly characteristic of a given molecule, in part because of the high resolution created in the 2D NMR data set between all hydrogenated carbons and their respective hydrogens, and in part because there are fast NMR methods, such as Non-Uniform Sampling (NUS),192 ultrafast NMR,193 and Ernst angle-based signal intensity optimization methods194 for acquiring full 2D data sets. Further, the use of a deep Convolution Neural Network (CNN) with a Siamese architecture has a more robust ability to learn the features of different classes of images even when there are only a few images per image class, as well as to recognize patterns or objects in images even in the presence of artifacts (Fig. 22).195
However, to provide the deep CNN with an adequate training set, required the accumulation of a few thousand of such 1H–13C HSQC spectra, which were found in the ESI† pages of the Journal of Natural Products. While the spectra are there, they are present in many different formats, with grid lines or without, with assignment annotations, and presence of signal color for phase-edited HSQC experiments. In order to use these to teach a deep CNN, they needed to be extensively cleaned of this extraneous content. Whereas this could be achieved using post-processing image modifying software such as GIMP (GNU Image Manipulation Program; gimp.org), it would have been highly desirable to have direct access to the raw untransformed data, in which case it would have been possible to optimize transformation and plotting parameters to produce standardized image files of the highest comparability (i.e., neat 2D HSQC spectra with a fixed scale in each dimension).
Nevertheless, a modified deep CNN, was populated and designated the Small Molecule Accurate Recognition Technology (SMART) platform, with these refined HSQC spectra, and then this trained system was utilized to analyze new spectra and place them in a location within the SMART map that assists in their structure identification.196 To demonstrate and authenticate SMART (Fig. 23), a series of molecules isolated from two different marine cyanobacteria, a Rivularia sp. from Vieques, Puerto Rico, and a Moorea producens from American Samoa, were analyzed by NMR and their HSQC spectra rapidly recorded using NUS pulse sequences. When queried by SMART, these were placed in close proximity to a couple of series of related cyanobacterial cyclic lipopeptides, namely the viequeamides197 and veraguamides. Ultimately, the compounds were fully characterized by a variety of spectroscopic methods, and their structures shown to be closely related to the viequeamides (Fig. 23).198
5.3 Dereplication during fractionation
The NMR spectrum of a fraction is a fingerprint of its entire chemical composition and, therefore, never lies about the composition of fractions. While 1H-NMR has been frequently used in metabolite fingerprinting of NPs, the advent of high field instruments together with cryoprobes and small volume tubes (3 or 1.7 mm NMR tubes) have addressed the previous limitation of low sensitivity so that NMR spectra of fractions can be directly analyzed to identify constituents. The deposition of raw data and the associated FID data will allow complete analysis of fractions. The advantages can be two-fold: to easily and quickly identify known compounds within fractions without further isolation, and to identify signals that are not within known compounds in the search for novel molecules.The aim of this example was to develop NMR fingerprints to identify novel compounds by first demonstrating the value of NMR fingerprints of fractions to identify novel compounds from a set of 20 sponges from the order Poecilosclerida. The presence of a unique 1H NMR spectral pattern in only 5 of the 220 spectra allowed the isolation of the novel compound iotrochotazine A (76) that was shown to have phenotypic activity on cells from Parkinson's Disease patients.199
The NMR of an active fraction with LAT3 inhibition ensured that the four compounds in the fraction were isolated. In this case, LC-UV-MS proved to be of limited value as the compounds had little UV absorbance and the ESI mass spectrum contained mainly fragment ions. The 1H NMR spectrum, on the other hand, revealed the presence of multiple compounds, providing a comprehensive fingerprint of all of the small molecules contained in the fractions. This resulted in the isolation of four novel compounds, venulosides A–D (77–80), whose structural relatedness had the advantage of providing SAR information.200
The metabolome of a termite-gut associated actinomycete using NMR fingerprints identified six new NPs, namely, the actinoglycosidines A and B (81 and 82), actinopolymorphol D (83), and the niveamycins A, B, and C (84–86).201 The metabolic fingerprinting approach in this publication reports the methodology. It consisted of the generation, through RP-HPLC, of five LLE fractions for each of the eighty-four crude extracts (21 strains/four crude extracts: OMA, LFA, RFA, and GYES) using parameters such as log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P < 5 that permitted the retention of molecules with lead and drug-like properties.202,203
P < 5 that permitted the retention of molecules with lead and drug-like properties.202,203
NMR fingerprints allowed suppression of metabolites, induction of new metabolites, and increased production of minor compounds to be determined after treatment with N-acetyl-D-glucosamine in three sponge-derived actinomycetes.204 These examples demonstrate the need to establish a 1H NMR NPs database of raw data that can be freely accessible in order to focus on novel NPs. Moreover, they exemplify the need for NMR raw data to allow NMR fingerprints to become a universal tool. Typical NMR fingerprints of fractions are shown in Fig. 24 and 25, and can be analyzed using the proposed database of raw files.
5.4 The configuration of lanciferine
The monoterpene indole alkaloid (MIA), lanciferine (87a), was isolated in 1973 from the aerial parts of the New-Caledonian plant, Alstonia boulindaensis Boiteau (Apocynaceae)205 and belongs to the akuammiline family.206 Engendering numerous complex scaffolds, the akuammiline MIAs have received much attention by synthetic chemists owing to their molecular structures and a broad range of biological activities.207 The oxidized furoindoline motif in 87a is embedded within a polycyclic framework, referred to as “indolinolid” in the original report.205 Although the molecular framework of 87a was the first of its kind, the akuammiline MIAs have since been expanded by nine congeners: picranitine,208 alstolactines A, B, and C,209 alstoniascholarines L and M,210 as well as scholarisines K, L, and M.211Research concerning the akuammilines has focused on isolation and pharmacological studies212 with relatively less emphasis on synthetic chemistry. However, synthetic endeavors spanning the past 30 years have resulted in the design of elegant and successful total syntheses. The asymmetric total syntheses of the three akuammiline alkaloids, aspidodasycarpine, lonicerine and the proposed structure of lanciferine (87a), was completed recently by Li et al.213 According to the authors, the structural reassignment of their product was hampered by the ambiguous and incomplete 1H NMR data disclosed in the isolation report. In addition, the 13C NMR data were also missing (in the mid 1970's, 13C NMR analysis was still very much a specialist's technique and widely inaccessible to NP research groups). However, a thorough analysis of just the 1H NMR spectrum, enabled by the availability of the raw data, would have revealed any inconsistencies with Ang Li et al.'s interpretation. Indeed, the 1H NMR chemical shift of C18 methyl of the synthesized compound (87b, 19S) (1.4 ppm) differed from that reported for natural lanciferine 87a (1.2 ppm). Furthermore, for the original isolation of 87a, the authors reported the unambiguous assignment of the configurations of all its chiral centers except that of C-19.214
In light of these data, it would seem that Ang Li et al., actually, did not synthesize 87a but a diastereoisomer, 87b. Continuing interest of Beniddir's group in MIA chemistry led to the development of a spectral database of a cumulative collection of alkaloids, for dereplication purposes.215 Hence, it was possible to retrieve the original sample of 87a and reacquire reliable 1D and 2D NMR spectra. These data in conjunction with a detailed NMR-based computational study using the CP3 parameter209 shed light on the configurational assignment of lanciferine and confirmed the 19R and 19S configurations for 87a and 87b, respectively.216
In conclusion, this case of ambiguity would have been removed if the raw data (i.e., FID) of the NMR of 87a had been made accessible.1 Indeed, FIDs or spectra availability, would have enabled the structure verification of 87a through computer-assisted spectral assignment approaches.15 Finally, this example brings out the need for new reporting standards for NMR data and more globally, NPs' spectral properties.
5.5 Unraveling the J values of mycothiazole
Mycothiazole (MYC, 88)217 is a bioactive sponge-derived polyketide-nonribosomal peptide synthetase (PKS/NRPS) hybrid product of continuing interest as a lead for an anti-cancer therapeutic.218,219The current understanding of the exact pharmacophore needed for its nM profile in cytotoxicity screening is incomplete and is the subject of continuing study of analogs. MYC 1H and 13C NMR data acquired at 300 MHz in CDCl3 were misinterpreted. A subsequent re-evaluation took place prompted by discrepancies in the 13C shifts and optical rotation data between natural and synthetic products.220,221 Further evaluation involved data collected at 600 MHz.220 Shown in Fig. 26 is that several resonances are broadened and overlapping. This confounds the task of extracting many J values, so many signals were listed as “m” in the original publication.217 The second generation analysis at 600 MHz220 included obtaining NOE data and remeasuring the J values for H-15 (5.62 ppm) as a dtt (J = 10.7, 7.5, 1.5 Hz) prompting the reassignment of the C-14, C-15 geometry from E to Z (Fig. 26).
 | ||
| Fig. 26 Mycothiazole (88) full 1H NMR spectra (CDCl3, 600 MHz) annotated with atom position numbers with output obtained by classical FID work-up. | ||
New FIDs have been obtained for MYC and are available as electronic information. Presented below are examples for which obtaining new FIDs enable accurate measurement of JHH and JHC values for the first order or non-first order multiplets. The first example involves closely overlapping resonances of olefinic hydrogens H-6, H-14 and H-15. Shown in Fig. 27 is a before-and-after data set with the new data provided by the two methods of post-acquisition processing. This allowed the accurate measurement of nine J values as shown in each of the panels. The principal tool used here was the second derivative/nonlinear fitting algorithm “Resolution Booster” developed by Mestrelab Research SL to reprocess the 1D NMR FID. Using this algorithm along with the post-acquisition Resolution Booster option, it was possible to clearly resolve all 16 multiplet lines of H-15 with surprising improvement of resolution without introducing artifacts or shifts in the spectrum. This enabled confident multiplet assignment along with accurate measurement of 3JH-15–H-14 and 3JH-15–H-16 data shown (Fig. 27) that differed from those reported in 2006 (see above). The data in Fig. 27C and D provide additional coupling values for H-14 and H-16 previously described simply as multiplets.217
Similar outcomes are shown in Fig. 28 and 29 that more accurately describe the coupling patterns of olefinic hydrogens (H-5, H-17) and aliphatic hydrogens (H-3′, H-7, H-7′). The previous data from measurement in CDCl3 reported most of these resonances as multiplets. Alternatively, analysis of these resonances by either first order or non-first order signal fitting accurately provided the eleven J values shown. These data should be useful in the future as new MYC analogues are isolated or synthesized. The value of obtaining and using HMBC-derived 1JCH data to make functional group assignments for compounds possessing ratios of H/(C + Z) < 0.5 was recently demonstrated.222 It appears that accessing such data has become a “forgotten art”, yet the measurement shown in Fig. 30 illustrates that this process can be done accurately and rapidly when raw data is available. The coupling value shown here now provides a more accurate estimate of the 1JC-15, H-15 = 186.9 Hz vs. the published value of 194 Hz.217
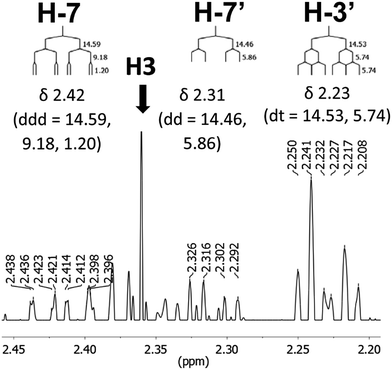 | ||
| Fig. 28 Mycothiazole (88) expanded 1H NMR spectra regions (CDCl3, 600 MHz) for H-7/7′ and H-3/3′ obtained from FIDs processed using second derivative/nonlinear fitting. | ||
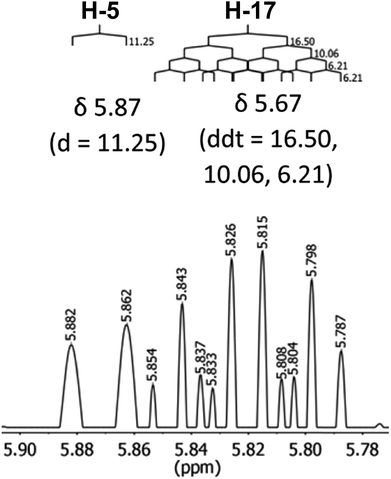 | ||
| Fig. 29 Mycothiazole (88) expanded 1H NMR spectral regions (CDCl3, 600 MHz) for H-5 and H-17 obtained from FIDs processed using second derivative/nonlinear fitting. | ||
As shown in the next section, there are other direct and indirect methods to obtain 1JC,H values from reprocessed FIDs, representing another rationale for the collection and dissemination of raw NMR data.
6 New methodology
6.1 Data mining the one-bond heteronuclear coupling constant, 1JCH
Assembling and assigning the common 1H NMR data parameters is a typical prelude to linking nuclei (bond connectivity) by 2D and 3D NMR methods; a process familiar to chemists conducting integrated structure elucidation.223 The undisputed value of chemical shift for establishing the electronic environment of nuclei has driven the development of NMR instrumentation to higher fields to maximize dispersion. Assembling molecular structures by interpretation of HSQC and HMQC, the most widely-available heteronuclear 2D NMR experiments, gives direct bonding information of 13C–1H couplets. The latter are identified by the presence of cross correlations, but lost in the process is another powerfully informative parameter: the magnitude of 1JCH, itself. Out of a necessity to maintain the signal to noise (S/N) in heteronuclear correlation experiments, the latter is sacrificed by abolishing the couplets through 13C-broadband decoupling during acquisition of the FID. Nevertheless, 1JCH can be recovered, as has been amply demonstrated through structure elucidation of numerous NPs, by the simple expedient of recording the FID with no 13C-broadband decoupling. The so-called coupled HSQC experiment replaces single cross correlations of each 13C chemical shift (or two, in the case of diastereotopic CH2 groups) with two component-cross-peaks, the 1JCH C–H couplets, where the value of the coupling constant is revealed by their separation in Hz.Often overlooked in 1H NMR spectra, is the cryptic presence of the one-bond heteronuclear coupling constants, 1JCH, seen as ‘13C-satellites’ of the 1H signals at the natural abundance of 13C, ∼1.1%. In fact, the utility of 13C satellites in 1H NMR spectra was recognized by Truner and Sheppard as early as 1959, when they analyzed the fine structure of the 13C satellites to determine the coupling constants of hydrogen nuclei of adjacent carbons that are chemically equivalent.224 Most likely, and especially for NP applications, the low abundance of the 13C satellite signals and the associated sensitivity challenge has been a major impediment for a broader implementation of this approach. Direct detection of 1JCH from uncoupled or ‘gated-coupled’ 13C NMR spectra still requires inordinately large samples and/or X-nuclei direct detection cryoprobe instrument. While indirect detection of 1JCH from HSQC spectra is relatively time-consuming, the 13C-satellites of 1H signals reveal heteronuclear couplings, in favorable cases, within the 1H NMR spectrum, requiring no special treatment beyond inspection, or facile post-acquisition processing of the FID at most. The extraordinary value of the 1JCH magnitude and its application in structure elucidation is underestimated and can be summarized as follows:
(i) Hybridization at carbon. The magnitude of 1JCH is directly proportional to the amount of s-orbital character (%s) in hybrid atomic orbitals (%s for sp1 = 50%; 33 1/3% for sp2; for 25% in sp3) that combine to form the molecular orbitals of sigma bonds. For olefins and arenes, unlike ‘normal’ aliphatic compounds, sp2-hybridized C have larger heteronuclear couplings (1JCH ∼ 150–170 Hz), while the sp2-hybridized C in terminal acetylenes consistently exhibit the largest magnitudes of any 13C–1H couplets (1JCH ∼ 250 Hz). For example, the terminal acetylene residue 3-hydroxy-2,2-dimethyloctynoic acid (Dhoya, first found in pitipeptolide (89)) from Lyngbya majuscula225 and several variants, from other cyanobacterial NRPS-PKS NPs226 is a group which shows an unremarkable 1H NMR chemical shift (1.96 ppm) due to diamagnetic shielding, but a large 1JCH ∼ 250 Hz. A vexing technical issue in HSQC spectra of terminal acetylenes is the acetylenic correlation signal is often ‘missing’. This is due to the large deviation of 1JCH in terminal acetylenes from the nominal value of the one-bond ‘J filter’ (1JCH = 140 Hz) used in standardized parameters of the pulse sequence, but the cross-peaks can be recovered with appropriate re-parametrization. A combination of resonance energy and electronegativity effects (see below) leads to exceptionally large couplings for five-membered hetero-aromatic rings (1,3-oxazole, imidazole, thiazole, etc.), compared to arenes, which can be readily identified from the 13C-satellites of their 1H signals. For example, the H-5 signal (azole numbering) in each of the three 1,3-oxazole rings of the trisoxazole macrolide (90) from the nudibranch, Hexabranchus sanguineus, as well as that of the thiazole ring of jamaicensamide A (91) from the sponge, Plakina jamaicensis, have 1JCH values of 198 and 190 Hz, respectively. It was no small feat that the 1JCH could be measured from 13C-satellites of a 33 µg sample using a microcryoprobe at 600 MHz.
(ii) C–H groups associated with electronegative elements. Whereas the one-bond homonuclear coupling constants of unconstrained hydrocarbons and alkyl residues vary little from a nominal and almost invariant value of 1JCH = 125 Hz, substitution by electronegative N, O, halogens and even the polarizable S atom, increases the magnitude to 140–150 Hz. For example, N-Me, O-Me and S-Me groups can be distinguished from C-Me groups (e.g., an acetyl group, CH3(CO), J = 128 Hz) and assigned independently of the corresponding 1H NMR Me chemical shift in non-obvious examples where interpretation is equivocal, e.g., the assignment of a methylthio group (S-Me) in varamines A and B, 92a, 92b (1JCH = 140.5 Hz) and lepadines I (93, 1JCH = 140 Hz).227 In the latter cases, elimination of alternative C-Me constitutional isomers was confounded by predictions of similar 1H NMR chemical shifts for the Me groups; a more common occurrence than generally assumed. An object lesson is provided by synthetic compound, 94 (Fig. 31),228 which has four Me groups – two attached to S, one to O and the fourth, to C. The assignment of the O-Me group from 1H NMR chemical shift, alone, is trivial (3.80, ppm), but the 13C-satellites also reveal the largest associated coupling constant (1JCH = 147.6 Hz) of the four. The remaining three signals are clustered and not readily assigned by chemical shift, alone, however, their identities are revealed by heteronuclear coupling constants. The resonances of the two S-Me groups are overlapped and have essentially identical heteronuclear couplings (2.43 ppm s, 6H, 1JCH = 141.3 Hz) that, incidentally, integrate for roughly twice the O-Me 13C-satellites. Therefore, the remaining Me signal, slightly more shielded group than the latter two, is associated with the smallest heteronuclear coupling, and can be assigned to the acetyl group (2.33 ppm, 3H, 1JCH = 128.3 Hz).
(iii) Identification and assignment strained 3-membered and 4-membered rings in monocyclic, bridged and fused polycyclic structures where, again, the coupling constants in cyclopropanes, cyclobutanes and heterocyclic small rings depart from a nominal 1JCH = 125 Hz to magnitudes of up to 1JCH ∼ 180 Hz in the case of a di- or tri-substituted epoxide (oxirane) found in meliatoxins A1 (95a) and B1 (95b) from Melia azedarach,229 or the oxetane ring of paclitaxel (96) ex post facto of the original X-ray structure.230 The latter method is particularly powerful as no other reliably and independently establishes ring size in cyclic NPs, and in many cases, can be used to resolve constitutional isomers (e.g., the isomeric products of a Payne rearrangement). Finally, electronic and ring strain factors that contribute to the magnitude of 1JCH are additive. For example, the 1H and 13C NMR spectra of the unique trans-chlorocyclopropyl ring in muironolide A (97), a macrolide from a Western Australian sponge, Phorbas sp., is associated with four large 13C–1H couplets (H-21, 1JCH = 177 Hz; H-22a, 1JCH = 173.4 Hz; H-22b, 1JCH = 173.4 Hz; H-23, 1JCH = 200 Hz)231 that uniquely identify strain and electron-withdrawing effects within the ring. A useful trend in the of 1JCH of the diastereotopic CH2 group of the imidazolone ring found in the cyclic peptide, N,N′-methylenodidemnin A from the Caribbean cyanobacterium Trididemnum solidum observed, expanded by measurements of 13C-satellites in the 1H NMR spectra of several imidazolone and oxazolidine models.232 An unusual finding was that 1JCH in the 13C–1H couplets of the diastereotopic CH2 are often non-equivalent and, therefore, dependent on relative orientation.
Exploitation of 1JCH can be useful in alkaloid assignments; for example, the presence of a 2H-azirine ring (azacyclopropene) in dysidazirine (98),233 and related compounds234 is confirmed by observation of the exceptionally large coupling constant (1JCH = 189 Hz) of the corresponding CH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N couplet. It is expected that the extraordinary structure of cyclopropylazetidinone (99), an ‘alkaloid’ obtained by Rainier and coworkers as an intermediate in the synthesis of natural pyrroloindolines and confirmed by X-ray crystal structure analysis, is expected to be associated with an unusually large 1JCH for H-2 (5.85 ppm CDCl3),235 interesting to measure, to say the least (in the publication,235 the 13C-satellites [1H NMR, 500 MHz] are too weak to be visible in the current PDF print format of the ESI†).
N couplet. It is expected that the extraordinary structure of cyclopropylazetidinone (99), an ‘alkaloid’ obtained by Rainier and coworkers as an intermediate in the synthesis of natural pyrroloindolines and confirmed by X-ray crystal structure analysis, is expected to be associated with an unusually large 1JCH for H-2 (5.85 ppm CDCl3),235 interesting to measure, to say the least (in the publication,235 the 13C-satellites [1H NMR, 500 MHz] are too weak to be visible in the current PDF print format of the ESI†).
Extraction of 1JCH values from 13C-satellites of 1H NMR spectra is limited by several instrumental and sample-related factors that militate against their observation. Nevertheless, access to the original FID of the spectrum can mitigate some of the difficulties in ways that are illustrated in three major groups:
(i) Poor S/N in 1H NMR spectra of small-sized samples. In order for the 13C-satellites to ‘rise’ above the noise level, a good quality 1H NMR spectrum of a ‘strong sample’ is required such that the signal due to the natural abundance of 13C in the sample exceeds the amplitude of random noise. With limited sample, this can be challenging, but as mentioned elsewhere in this review, the data content of the time-dependent periodic function that constitutes the FID is a fixed product of S/N and resolution: one can trade one for the other, to some extent, by judicious reprocessing. Careful use of apodization functions prior to FT of the FID may regain S/N at the expense of resolution (line width) to reveal 13C satellites that are invisible from first inspection and in printed documents such as PDF files in traditional ESI† format. As loss of resolution is almost always inconsequential for measuring 1JCH, except for very weakly dispersed signals, this can be an effective way to tease out important information from FID data made available in digital format.
(ii) Spectral overlap or complex multiplet structure. 13C satellites that exhibit complex multiplet structures, due either to overlaid homonuclear coupling (nJHH with n = 2, 3, etc.), or symmetry-related reasons, may completely ‘disappear’ beneath the noise or be obscured by nearby 1H signals. Fortunately, only one half of the 13C satellite doublet signal needs to be observed as the 1JCH is reconstructed from twice its separation from the dominant centroid 12C–1H signal (ignoring the slight isotope shift of the former). Here, a caveat should be stressed: the sample should be sufficiently pure that spurious impurity signals are not mistaken for genuine 13C satellite signals. Regrettably, with very noisy spectra, ‘there is no such thing as a free lunch’: little can be done if apodization of the FID, even at an extreme level prior to FT, does not result in reliable appearance and identification of the 13C satellites. In this case, salvaging the 1JCH may only be achieved by re-recording the 1H NMR with a more concentrated sample, in which case it is far preferable to record the coupled HSQC.
(iii) Line-shape. In order to separate the 13C-satellites from the base of the dominant 12C–1H signal, good NMR signal line shape is required, especially at higher fields.
For the foregoing reasons, readily measurements of 1JCH from 13C-satellite signals is most practical from 1H NMR signals where signal complexity does not exceed singlet or doublet splitting. Here, the low-abundance 13C–1H couplets can be exploited best, delivering valuable new information on electronic environment, hybridization and ring strain for molecular structure determination of an NP. All this, from no more than a re-processed 1H NMR spectrum, accessed from archived digital FID data. An enhanced HSQC experiment for an accurate and more rapid assessment of one-bond proton-carbon coupling constants has been reported very recently.236
A variety of 2D NMR methods have been developed that enhance the utility of C,H-coupling information in NP research, covering both direct (1JC,H) and longer-range (≥2JC,H) coupling relationships. Examples are the ASAP variant of HSQC237 and the establishment of NOAH supersequences238 for accelerated acquisition, non-uniform sampling (NUS)239 and CRAFT 2D processing240 techniques for enhanced resolution, as well as LR-HSQMBC and HSQMBC-TOCSY for improving the detection of long-range correlations.49,50
6.2 New analysis of published data by optimal processing of the FID
NMR data can provide a wealth of information regarding a given chemical structure and much of this information is frequently overlooked. For instance, coupling constants (J) provide key information, especially in configurational aspects. Consequently, valuable details about structural identity are often misinterpreted and/or lost. In fact, deep analysis of a 1H NMR spectrum often obviates the acquisition of further experimental data and enables a more efficient use of the NMR spectrometer. In this regard, the availability of the raw NMR data plays a key role in both the verification of interpretation and the extraction of new information that otherwise is lost.This affirmation can be illustrated by the measurement of long-range (4–5J) coupling constants such as the ones between a hydrogen nucleus of an aromatic ring and those of a side chain. To access this information, FIDs should be multiplied by resolution-enhancing window functions such as Gaussian or sinebell. This is enabled by the availability of the digital NMR raw data. This approach has been used by Lima et al. (2015 and 2016),241,242 Pederoso et al. (2008),243 Amoah et al. (2015),244 and da Silva et al. (2015),245 for establishing the connectivity of aromatic and side chain moieties of several NPs. In the case of butein (100),242 for example, the shifted sinebell multiplication (SINM) followed by an exponential multiplication (EM) of the FID with a Lorentzian line broadening factor of 0.3 Hz instead of the simple EM (default setting on most NMR spectrometers; see also Fig. 5) prior to Fourier transformation revealed a small additional coupling constant (J = 0.5 Hz) correlating H-6 with H-α (Fig. 32). This finding is supported by the reciprocal analysis of the signal of H-α. Thus, the molecular connectivity between the aromatic ring with the double bond side chain in butein could be established based only on the 1H NMR spectra without the need of two-dimensional (2D) NMR experiments.
Furthermore, the processing of the raw NMR data can bring information from even longer conjugated chains. The polyacetylenes found by Buskuhl et al.246 and Pollo et al.247 are good examples of this application. In these cases, the employment of enhanced line shape processing permitted the correlation of a long distance coupling (9J; Fig. 33). Such long-range correlations can only be observed in situations where the electronic density is high, such as on conjugated triple bonds. Thus, advanced raw NMR data processing permitted not only connecting moieties, such as in 100, through the correlation of H-1′ and H-8′, but also determining the presence of triple bonds in the polyacetylene structures as in vernonyine (101).
The same strategy can be used in 2D NMR correlation maps, such as HMBCs. The original file, containing the raw NMR data is of great importance once it allows counter level editing, which permits observation of a correlation or lack of one. The advanced processing of HMBC allowed the unequivocal establishment of the 13C NMR chemical shift assignments from C-2′ to C-7′ from the long-range 1H–13C correlation of H-1′ and H-8′ in these polyacetylenes (Fig. 33).
NMR-based techniques248 have enormous potential for NP investigation since they provide unique and comprehensive information for structure determination and dynamic of chemical compounds. Therefore, advanced NMR processing strategies can be valuable on those spectra acquired directly from raw material as in gel-like systems through HR-MAS NMR spectroscopy,249,250 because in these cases the spectral resolution is naturally lower due to restricted molecular mobility.
Nevertheless, the quality of the results from advanced NMR data processing depends on spectra being acquired with sufficient signal-to-noise ratio (S/N). This requires an appropriate number of scans and high time-domain resolution (at least 64 K data points). Additionally, the 1D spectra and nD correlation maps need to be processed using a large number of zero-filling (at least 128 K in 1D and 4 K per 1 K in 2D).
6.3 In-depth analysis of 1H and 13C NMR data of smenospongidine
Smenospongidine (102), a biologically active quinone sesquiterpenoid, was isolated from the sponge Smenospongia sp. by Kondracki and Guyot in 1989.251 The authors proposed the structure of 102 based on analysis of HRMS, 1H NMR and 13C NMR data, but omitted carbon chemical shift data from the manuscript. In 1992, Rodríguez et al. published the first tabulated 13C NMR data of 102.252 In the intervening years, 102 was isolated from various sponge sources253–258 each reporting close agreement to the published data. In 2002, an enantioselective total synthesis of 102 was reported by Ling et al., which, in addition to the usual statement about good agreement with published data, was accompanied by ESI† with 1H and 13C NMR spectra of the synthesized molecule.259 Recently, the Williams' group also isolated 102 from Dactylospongia elegans and found significant discrepancies between their spectral data, and previous reports. Herein, they summarize the discrepancies and report corrected data for 102. This case story demonstrates the value of depositing raw NMR data by showing how errors (omission, assignment, typographical, etc.) propagate through the literature when forced to rely on reproduced, tabulated, or listed data. Moreover, it points out the difficulties in locating original NMR data decades after publication. Although 102 is a specific example, the problems are nonetheless widespread and persistent in the literature.The spectral data of 102 obtained from this study are tabulated (Table 4) against those reported by Rodríguez et al., who did not report signals for the non hydrogen-bearing carbons C-10, C-16 and C-18 in 102. Aside for that, the only major difference (>2 ppm) between the two 13C NMR data sets occurs at C-20 (150.0 vs. 154.7 ppm), with Williams' value of 150.0 being more consistent with their data for the C-5 epimer of 102. The partial 1H NMR data reported in that manuscript has two main inconsistencies. First, a singlet reported at 5.41 ppm assigned as the hydroxyl hydrogen may instead be the olefinic hydrogen H-16 in the quinone ring. Second, a doublet at 0.77 ppm assigned to methyl hydrogens (H-13) is more characteristic of H-10, an axial methine hydrogen at the trans-decalin junction in quinone-containing analogs of 102 with identical configuration.251 With access to the original spectra, these issues of unreported or possibly misassigned signals are easy to resolve. For example, the last issue (H-10 vs. H-13) could possibly be distinguished by the integrals, multiplicity (d vs. dd) or the magnitude of observed coupling as the axial methine H-10 should display a larger J value (>10 Hz), due to coupling with the neighboring axial hydrogen (H-1), than the typical 7 Hz observed from methyl doublets. It should be noted that it is highly unlikely that even contemporary spectra exhibit adequate resolution or sufficient peak-picked expansions to resolve the matter when disseminated as ESI† material in the currently customary PDF format.
| a Assignments were made by matching the hydrogen and carbon nuclei with the closest reported chemical shift values. None of the reported chemical shift values could be assigned to carbon nuclei at positions 16, 20, and 3′, whereas the signals at 69.0, 65.9 and 31.0 ppm were deemed extraneous. |
|---|
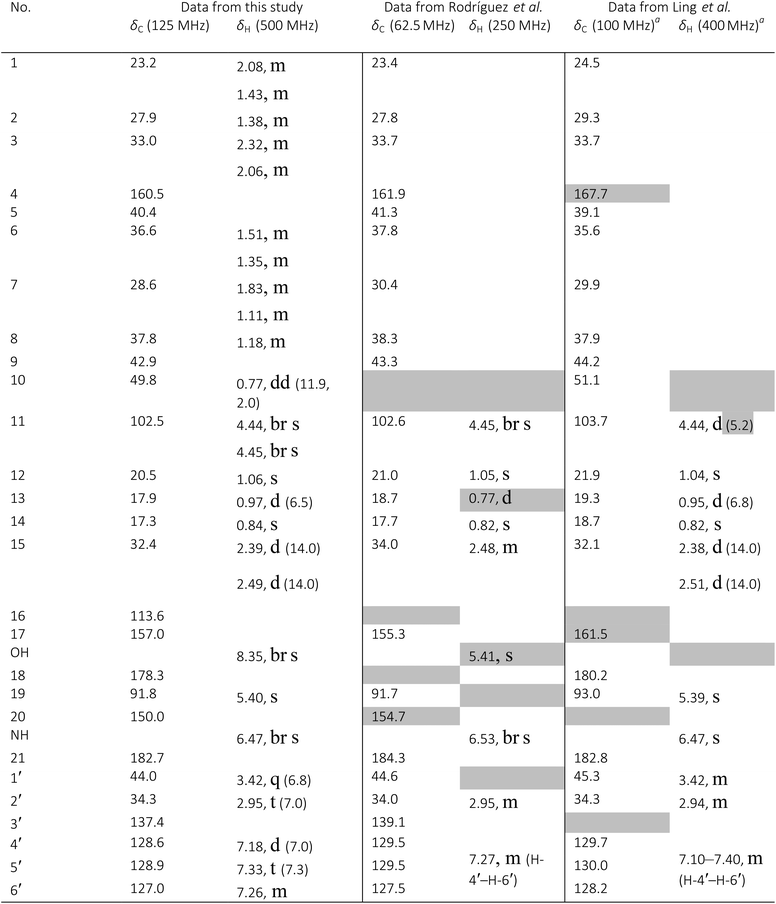
|
To resolve these issues and clarify the identity of Williams' sample of 102 with only hydrogen and carbon data of the sample on hand, the reported synthesis was repeated. As is typical, no specific hydrogen or carbon assignments are reported in the manuscript describing the synthesis of 102 for the listed chemical shifts, so they have been assigned as seemed best for this comparison (Table 4) with the major differences highlighted in gray. Unfortunately, this data raised more questions. Their listed 1H NMR data on S19 does not include the signal for H-10, the hydrogen at the A/B ring juncture possibly misassigned by Rodríguez et al., but does include a signal at 0.97 ppm (d, 3H, J = 6.0 Hz), here assigned to H-13; a signal missing altogether from Rodríguez et al.'s paper. Despite the inclusion of 1H NMR spectra in the ESI,† the presence of these signals could not be conclusively confirmed because of the unavailability of an appropriate expansion of the spectrum. Other issues apparent from the listed 1H NMR values are the mischaracterization of resonances here assigned to the terminal exocyclic alkene H-11 (reported as 4.44 ppm, d, 2H, J = 5.2 Hz) and H-2 (2.94 ppm, 2H, m). The latter resonance should be a triplet as the hydrogens responsible for the signal are adjacent to only two equivalent hydrogens, while the characterization of the 4.44 ppm (d, 2H, J = 5.2 Hz) resonance is clearly erroneous, as a J value of 1.2 Hz, typical of the coupling between two non-equivalent hydrogens of the exocyclic terminal alkene, can be calculated from peak-picking in the ESI.† The 13C NMR spectrum provided in the ESI† and the chemical shift values extracted from the spectrum raise further questions on the interpretation of the NMR spectral data. Twenty-seven unique 13C NMR resonances are expected for 102. The ESI† of Ling et al. lists 25 signals for 102, omitting two carbonyl signals. Of these 25 signals, only 7 out of 12 required sp2 carbon signals are reported and the list includes signals at 69.0 and 65.9 ppm clearly inconsistent with the proposed structure as it lacks oxygenated sp3 carbons. The 13C NMR spectrum with the poor signal/noise included in their ESI† sheds some light on the situation, but also raises questions as it includes the two carbonyl signals omitted from their list. The two carbonyl signals are labeled at 182.8 and 180.2 ppm but both appear between the chemical shifts of 181 and 182 ppm in the 13C NMR spectrum perhaps due to peak-picking errors.
There is little question 102 was isolated or synthesized, as published in these articles. The Williams' group has in fact synthesized 102 from ilimaquinone using the method described by Ling et al., and independently confirmed the structure. Throughout the process, the corresponding authors of those reports graciously offered assistance and searched for their original data at Williams' request, but decades later were unsurprisingly unable to locate it. The difficulties of individual labs or departments maintaining NMR records over 40 years are significant. The staff at University of Hawaii, Manoa, receives frequent requests for copies of NMR data generated by the late Paul Scheuer and Richard Moore with a success rate of less than 50%. Most recently, a request for data on the cyanobacterial compound micromide could not be fulfilled due to degradation of the CD backups. The fact remains that our community's reliance on tabulated or summarized data introduces the possibility of a litany of errors into the literature. Availability of raw NMR data would undoubtedly play a major role in curbing propagation of these errors.
7 Other nuclei
7.1 Fluorine: paramagnetic and diamagnetic effects
Fluorine is commonly used in organic chemistry, especially in medicinal chemistry and materials, because it is both small and much more electronegative than the H and C atoms that make up a good portion of organic compounds.260–262 Fortunately, 19F is 100% naturally abundant and NMR active. 19F has a gyromagnetic ratio close to that of 1H, and a nuclear spin of ½, but covers a much larger chemical shift range than 1H (∼400 ppm for organofluorines), meaning that signals tend to be well resolved.263 Unfortunately, the shifts of these fluorines can be difficult to assign if multiple fluorines on a molecule are in similar environments.The shielding that leads to observed 19F shifts arises, in part, from both diamagnetic, and paramagnetic effects. The diamagnetic term is based on the electron density around the nucleus, while the paramagnetic term is based on the excitation of electrons in fluorine's p orbitals (not an issue for 1H). Consequently, 19F NMR shifts cannot be thought of as reporting on the “nakedness” of the nucleus in question, as 1H NMR and 13C NMR shifts often are. Computational work by Christe and coworkers confirmed that the paramagnetic shielding is significant, and can be crudely estimated by the computed anisotropic shielding, although this value is dependent on interactions between the fluorine atom and solvent.264
These differences between 19F and 1H/13C shielding contribute to the difficulty of assigning 19F signals, and associated data reporting issues and errors in assigned structures. E.g., Burdon and co-workers synthesized functionalized perfluoroanthracenes and, based on the 19F NMR spectra of the products, decided that they were able to substitute “mainly or entirely in the 2 position”.265 Although 19F chemical shifts and splitting patterns were discussed in the text, no spectra or FID data were provided. In a subsequent study by Baker and Muir, computational results indicated that the initial experimental data more closely matched computed data for products of substitution at the 9 position, but direct comparisons with the experimental data was not possible and ambiguity about the structures still remains.266 This ambiguity could be resolved through a comparison of raw data with that generated from higher level quantum chemical computations. There are many more recent examples in which only 19F shifts are reported, with no spectra reproduced or raw data made available. It is hoped this situation will change soon, especially given the rise in importance of fluorine-containing organic molecules.260–262
7.2 Fluorine and its role in ADME
A growing area of research interest in the NP community is the generation of “non-natural NPs” by using synthetic biology approaches.267,268 The idea is to use the privileged scaffolds269 afforded by nature, and modify them to incorporate moieties and/or atoms not commonly found in NPs.270–273 In particular, the incorporation of an F atom is highly desirable, likely due to its positive impact on the biodynamic properties of biologically relevant molecules. Such analogues may affect one (or more) properties, such as protein ligand recognition and interaction, absorption, distribution, metabolism, elimination and toxicity (ADME-Tox).262,274–276 Moreover, as true NPs, organofluorine compounds are exceedingly rare, with less than ten reported.276 Hence, the way in which this atom affects a NP's biological activity and/or spectroscopic properties is rarely explored.The chemical properties of 19F, including the atomic radius, electronegativity and polarizability of the C–F bond262,275 all contribute to its use in a suite of fields (i.e., pharmaceutical industry, organic materials, and agrochemicals).277 In addition, the magnetic properties of the 19F nucleus, outlined make this nucleus an important tool for studying relevant biological processes particularly via the use of NMR, in the study of structure and function of biomolecules, enzymatic mechanisms, metabolic pathways, and ligand protein recognition.278,279
Some NP groups are striving to incorporate a fluorine atom.271–273,276,280–284 While most NP chemists are quite adept at analyzing NMR data, there are some spectroscopic properties of the molecule that change, sometimes dramatically, upon incorporation of 19F. As such, having the raw NMR data available serves to educate this research community on how to work with this nucleus in structure elucidation. For example, due to the nuclear spin of ½, the 19F nucleus couples to 1H and 13C, yielding signals with characteristic splitting patterns, many of which can be analyzed to further verify (or refute) a potential structure. Moreover, due to the high gyromagnetic ratio, the dipolar couplings are stronger, giving origin to enhanced 1H–19F NOE effects. Finally, the coupling constants (JCF) for 13C–19F are quite large (up to 250 Hz), providing information about the location of the F atom and the connectivity of adjacent atoms.278,285 In fact, these large JCF couplings are very helpful in structure elucidation, akin to using HSQC data to assign how a 13C signal can be correlated with its attached 1H signals.280,286 Additionally, relatively simple experiments, such as a 1H decoupled 13C experiment, will display splitting due to the 13C–19F coupling, and upon first inspection, such data may be quite foreign, especially to a student. In summary, with respect to incorporating 19F into a NP, some changes to the NMR spectra are modest, while others can be quite profound and/or even unanticipated; access to the raw NMR files would facilitate a more thorough evaluation and dissemination of such data.
A recent example highlights the value of 19F NMR in structure elucidation, where two fluorinated peptaibols (analogues of alamethicin F50) were biosynthesized via a site directed building incorporation approach.280 In that study, Trichoderma arundinaceum, a well know alamethicin F50 producer, was fed with fluorinated building blocks (o/m/p-F-DL-Phe), and the biosynthesis of the fluorinated analogues was monitored via in situ MS and 19F NMR. The structure elucidation of the fluorinated analogues was carried out using a set of spectroscopic techniques, including 1H, 13C, and 2D NMR data. The incorporation of fluorine in the final product was confirmed by 19F NMR, analysis of the prominent 13C–19F JCF values in the 13C spectrum, and by comparison of these data with those obtained for the synthesized standards (Fig. 34 and 35). The close match between the 19F and 13C NMR data of the synthesized monofluorophenylalinols (MW 165) and that of these moieties within the large peptaibols (MW > 1900) is remarkable. While members of this research team have performed thousands of NMR experiments over the years, the 19F NMR experiment was somewhat foreign. However, those data were extremely straightforward to analyze, and it is easy to envision deriving value from sharing those raw NMR files.
As noted previously, fluorine containing secondary metabolites are extremely rare in nature.276,287,288 Thus, when they are reported, thorough peer review is needed to insure the validity of the structure, (another compelling argument for the sharing of raw NMR data). A recent report highlights where some knowledge about 19F NMR would have likely prevented a mistake in the literature.289 The organofluorine compound [3-(3,5-di-tert-butyl-4-fluorophenyl)propionic acid] was reported isolated from a Streptomyces sp. TC1,289 which suggested the existence of an enzyme capable of mediating an aryl fluorination reaction. This report attracted the attention of two different groups, who via synthesis of the putative fluorinated natural product, and based on the analysis of 1H, 19F and 13C NMR spectra, both demonstrated the absence of fluorine in the secondary metabolite.290,291 While those follow up studies essentially refute the initial study, perhaps a more thorough analysis of the NMR data at peer review, including examination of raw NMR data, would have prevented the need for such research.
These examples show the importance of a detailed analysis of the NMR data, both when striving to generate fluorinated analogues and if/when naturally occurring organofluorine compounds are reported. A solid understanding of the NMR properties of the 19F nucleus is needed to rationalize the structure elucidation, and the raw NMR files would serve to both document and disseminate these information, possibly giving fodder for more detailed analysis as more advanced tools are developed.
7.3 The complex 19F NMR spectrum of 4,4-difluorinated proline
Amino acids and peptides are important lead compounds in drug discovery. However, such compounds usually undergo some form of structural modification before they can be considered viable drugs. Fluorination is one way to achieve this.292 The benefits of fluorination in terms of ADME properties are well known, and have been outlined in the previous section. In addition, fluorination can potentially enhance the target-binding properties of lead compounds, through conformational control.293 For example, fluorinating the 4-position of the amino acid, proline, can effectively stabilize either the exo- or the endo-pucker, depending on the fluorine configuration (an example of the “fluorine gauche effect”).294 This conformational biasing of proline has been exploited in the design of collagen mimetics,295 enzyme inhibitors,296 and organocatalysts.297Surprisingly, the closely related scaffold 4,4-difluoroproline has been little studied. The derivative 103 (Fig. 36) has previously been synthesized, and the 1H and 19F NMR spectra of this compound have been recorded,298 but these NMR data were reported only in condensed form with most signals simply described as multiplets, and no raw NMR data were made available at the time of publication. It would seem to be worthwhile to undertake a full analysis of the NMR spectra of 103, in order to ascertain all of the J-values and thereby gain information on the conformational behavior of this compound.299
Accordingly, the Hunter group recently synthesized 103 following a published protocol,298 and re-acquired the 1H and 19F NMR spectra (Fig. 36). The spectra are complicated by the presence of Boc rotamers, giving twin sets of signals and possibly explaining why a full analysis was not reported previously.298 With raw data now in hand, Hunter and co-workers performed an in-depth analysis of the spectra through DAISY simulations, and this revealed an unusual pattern of J-values of 103 (Fig. 36). The two diastereotopic fluorine atoms of 103 have identical chemical shifts; hence, the fluorine atoms do not couple to one another, and together they cause each of the signals corresponding to the four vicinal hydrogens to be split into a higher multiple of an n + 1 triplet. Nearly identical sets of J-values are observed for both rotamers of 103. Finally, Hunter and co-workers validated their analysis by also acquiring 1H-decoupled 19F and 19F-decoupled 1H spectra (Fig. 36), which were also found to be accurately simulated using the same J-values.
This elucidation of the J-values of 103 (Fig. 36) is a first step towards understanding the conformational behavior of this potentially valuable fluorinated building block.299 This information may inform the ongoing development of drugs and bioprobes that contain conformationally-biased proline residues.
7.4 Nitrogen: an underrepresented nucleus in the structural investigation of natural metabolites
Nitrogen containing metabolites occur naturally in essentially all terrestrial and marine organisms, and many of these compounds exhibit important biological functions related to their N-substitution. Unlike other nuclei, and despite the importance of nitrogen in natural metabolites, the 15N NMR of these compounds is rarely reported. In most, the nitrogen is a biologically essential element. Therefore, while nitrogen plays an important role in NP chemistry and biology (e.g., labeling of non-proteinogenic amino acids), the NMR-detectable stable isotope 15N represents a spin ½ nucleus which has very low natural abundance of only 0.35%.300 Lowering NMR sensitivity even further, 15N has a gyromagnetic ratio of only about 1/10th of that of 1H. These intrinsic properties make 15N difficult to observe directly. However, its enormous chemical shift dispersion of ca. 800 ppm offers a powerful source of structural information. Additionally, the orientation of the nitrogen lone pair of electrons is sensitive to the chemical and magnetic environment of the rest of a molecule. This has large effects on the observed coupling constants with nearby hydrogens. These coupling constants can have relatively large values, both negative and positive in a Karplus relationship, and can be used as evidence to distinguish two identical planar structure that only differ in the orientation of the lone pair of electrons.As NMR hardware, software, and experimental techniques have advanced, it has become possible to detect 1H–15N correlation of sub-milligram samples of NPs by using inversed-detected pulse sequences. Martin and Hadden301,302 as well as Marek et al.303 have provided excellent general guidance in their comprehensive reviews. While 15N chemical shifts are often determined indirectly using 1H detected HSQC and/or HMBC experiments to enhance sensitivity, this approach is limited in terms of precision and often also accuracy (lack of reference marker). While DEPT and INEPT based experiments for direct detection can overcome this limitation, they are not widely used and pose specific sensitivity challenges for nitrogen atoms that do not bear a hydrogen. A third approach for 15N detection is to use the CIGAR-HMBC experiment introduced by Hadden et al.304 and modified by Kline and Cheatham.305 By sampling a range of 15N–1H coupling constants in a single spectrum, the CIGAR-HMBC sequence minimizes the risk of missing key correlations.
Importantly, as new techniques emerge and become part of routine operations, preservation of the raw data also becomes increasingly important, as a means of safeguarding the valuable structural information of the 15N spectra. As such, raw data sharing of this heteronucleus is not only about the documentation of experimental information, but more importantly a means of expanding the utility of (15N) NMR in structural analysis and, thereby, enhancing the reproducibility of NP and chemical science.306 Additional rationales for the importance of preserving raw 15N NMR data relate to the methods, precision, and accuracy of 15N chemical shift reporting, the value of structural information encoded in 15N NMR spectra, and the relevance of the more abundant 14N nucleus for explaining 1H NMR spin–spin coupling networks.
7.5 Nitrogen: chemical shift referencing, accuracy, and precision
Unlike 1H and 13C chemical shifts, which are reported relative to TMS as the accepted reference, (frequently via residual solvent signals), 15N NMR has no widely accepted single compound that serves as the universal reference standard in both the small molecule and the biomolecular NMR communities. Currently, most reports reference 15N chemical shifts via liquid ammonia (NH3/NH4OH), either via direct measurement or by application of a series of frequency conversion factors. The benefit of referencing with NH3 is that the resonance appears in the high-field portion of the 15N spectrum, avoiding resonance overlap in the more populated lower field. This approach explains why almost all reported δN are positive numbers. However, liquid NH3/NH4OH is typically used as an external reference, and temperature will affect the calibration result by as much as 40 ppb per degree, which compares unfavorably with the 4 ppb per degree variation of TMS in organic solvents.307 This is one main reason why other reference compounds are used. Varying in solubility and the effort required for sample recovery, nitromethane (90% in CDCl3; IUPAC recommendation for both 14N and 15N), nitric acid, ammonium chloride, formamide, or ammonium nitrate are often used in NP research. Two 15N resonances exist in ammonium nitrate, and both are used as reference signals. Notably, in the NP literature, almost all 15N chemical shifts have been determined from the indirect dimension, via inverse (1H) detection. Collectively, this explains the substantial variation of reported δN values as a result of inconsistent referencing. Accordingly, the reported δN should be considered approximate values, and the availability of raw data is one element that could help resolve this situation.Another significance of raw data in 15N NMR relates to the accuracy of δN values, which are affected by the following factors:308 (a) the magnetic susceptibilities of the solutions from which the compared δN values originate are typically not identical. (b) The nature of the lock substance introduces a systematic variation/error. When using D2O, ND3NO3 or similar NMR solutes to lock the field frequency ratio, line-widths will broaden, which causes δN variations in the range of 0.1 ppm. (c) The temperature will affect chemical shifts not only in 15N but also other nuclides. For 15N, 0.4 ppm variation will be observed in two experiments when they have 10 K difference in temperature.
7.6 Nitrogen: NMR structural information encoded in 15N NMR spectra
Although the 15N chemical shift range is almost four times wider than that of 13C, the most interesting range of 15N resonances of NPs falls into the 20 to 420 ppm window. Notwithstanding the very low inherent sensitivity, it is also difficult to generate excitation pulses that are short enough to cover a 300+ ppm spectral window effectively, especially at the relatively low observation frequency of 15N. On the other hand, with the exception of oligo-(cyclo-)peptides, NPs often contain only one or very few 15N nuclei. For these collective reasons, the F1 range of a 2D NMR experiment is preferably limited to a narrow spectral window. In order to achieve this goal, raw data become invaluable as researchers can search available data(bases) and/or perform in silico prediction with increased precision provided raw data is available. Martin and co-workers have demonstrated several such cases, using both an NMR database and a commercial algorithm to predict 15N chemical shifts with only 2 ppm error, while most unfavorable result produced a variation as large as 50 ppm difference.309 Even long-range 1H–15N coupling constants are accessible through this method, with analogous limitations of currently achievable accuracy. As predictor algorithms depend on spectra derived from (manually) interpreted data, this method will become more accurate if the calculations could be based on raw NMR data.Consideration of 15N coupling constants adds another layer to structural elucidation of nitrogen-containing molecules. Although 1H–13C heteronuclear long-range couplings are generally uniform, the same cannot be said about the corresponding 1H–15N couplings. This is mainly due to the effect of the lone pair electrons of nitrogen. The direction of the C–H bond of a hydrogen that exhibits a long-range coupling to 15N can have a significant impact on the value of the 1H–15N coupling constant. When the C–H bond direction is synclinal to the orientation of the lone pair, the 1H–15N couplings tend to be stronger and its long range HMBC correlations are detected readily. In contrast, when the C–H bond direction is anticlinal, the couplings will be much weaker and are more difficult to observe in HMBC experiments. The large variation of long-range 1H–15N couplings makes it more difficult to observe all 1H–15N correlation in a single HMBC experiment, because of the challenge in optimizing the magnetization transfer delay between 1H and 15N. So far, a universal approach has not been established. In current practice, the coupling values are either predicted in silico,309 or two different coupling values are chosen as distinct magnetization transfer delays implemented into two HMBC experiments.310
While 15N is the primary isotope related to the acquisition of nitrogen NMR spectra, it should be pointed out that the prevalent nitrogen isotope, 14N, also plays a role in NMR spectra of natural metabolites, namely in the spectral interpretation of 1H NMR spectra. One recent example is the observation of a 1H–14N coupling in the 1H NMR spectrum of ambiguine N isonitrile,311 a hapalindole alkaloid (104). This was discovered when analyzing preserved raw data via1H iterative full spin analysis (HiFSA) with the PERCH software tool, an algorithm based on quantum mechanical calculations and iterative fitting procedures.62
The signal of the axial hydrogen, H-26a shows an unexpected and rather complex splitting pattern. Only when considering heteronuclear coupling, was it possible to explain the involvement of H-26a in a 3J-coupling with the 14N nucleus of the isonitrile group. The coupling of the hydrogen with 14N, representing a spin-1 nucleus, leads to an additional signal splitting to (pseudo-)triplets with a relative ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. After including the 14N spin-particle and its coupling into the spin simulation, a fully matched spectrum was obtained. Moreover, the coupling constant of H-26b with the isonitrile nitrogen could be determined to be as small as 1.13 Hz, which was required to achieve convergence during the HiFSA iteration. It is a reasonable hypothesis that similar evidence is prevalent to other nitrogen-containing small molecules. Access to raw heteronuclear NMR data would much facilitate the analysis of high quality original 1H NMR data of N-containing NPs. However, most current databases do not support this kind of data mining, because the stored data is interpreted information rather than raw data. Recently developed repositories such as Protein Chemical Shifts312 are no exception. Other typical examples of obscured NMR information is the use of “multiplets” (m) to describe signals with more than two or three spin–spin couplings. This particularly affects signals with small couplings, such as the long range 1H–14N couplings, which otherwise could help exploit the C–H orientation in three bond anticlinal or synclinal arrangements relative to the lone pair electrons.
1. After including the 14N spin-particle and its coupling into the spin simulation, a fully matched spectrum was obtained. Moreover, the coupling constant of H-26b with the isonitrile nitrogen could be determined to be as small as 1.13 Hz, which was required to achieve convergence during the HiFSA iteration. It is a reasonable hypothesis that similar evidence is prevalent to other nitrogen-containing small molecules. Access to raw heteronuclear NMR data would much facilitate the analysis of high quality original 1H NMR data of N-containing NPs. However, most current databases do not support this kind of data mining, because the stored data is interpreted information rather than raw data. Recently developed repositories such as Protein Chemical Shifts312 are no exception. Other typical examples of obscured NMR information is the use of “multiplets” (m) to describe signals with more than two or three spin–spin couplings. This particularly affects signals with small couplings, such as the long range 1H–14N couplings, which otherwise could help exploit the C–H orientation in three bond anticlinal or synclinal arrangements relative to the lone pair electrons.
Collectively, the above points clearly support the importance of raw NMR data for heteronuclear NMR in the nitrogen domain, encompassing both 14N and 15N effects.
7.7 Phosphorus: 31P NMR in natural product structural investigations
The structural nature of phosphorus present in naturally occurring molecules may be identified by 31P NMR spectroscopy.313–317 The 31P nucleus being 100% abundant, having a broad chemical shift range of ∼800 ppm, and with a receptivity 377 fold that of 13C,317 represents a sensitive tool for deducing the structural nature of phosphorus when present. The two main parameters associated with 31P NMR that are used for assessing structural content are: (1) 31P chemical shift which is typically used to identify the type of phosphorus (e.g., phosphate, phosphonate/phosphonic acid, or phosphinate/phosphinic acid); and (2) 31P coupling constants to both 1H and 13C. The latter information serves to pin down the “local neighborhood” in which the phosphorus-containing structural element resides. The information deduced from the 1H/31P J-couplings, is useful for assessing 2- or 3-bond relationships including configurational criteria, and may be extracted directly from the 1-D 1H NMR data with the assistance of HiFSA. Selective heteronuclear decoupling of the 31P contribution to the 1H spin system would facilitate the HiFSA interpretation of the 1H NMR spectrum. Moreover, the use of 1H homonuclear-2D-J-spectroscopy can also be used as orthogonal NMR methodology where, provided the digital resolution along the F2 dimension is optimized, the F2 projection results in a 1H-decoupled 1H NMR spectrum but one which retains the 1H/31P J-coupling information. Examination of the fully coupled 31P NMR spectrum produced by gated decoupling of 31P will also reveal the 1H couplings to phosphorus. Since most phosphorus-containing small molecule NPs contain but a single phosphorus atom,318–323 this represents a very straight forward approach to spectral analysis. The coupling of 31P to the 13C signals in the molecule further defines the structural environment of the phosphorus substituent and can be evaluated directly from the proton-decoupled 13C spectrum. If the NMR spectrometer is so equipped, decoupling of 31P from 13C can be achieved which unambiguously defines which carbon signals are coupled to 31P, including very small couplings which may reside within the linewidth of the carbon signal. Large couplings will be collapsed and removal of small couplings will lead to a sharpening of resonance lines with concurrent reduction in the linewidth-at-half-height (w1/2). Because of the large difference in the observation frequency between 13C and 31P nuclei in a molecule with only one phosphorus atom, with broadband 1H decoupling, spectral interpretation of the 31P coupling to the 13C is first order. The coupling of 31P to 13C can also be deduced from a 31P-detected 31P,13C-HMBC or 31P,13C-HSQMBC experiment. If, however, there is more than one phosphorus in the molecule and the phosphorus atoms happen to be spin coupled, and have the same or similar chemical shifts, the spin pattern observed for the proton-decoupled 13C spectrum arising from coupling to 31P will exhibit higher order spin coupling effects (virtual coupling). Examination of 1H fully coupled 31P NMR spectra can reveal the number and kind of aliphatic groups attached to the phosphorus. For example, the proton-coupled spectrum of the 31P signal of trimethyl phosphate exhibits a 10-line pattern arising in this case from spin coupling to nine equivalent protons (n + 1). This provides additional structural hooks.Finally, it should be noted that the relatively high sensitivity of the 31P nucleus make it attractive for the establishment of quantitative 31P NMR (qPNMR) methods. This enables the determination of impurity profiles with high selectivity, as has been demonstrated for phosphonomycin, 105, (now fosfomycin), a broad spectrum antibiotic discovered from a Streptomyces species in 1969.324 It is used parenterally as the sodium salt, or orally as the calcium or more commonly, as the tromethamine (trishydroxymethylaminomethane) salt. In either of the first two cases the only significant degradation product arises from opening the epoxide ring to give a mixture of the (1S,2S and 1R,2R) diols 106a and 106b, collectively referred to as “impurity A”. The lack of UV absorbance and the high hydrophilicity of these compounds confounds the usual HPLC quality analysis procedures and Jiang et al. have developed a quantitative 31P NMR (qP NMR) method circumventing these problems.325
Considering that the majority of investigated NPs are devoid of phosphorus, it is even more important to realize that (selective) 31P derivatization and subsequent qP NMR has great potential to advance the analysis of complex NPs. One smart concept targeted at expanding the utility of 31P NMR to oxygenated NPs involves the in situ labeling of labile hydrogens (aliphatic as well as phenolic and carboxylic hydroxyl groups) with a phosphitylation reagent. Using 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane (Cl-TMDP) as reagent, the proof of concept was demonstrated for the analysis of lignins, which consists of condensed and uncondensed polyphenols.326 Recently, this method has been developed further into a simultaneously qualitative and quantitative 31P NMR method for the analysis of complex mixtures of condensed tannins (proanthocyanidins, such as 75) in Acacia and Schinopsis species.327 The method takes advantage of the large 31P chemical shift dispersion of the derivatized groups, the structural information from HSQC spectra of the derivatized materials, and the favorable sensitivity and selectivity of qPNMR. Collectively, this allowed the comprehensive characterization of complex proanthocyanidins from crude mixtures, including the quantification without the need for identical calibrants, both of which represent major phytochemical challenges. Considering the chemical complexity of such analytes, the availability of raw 31P data will predictably advance the knowledge base for interpretation of qualitative and quantitative 31P NMR spectra.
In the context of the raw data focus of the present review, it should finally be pointed out that 31P NMR reference spectra have the potential to inform subsequent studies aimed at solution structures of drugs binding to molecular targets. One example is the complex of the antibiotic, nisin, and a shortened version of the bacterial cell wall precursor lipid II (3LII), which show differences in the 31P chemical shifts of the free versus nisin-bound forms of 3LII, as a result of intramolecular hydrogen bonding.328 In the same study, overlaid 15N HSQC spectra were also employed to map nisin binding.
8 Databases
8.1 Database introduction
Both the quality and resolution of NMR spectra have improved since the introduction of the first Varian commercial spectrometer, the HR-30, in 1952. However, most of the contributors to this review never experienced the beginnings of NMR before computer screens replaced the long scrolls of paper sheets that were used. The digitization of NMR did simplify tasks such as peak-picking, integration, phase correction, and apodization functions. It also enables the sharing of spectra over networks.But this digitization also came with adverse side effects. As every manufacturer developed new features, used different digitization technologies, and different acquisition methods, the complexity of the file formats increased. Current multi‐vendor NMR software accommodates up to 20 raw data formats, not counting the different flavors of each of these formats. In addition to these differences, the storage methods are different: some use a single file for the acquisition, others require multiple files, whereas even others require a particular directory structure to be functional. Current conversion solutions often involve juggling between several file formats and software tools in order to obtain input data that is compatible with a given NMR software. Such an elaborate process is detrimental to the integrity of both data and associated metadata, as it implies conversion between different floating point and/or integer encoding schemes and introduces rounding errors. The Holy Grail is software that will convert all of the other formats into readable files without the loss of data. Unfortunately, no such software exists.
As long as a user can access a repository of data, and dig in archives (implying the storage support remains active), and can still open those files, everything should be fine, except when it comes to sharing results. As this typically is done through publications, it opens the question of how NMR results are actually reported. Most frequently, as tables, which just may include HSQC, HMBC, and NOE correlations, but sometimes just as text (listings of chemical shifts, coupling constants, multiplicities, and assignments). Recently, it has become customary to include printouts of spectra as ESI.† As this has been limited to PDF format, these published spectra have been “filtered” through various convoluted conversion processes such as screen captures, lossy bitmap compression and/or presentation software, and other operations that involve format changes and/or are associated with degradation of information. The result is often a small, highly pixelated bitmap picture, which hides the details needed to examine a proposed structure (coupling constants, satellites, purity). Beyond data sharing, lurks the problem of the minimal information required to elucidate and describe unambiguously a structure. While publication platforms differ in their requirements, NMR users would benefit greatly from initiatives similar to those developed in the Mibbi project,329 aimed at producing minimal reporting guidelines for the biological and biomedical investigations.
Recently, pharmaceutical companies, manufacturers, software companies, universities, and others joined the Allotrope foundation effort (allotrope.org). The foundation's objective is to develop a single universal data format, linking the three main scientific productions: raw data, results and evidence. The single file using the Allotrope Data Format (ADF), contains the original data file, and the treated data (in a standardized but still evolving form). Further, it provides a way for the equipment, people, processes, geographical locations, and projects to be linked and described. The foundation develops standardized vocabularies and data descriptions. With the use of ontologies and semantic web technologies, all of these elements can be linked to other resources (online databases or internal repositories) and annotated appropriately. This joint effort aims to unify current analytical data, including NMR data, and allow NMR records to survive the test of time, crashing hard drives, and the confusion of a myriad of formats.
8.2 The urgent need for spectral repositories and automation support for peer-reviewing of spectral data
Structure elucidation of organic compounds starts with experimental data, transformed into spectra, and translation into present/absent structural fragments. The final step combines these fragments into a structure proposal fitting the given constraints derived from the experimental data. This process can fail and lead to a wrong structure, because this sequence can be broken at any point.19,121,330,331 In order to detect wrong conclusions, it is often necessary to trace back to the raw data.Furthermore, there is a major need for validated reference materials of authenticated chemical structures in order to build spectral databases that can fully support the process of upcoming structure elucidation problems. As long as the scientific community relies on non-validated reference materials with potentially wrong structures, conclusions derived remain uncertain. The unwanted consequence of this domino effect is that the impact of non-validated results increases, rather than decreases, by contributing to an increasing number of potentially wrong structure proposals.
The following two case studies exemplify this tight relationship between research quality and the availability of raw data: (i) aglalactone isolated from Aglaia elaeagnoiea, and (ii) the identical NMR-data published for orientanol A and eryvarin A. The wrong structure proposal for aglalactone, (35)118 has been revised to 36 in a subsequent paper.122 The following situation has not been corrected. Orientanol A (CAS-RN: 190381-82-9; C21H24O7, 107)332 was published by Tanaka et al. for the first time having a 2,3-dihydroxy-3-methylbutyl side chain showing 13C chemical shift values of 27.1, 78.6, 72.9, 26.1 and 25.0 ppm. In a later paper333 published by the same group, a new isoflavonoid named eryvarin A (CAS-RN: 302928-70-7, C21H22O6,108)333 was described having nearly identical carbon chemical shift values (9 positions differing by 0.1 ppm each). The 1H chemical shift values are also identical within 0.02 ppm; and the coupling constants are within the range of the digital resolution of a standard 1H NMR experiment. It is interesting to note that even the labile hydrogens of both OH-groups in 108, are identical to those in 107. Formally, 108 is created from 107 by cyclization of the side-chain and elimination of H2O. Despite this cyclization, the chemical shift values of the carbons and hydrogens in the sidechain of 107, which is now converted into a six-membered dihydropyran ring system in 108, remain unchanged. Table 5 compares the 13C NMR experimental values of the five carbons within the sidechain against their expectation ranges and the expectation ranges of the 2 possible cyclization products. Table 5 also shows that the experimentally determined chemical shift values fit best to the sidechain product named orientanol A (107), whereas the dihydropyran-derivative 108, is a reasonable alternative structure to the given data under the assumption that the resonances at 78.6 and 72.9 ppm have been misassigned despite measuring 2D NMR-spectra. The spectral data of eryvarin A have been repeated (compound 7 in ref. 334). There is no claim that either orientanol A or eryvarin A is the correct structure proposal to this set of spectral data, but the severe inconsistency in the underlying data material is clearly visible. It is also clear (Table 5) that the alternative ring closure structure (109) is not viable.
This example demonstrates the urgent need to deposit raw spectral data in an electronic format in a repository in order to reinvestigate the whole process of structure elucidation starting at the very beginning and allowing chemists to follow the whole chain of decisions. It is also clear that there is not always an absolute solution to the interpretation from any one source of data. However, the availability of raw data offers a means of clarifying such a discrepancy.
The CSEARCH-database (nmrpredict.orc.univie.ac.at/) consists of some 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 13C-NMR spectra and a sophisticated software-package. The examples given here have been found, when searching for identical spectra published by at least one common author occurring in different literature citations associated with different structures.
000 13C-NMR spectra and a sophisticated software-package. The examples given here have been found, when searching for identical spectra published by at least one common author occurring in different literature citations associated with different structures.
8.3 Databases for dereplication
The number of known NPs has grown, and continues to grow at a remarkable rate, swelled by over a century of research into terrestrial and marine plants, animals and microbes. A significant proportion of these NPs were first described in the scientific literature prior to, or in the formative years of NMR-enabled structure elucidation. Notwithstanding the many impressive feats of structure analysis achieved in those early years, where available, published accounts of NMR data from this era predating modern high field and 2D NMR spectroscopy, are limited to tabulation of 1H NMR (and later, possibly 13C NMR) resonances. By necessity, NMR assignments were often either incomplete or tentative, with some resonances described merely as broad singlets or unresolved multiplets, and overlapping resonances collectively listed as “envelopes”, or not listed at all. In an effort to enhance NMR characterization, early authors sometimes published accompanying unannotated images of NMR spectra, although these rarely offered added insights into molecular structure. The limited NMR characterization of legacy known NPs remains an enduring problem to this day, especially as current and future researchers seek to re-investigate these structures, as a prelude to exploring their chemical and biological properties. For example, modern researchers typically re-isolate a known natural product, and use this as a launchpad to explore a rare and unusual structure class. The justification for such an investment is based on the view that known NPs represent a legacy resource of considerable value, capable of accelerating the discovery and development of new molecular products (e.g., as pharmaceuticals and agrochemicals).Illustrative of just such an investment, during a search for new antifungals the Capon group recently isolated a large and structurally complex (C56H102N3O15) natural product from cultivation of a sheep-feces-derived Streptomycete. Based on a preliminary spectroscopic analysis they determined that this metabolite was most likely the guanidyl polyketide macrolide, amycin B (110), first reported last century from a Greek soil-derived Streptomyces spp.335 The amycins are a remarkable class of natural product that include niphimycin/scopafungin,336 copiamycin,337 the azalomycins338,339 and guanidylfungins,340 neocopiamycin A,341 malolactomycin A,342 RP 63834,343 the shurimycins,344 RS-22s,345 the kanchanamycins,346 and the primycins.346 Despite being known as NP antifungals for over 50 years, chemical knowledge of amycin B (110), and other members of this structural class remains limited to planar structures, supported by modestly annotated and tabulated 1D NMR data.
This is not an uncommon occurrence. There is without doubt a great deal unknown about a great many known NPs. To explore the antifungal potential of amycin B and related NPs it was first necessary to confirm (and if possible complete) existing structure assignments. Whereas the 1D and 2D NMR data acquired on a re-isolated sample of 110, was an excellent first step (Fig. 37), lack of access to comparable data for other members of this structure class severely limited the scope of these investigations. This dilemma is compounded by the fact that the original authentic samples of these and most other known NPs are generally lost, and commercial sources are largely non-existent (Fig. 37).
A possible solution to this problem lies in the observation that modern NP researchers routinely detect, isolate, characterize and identify known NPs, and in doing so acquire and analyze high quality NMR data, often vastly superior to published data (as evidenced by the reisolation of 110). However, as the constraints of modern scientific publishing preclude the reporting of known NPs, this NMR data languishes as unpublishable output in the archives of individual laboratories, companies and institutions, albeit a very valuable resource. With modern NMR data comprising electronic files that are readily shared, processed, and analyzed by any number of free and commercial softwares, there is a very strong case for establishing a global NP NMR data repository. This repository could accept, register, curate and facilitate free worldwide access. In due course, scientific journals could make uploading and registering of NMR data a condition of manuscript submission, much as is already the case for X-ray crystallographic and genetic sequence data. The same could apply to (post)graduate NP research theses, which are typically rich in such data. In this scenario, researchers uploading data could be acknowledged on a per data set basis, the registered entry could be cited by future researchers, thereby forstering a collegial culture of international, interdisciplinary, and intergenerational recognition.
8.4 The importance of raw data in databases
This Raw Data Initiative is a continuation of previous efforts1 to initiate the creation of a repository for raw NMR data dedicated to small molecules, especially those of biological origin. One major goal is to minimize the time taken to identify known molecules and therefore to avoid the duplication of structure determination efforts. Current practice involves extraction, isolation/purification, time-domain NMR data acquisition, conversion of these into the frequency domain, extraction of spectral parameters (chemical shifts and coupling constants) and proposal of a chemical structure by deductive reasoning. A search of available databases provides a high probability that the compound is novel or known. Even in the latter case the task is not finished until a search of the literature confirms that the spectral data of the isolated compound matches that of the known. The preservation of FIDs opens the doors for re-processing with other parameters and/or algorithms, which subsequently allows extraction of more or better spectral parameters from the same data. The same reasoning should encourage the community to constitute reference samples repositories, thus permitting the recording of new raw data sets by means of emerging methodologies. Non-Fourier transformation of FIDs are pertinent in many situations, especially for multi-dimensional NMR data processing, in contexts such as non-uniform sampling, covariance analysis or the extraction of relaxation and diffusion parameters. Even transformed data in the direct dimension may benefit from processing methods like reference line deconvolution. The possibility of reprocessing time domain data therefore warrants the possibility of obtaining better spectral data, better estimations of spectral parameters and finally more reliable structure determinations.347The Institute of Molecular Chemistry of Reims, France, is putting together a focused library of raw, time domain NMR data and transformed data that is linked with enhanced structure files, i.e., Structure Data Format (SDF) files in which atoms may be arbitrarily tagged. These tags are connected to chemical shifts values and paired to coupling constant values. Data was obtained from a small library of glucosinolates and of their desulfated derivatives using the PERCH software,62,348–352 in a process that is similar to the constitution of the MetIDB database. Such a protocol is the only one that ensures a realistic transposition of a 1D 1H NMR data from one static field value to another for comparison purposes.
The same research group has also designed a dereplication workflow based on 13C NMR data and is used for the analysis of complex plant extracts.353 The 13C NMR spectra of the samples produced by Centrifugal Partition Chromatography fractionation are binned and the bin contents are classified according to the resemblance of their chromatographic profiles. Sets of chemical shifts with similar profiles constitute keys to the search for known compounds in a locally developed and enriched database that links structures and 13C NMR chemical shifts. The latter are obtained by prediction from structures by means of commercial software. The availability of raw NMR data reference compounds would contribute immensely to the progression of efficient dereplication tools.
8.5 The breadth of databases and their use by chemists
The past 10 years have witnessed the development of a global network for data sharing of spectroscopic and chromatographic data in metabolomics.354,355 The principles behind this data sharing network have been established over the past 20 years by the molecular biology community in areas such as genomics and proteomics.356 Since the field of metabolomics is populated by a considerable number of analytical chemists there is now hope that the principles of free and open data sharing will be more widely adopted by the quite conservative chemistry community. This hope is supported by initiatives like the FAIR data movement,357 which advocates for research data being Findable, Accessible, Interoperable, and Reusable (FAIR), in order to properly support the scientific methods. These are gradually being adopted by major funding agencies such as the European Commission, the National Science Foundation, the National Institutes of Health and many other funding organizations. The fundamentals of metabolomics data sharing are not very different from those in NP chemistry. Data in Metabolites and other databases are composed of a core of raw data surrounded by metadata aiding in the interpretation of the experiment. Whereas the raw data consists of NMR, MS, and chromatographic data, the metadata covers the whole range necessary to understand how the study was conducted: the species under investigation, which organism part the metabolite was isolated from, which instruments were used, and which parameters were used.Every data point in the above-mentioned metadata is backed by a term in a commonly used ontology, and the question which type of metadata needs to be reported is dictated by the minimum information standard. For metabolomics these minimal information (MI) standards were created by the metabolomics standards initiative around the year 2007.358 Most of the minimum information principles established by the MSI are directly applicable to the NP community. Additionally, information from the 2015 initiative to establish the minimum information about a biosynthetic gene cluster are relevant.359 The molecular biology community, in establishing databases like MetaboLights and the metabolomics workbench, has laid the technological foundation for the archives necessary to establish a raw data NMR sharing in NP chemistry. MetaboLights, for example is completely based on open source technology, open data standards and open data formats, and community-based reviews on the topic have appeared.360
Following the establishment of MetaboLights, the COSMOS initiative361 has complemented the set of open data formats for mass spectrometry such as mzML with a sister format for the representation of raw NMR data, nmrML, which has been established very recently.362 With clear signals by the major NMR instrument manufacturers for the support of this new open format, nmrML has the power to replace the age-old JCAMP as a usable open data format in NMR spectroscopy. The computational frameworks to hold and describe raw data and metadata are equally in place. The ISA format, for example, is widely used across domains in molecular biology and metabolomics in particular.363 It is capable of holding all the necessary metadata of an investigation, study and its underlying assays (ISA = Investigation, Study, Assay), in a spreadsheet-like format, backed by a wide range of ontologies, including the NMR term ontology established as part of the nmrML work.
There are indeed few differences in describing an NMR-based metabolomics experiment and describing the isolation and identification of a NP, the most important of which might be the fact that the latter is hopefully based not on mixtures but spectra of pure compounds. In summary, it is anticipated that this work, embedded in a large, worldwide community interested in metabolomics data management over the past 10 years will be instrumental in establishing a network and movement for NMR data sharing in NP chemistry. Several publishers have already embraced open data sharing for the articles published in their journals, often at additional burden for the researcher, and dedicated data publications364 in certain journals are a viable alternative to the typical reports about the isolation of NPs found in more traditional outlets.
8.6 Raw NMR data formats
The recently introduced mzML, nmrML, sibling formats for MS and NMR, respectively, aim at integrating two analytical techniques that are essential for metabolomic analysis. The utility of NMR and the importance of open data formats in advancing the contextualization of metabolomic data in pharmacognosy research has been highlighted by some of the authors.365 While well-intended at the time of implementation, existing implementations of the IUPAC JCAMP-DX spectroscopy standard into NMR software vary in adherence to the standards and inter-operability. In practice, JCAMP data are not fully compatible between the various software platforms. More comprehensive data standards exists, such as the ISA363 and ADF (allotrope.org) formats that are fully capable of including NMR metadata and even provides the ontology of nmrML, but their broader implementation is pending and complicated by their broader scope.One very recent effort approaches the standardization of NMR data format for instruments and software through a process driven by an extensive consortium of manufacturers of analytical equipment and its user base, representing a variety of fields in research and application: the Allotrope Foundation (allotrope.org) has developed a universal raw data format, the Allotrope Data Format (ADF), which also accomodates the storage of derived results. Another effort, co-led by one of the present authors,366 is the NMReDATA initiative (nmredata.org)367 and combines forces from the NMR scientific community consisting of individuals, software manufacturers, and the journal, Magnetic Resonance in Chemistry (MRC). One tangible recent result is that MRC requires the dissemination of digital NMR spectra and data for assignment articles submitted since early 2018. Moving forward, the MRC editors also intend to require that authors supply raw NMR data as a means of result verification.368
The establishment and widespread implementation of universal data formats in science is a major challenge. Creation of the actual formats and their acceptance are both evolutionary processes, which can be predicted to take time. In fact, progress may depend more heavily on the success of consensus building mechanisms than on the scientific mechanics of the actual format definition, for which the above initiatives have already paved the way. As this process continues to unfold, it is important to realize that the data produced by NMR instruments already represent a “native” form of raw NMR data and are readily available for use. One key message of this Raw Data Initiative is that there is no reason for procrastination. Archiving and dissemination of raw instrument data is feasible and practiced by an increasing number of scientists. Albeit somewhat proprietary, the single FID/SER files and pre-defined folder structures can be read by many software tools, even when produced by older hardware, and transcription to the future raw data standard(s) will almost certainly be straightforward via automated conversion tools.
9 Clinical uses
9.1 Expanding raw data concepts from chemistry to clinics: moving from NMR to MRS
Thirty years ago, the idea of bringing NMR into a clinical setting using magnetic resonance spectroscopy (MRS) generated considerable excitement. Despite this enthusiasm, MRS is still not widely used, in part due to financial considerations (billing for time on scanners), lack of expertise in radiology departments, vendor reluctance to develop new software, and possibly foremost, because the number of metabolites identifiable is small. Today, the main use for MRS is to distinguish a brain lesion as a tumor or non-tumor. The most common metabolite signals used to identify a tumor are a decreased N-acetylaspartate/choline and increased lactate-to-lipid ratios.Reflecting the belief that the power of MRS for chemical imaging of the brain and other organs is enormous, raw MRS data is being revisited with machine learning and other mathematical tools, beginning with a large database of pediatric brain tumors. Starting with this particular database is important as recent technical improvements in MRS allow the identification of other tumor-specific markers that can help classify tumor biochemistry,364,369–372 and by progress in applying new analytical tools to increase the number of identifiable signals. Using newly developed normalization and other mathematical tools,373 proof of concept has been generated for the identification of >90 signals from brain MRS data of pediatric concussion subjects.374 The MRS fingerprints enabled differentiation of healthy children from those with concussions. The tools were applied to processed, post-FT data, neglecting the imaginary numbers in the data, so that pre-processed data represents a largely untapped source of information. While optimization of the data analysis tools is work in progress, the results already suggest the value of revisiting raw MRS data, which are often not stored and, thus, lost.
Current approaches to MRS data analysis carry assumptions about which chemicals contribute to an in vivo spectrum. However, these are incomplete or even flawed. Importantly, they diminish the capability of detecting metabolic features that are not inserted a priori in the underlying MRS models. Future work will group patients into clinically relevant subgroups (responders vs. non-responders to certain therapies) and look for common chemical signals, thereby bypassing any assumptions. If successful in the long-term, this research will provide readily obtainable (noninvasive 30 min scan on any state-of-the-art MR scanner) metabolic signatures at the time of diagnosis that lead to personalized therapy.
Collectively, the availability of raw MRS data is crucial for the ability to extract new insights from existing measurements that are performed daily, on a routine basis. Similar to NMR in chemical analysis, raw MRS data contain a plethora of untapped information, which can be unraveled. Notably, because NMR and MRS share the same underlying nuclear resonance mechanisms, insights derived from chemical NMR analysis could potentially inform clinical MRS applications, and vice versa. Similar prospects for the utility of raw NMR data disseminated via an open database concern other forms of in vivo NMR spectroscopy, including a 1D 1H or 31P experiments aimed at the chemical analysis of tumor and other pathological tissues. The ability to quantitatively assess contributions from certain identified metabolites can provides valuable information for subsequent patient treatment and open opportunities for individualized medicine.
10 Conclusions & outlook
10.1 Decades of manual mining prove the concept
Comparing the development of mass spectrometry (MS) and NMR spectroscopy in terms of databases and computational tools clearly indicates that data simplicity has been the main driver of the use of such data: both the ions in MS and the singlets of 1H broad band decoupled 13C NMR data can be represented as x,y-pairs (MW or chemical shift, respectively, and intensity) and, thus, can readily be transformed into search algorithms. As coupling or other connectivity/spatial relationship information is invovled in nearly all other 1D and 2D, NMR spectra are inherently more complex and mostly evade a simplistic treatment.However, the development of the CSEARCH database (http://nmrpredict.orc.univie.ac.at/) for the systematic mining and use of 13C NMR data can serve as an excellent example of the information content of NMR spectra in general. Especially for 13C NMR, the chemical shift value of a given carbon atom is highly characteristic of its chemical environment in a given molecule. In fact, deviations are so small (in the low ppb range) that even the absolute configuration of monomeric building block in oligomeric compounds can be achieved183,184 and subtle differences in the diastereoisomerism of closely related congeners can be recognized.60 Importantly, for general applications, 13C NMR enables structural dereplication with extremely high degree of certainty, provided that adequate acquisition conditions are employed to ensure comparability of the data sets (e.g., concentration range, solvent, temperature). The CSEARCH database clearly highlights both, the dereplication of 13C NMR and the necessity to make NMR raw data accessible to the scientific public. The database has been built over decades by transferring tens of thousands of assigned NMR data sets in combination with the structures derived, from peer reviewed journal sources into a digital format. From this starting point, data comparison, and shift value statistics and shift value-structure motif correlations were made possible. Taken together these two contributions, which do cover more than a decade of scientific progress, prove that improvements in NMR data handling, data interpretation and data presentation are still needed. It must not be overlooked, that the mere presentation of processed NMR spectra in the ESI,† as advocated by many scientific journals, was only a first step forward. The shortcoming of printed ESI† has been a subject of discussion in other scientific communities, such as genetics.375 It does not sufficiently address the problem, since both spectral overlap and low resolution graphics usually allow no unequivocal analysis of spectral identity or special spectral features. Raw NMR data, especially for 2D NMR spectra, are usually a few megabytes only and even desktop-grade IT infrastructure will allow its swift dissemination. The “soft revolution” in NMR technologies allows the processing of such data independent of the instrument platform involved in their recording. Hence, raw NMR data deposition is needed urgently, for the following four key reasons. (i) It is vital to present raw NMR data of substances isolated from natural sources or synthesized in a total synthesis approach aiming to verify a structure hypothesis. This obligation is especially important if a new NP discovery claim is made. (ii) It is equally important to present raw NMR data of substances/substance mixtures administered in pharmacological in vivo and in vitro studies; especially if isolates from natural sources are investigated. Only if certified material (with NMR data) is utilized can this obligation be waived. (iii) Raw NMR data should also be made available for substances/substance mixtures that are utilized as calibrants in quantitative measurement campaigns; especially if isolates from natural sources are investigated. Only if certified material (with NMR data) is utilized this obligation can be waived. Finally, (iv) industry, especially outfits that bring measurement platforms to clinical use (under FDA or IVD-CE clearance), monitor drug (metabolites) and/or raw materials used for calibrant production by NMR spectroscopy should always provide the respective raw data.
10.2 The urgent need for public dissemination of raw NMR data
This review aims to increase the awareness of NP scientists, and the entire chemistry community, to the advantages of modern software tools to extract meaningful data from raw FIDs and avoid the loss of information in the process of NMR data interpretation and documentation. This urgent call for a change in scientific dissemination practices is supported by a global group of scientists, who contributed to the present review (Fig. 38). Specifically, this group advocates universal depositing of these FIDs in free access databases, institutional repositories, or at least in investigator-initiated ESI† files so that the FIDs will be available to scientists worldwide, not the least to manuscript reviewers. Establishing the public sharing of raw NMR data as a standard practice will create both obligations and opportunities that engage authors, reviewers, and readers equally. This creates a mutually beneficial push–pull relationships that can only enhance the integrity of science and reduce the occurrence of incorrect published structures,19,22,23 especially when considering that researchers essentially serve in all three roles simultaneous, i.e., as authors, reviewers, and readers. | ||
| Fig. 38 Support for the call for disseminating raw NMR data comes from the global natural product research community, as shown by the locations of the authors who contributed to the present study. | ||
Although a number of databases exist, there is no universally accepted format, especially for crucial FID-associated metadata, such as, solvent, temperature, concentration, instrument, field strength, and charge (i.e., pH or more likely pD) for spectra of compounds with ionizable groups. This review reports on at least two examples where conclusions have not yet been reached because spectra of the same (or not) compound have not been identical, almost certainly because spectra were taken of samples with different degrees of ionization. In general, this is particularly a problem with peptides. Fortunately, the reporting standardization including metadata aspects may be addressed as IUPAC has put together a Project Task Force to address just this problem. A global, universally accepted database is an enormous task. Its feasibility will depend on an adequate combination of international coordination, funding, and sustainable mechanisms, most likely required by “first world” countries. Historically, funding and sustainability have restricted most existing databases. The rise of distributed databases, linked data, and data-interoperability consortia could provide alternative monolithic data-silos that are difficult to maintain. In fact, these approaches are more likely to ensure viability, accessibility, and achievement of overall project scope for a global, universally accepted database containing raw NMR data. The availability of metadata for raw NMR data (FIDs) becomes even more crucial for experiments that involve randomly generated parameters, such as the randomized t1 sampling schemes in non-uniform sampling (NUS) 2D NMR experiments. Collectively, metadata is a vital part of the raw NMR data and responsible for making data sets fully transparent.
The case is made here that 1H NMR data alone, if mined thoroughly, can go a long way to overcoming most of our current problems. Correct structure elucidation is an absolute necessity for the development of bioactive leads. The prevailing mantra is that absolute structure determination is only achieved through X-ray diffraction or total synthesis. The former can have difficulty in distinguishing O and NH and the latter is both resource intensive and not infallible, as is evidenced by the interesting case of elisabethin A, isolated in 1998 from a West Indian Sea Whip and assigned the structure 111.376
In 2004, the total synthesis was claimed,377 to be contradicted almost immediately,378 as beautifully summed by David Whitehead at www2.chemistry.msu.edu/courses/CEM958/FS04_SS05/whitehead.pdf. The structure of elisabethin A could well be that claimed by the original authors, but it has not yet been confirmed by synthesis. It seems likely that the synthetic product is a diastereomer of 111. This case supports the claim that there is no such thing as absolute structure proof. Nonetheless careful analysis of all of the data embodied in a simple, but accurate 1H NMR spectrum can lead to a structure, in which there can be high confidence, even when the spectrum has been acquired on microgram quantities of highly pure material.
The availability of raw NMR data would also serve as a catalyst for the increasing number of studies that utilize quantum chemical calculations for the purpose of structure elucidation. The computation of chemical shifts and coupling constants using quantum chemistry is now regularly included as a key component in the assignment and revision of the structures of complex NPs. While theoreticians have been developing methods for computing these values for decades, many organic chemists first became aware of the power of such approaches through Rychnovsky's reassignment of the structure of hexacyclinol (112 to 113).379 A variety of reviews have compiled examples (there are many) of similar studies,380–382 and Hoye and co-workers have even provided a tutorial for carrying out such studies.383 Several representative examples have been discussed in this review. The combination of experimental data and quantum chemical calculations has the potential to revolutionize structure determination, both its speed and accuracy.
10.3 Evolution of raw NMR data repositories
The term “database” is used frequently to refer to gathered digital content of any kind, stored in a binary or text format, using a more or less pre-defined structure, e.g., that of a relational or NoSQL database. For the purpose of this discussion, the “container” is defined as a database, the content as (raw NMR) data, and the combination of the two as a repository. Moreover, as the term “data” has a very broad meaning, in the present review, data refers to the combination of experimental data (i.e., the information/data obtained during the NMR experiment, in particular the FID) as well as the metadata that are necessary to reproduce the experiment (e.g., field strength, pulse sequence, solvent, concentration, temperature, etc.). Often, additional information is important or helpful, but not strictly necessary to reproduce an experiment. Such data can be referred to as ESI† and encompasses, e.g., patient information from the clinical trial that produced the NMR samples that enables statistical analysis.The establishment of a (raw NMR data) repository encompasses two principal steps: (i) definition of the information that is intended to be stored, including which experimental and ESI† is required and/or optional; (ii) conception, structure, and IT aspects of the repository itself. Both choices are critical as they have implications for the maintenance and evolution of a repository, especially when it is intended for long-term service. Migration of information from one database (container) to another is typically possible, with effort depending on the database technology. Despite this basic flexibility, it is not possible to recover information that has not been stored to start with. While this may sound trivial, it highlights in fact key points of the present article: (a) diligence and inclusion are paramount; (b) data which has not been stored in the past –as is the case with the majority of the experimental NMR spectra acquired since the inception of FT-NMR – is irrecoverable; and, therefore, (c) building of such a repository is a timely and urgent task.
Another conclusion from the general portability of a database is that, as long as the stored information is in definite format and structure, annotated, and accessible, the container itself is irrelevant to the data. However, the container is most relevant for the users as it is what scientists are interacting with. The availability of modular and publicly accessible APIs is mandatory to make the data meaningful. Under these conditions, the development of the repository, its data structure, and its storage technology can be handled separately, as long as the scope of each aspect has been defined. This will ensure that the scientific community, including NP researchers, can build the tools that can be integrated into the repository and are best suited for the particular needs of an application.
Some of the essential properties of a global repository are that it (i) provides the user with the ability to upload information and obtain a unique and permanent identifier (such as a DOI [Digital Object Identifier]) that points to it; (ii) ensures efficient access to the data, e.g., via batch downloading and programmable interfaces (APIs); (ii) guarantees the long-term availability of the data. The last point has been addressed very recently by the Organisation for Economic Co-operation and Development (OECD), which has developed recommendations for sustainable business models that balance policy regulation and incentives and can assist researchers, policy makers, and funders involved in repositories.384 A global repository should be able to deliver a permanent identifier, such as a DOI, for each deposited object including the NMR experiment, the relevant molecule(s), assignments, linked publications, etc. Any objects based on information that are already stored in the repository can be used to generate a permanent hyperlink-like structure that connects, e.g., assignment to the associated spectra and publications to assignments.
In addition to these fundamental functions, and depending on the particular research area, databases may offer more “intelligent” functionality to the repository, such as advanced browsing or interfaces for novice users. The NP community has a high demand for tools for dereplication and identification, including separation, isolation, structure elucidation, and metabolic profiling. As such tools evolve according to community needs and will most likely remain under permanent development, it is necessary to separate their design and maintenance from the construction of the repository. At the same time, the repository should foster an enabling environment for projects that advance NP research.
As the use of software tools in NMR analysis is becoming increasingly critical, all data resulting from software output should also include a permanent hyperlink that points to the version and ideally to the underlying code that produced the output. For instance, almost all FIDs recorded today are subject to digital filtering, and an error in such a central component of the software/hardware workflow could have confounding consequences, especially if the underlying algorithm is undocumented. This again emphasizes the importance of storing NMR data in an as unmodified form as possible (“raw”), similar to what is customary in digital photography. Depositing original, raw NMR data and obtaining a unique identifier for them is also the most straightforward approach.
Major efforts towards the development of repositories for raw NMR data have already been expanded. The following list compiles several of them, in no particular order: NMrb was launched in 2004 as a repository of raw NMR spectra for biosciences,385 and apparently has disappeared; SPECTRa (https://spectradspace.lib.imperial.ac.uk:8443/handle/10042/25) was a project for the sharing of raw NMR data, but is inactive since 2008; NMRShiftDB (http://nmrshiftdb.org); SDBSWeb of the National Institute of Advanced Industrial Science and Technology, Japan (http://sdbs.db.aist.go.jp) allows downloading of peak-picked data, assignments, and bitmap images; Chemspider (www.chemspider.com) is a free but not open database of chemical compounds that provides NMR raw files as subsidiary data for a limited number of compounds; The Human Metabolome Database (http://www.hmdb.ca/)386 focuses on human metabolites and contains raw NMR data for selected compounds; Biological Magnetic Resonance Data Bank (http://www.bmrb.wisc.edu/)387 seeks to provide qualitative and quantitative NMR data (processed, assigned; not raw) of biological macromolecules and metabolites; the Open Spectral Data Base (http://osdb.info/)388 is an open source project intended to be extended, enhanced, and used for open science data sharing by its users; C6H6.org is an open source project, built using recent technologies and running inside a web browser, offers means to store, share, analyze, and interact with raw NMR data.
10.4 Action items for implementation
A very recent initiative by the National Center for Complementary and Integrative Health (NCCIH/NIH) has solicited information from the research community regarding the development of an open-access NMR data repository (NOT-AT-17-015 at https://grants.nih.gov/grants/guide/notice-files/NOT-AT-17-015.html). Recognizing the repository gap for NMR data and the particular importance of NMR as an analytical technique for NP research, the initiative has been seeking input on a comprehensive list of topics which exemplify the breadth of parameters: purity standards for single chemical entities, value of spectra from complex mixtures and nuclei other than 1H and 13C; nomenclature (spectra, structures) and other key data standards; minimum metadata requirements; harmonization of publication standards; association with other analytical such as LC, UV, and MS; analytical tools required to achieve the most value; minimum size and diversity for maximum usefulness; key features and functionality from different perspectives including users, data contributors, and/or the research community.Considering the overwhelming evidence of the cases presented in this review for urgent need for raw NMR data, an improvement of the situation can be achieved by taking action at several different levels, as follows:
Independently from, or in parallel to, classical publications outlets, authors can take immediate action by depositing raw NMR data into publicly accessible repositories. Institutional (e.g., university and research institution based) and global (e.g., Harvard Dataverse; dataverse.harvard.edu) solutions exist already for this purpose and offer sufficient flexibility to share raw NMR data today, while allowing for their inclusion into a global repository envisioned for the future.
Such a unified repository should be all-inclusive with regard to the type of collected NMR data and avoid any bias towards certain approaches regarding the utility and/or future applications of the data. Importantly, the repository should support equally all methods for NMR-based structural dereplication such as 1D 1H, 1D 13C, 2D HSQC, and any hybrid approaches.
Notably, the foremost feature of the envisioned global repository is long term sustainability, as it represents the quintessential challenge research operations in general and databases in particular for environments that depend on extramural funding and lack independent revenue streams. The achievement of sustainability will greatly benefit from trans-institutional, trans-agency, trans-societal, international consortia and processes that actively involve (NMR) data-producing scientists.
Predictably, the designation of actual sharing mechanisms and data formats are more likely to produce controversial discussion than the identification of wish-list features. Whether the establishment of the sharing mechanisms is driven by a (predictably) lengthier consensus process or a balanced group of representative experts, the utilization of existing resources is a lesser consideration than the modularity of the chosen approach and, foremost, that lack of any further delay.
The pre-determined data formats of current NMR instruments have evolved and are widely supported by third party software tools. While they likely will be replaced, or at least be used in parallel with, standardized and open formats, they still represent a good start for data sharing, and there is no reason to wait for the development of standards as data can be shared right now.
10.5 Raw NMR and other data enhance the future of natural product research
It is highly likely that the availability of a global repository of raw NMR data will potentiate productivity. Representing a tangential aspect of the call for raw NMR data sharing, recognition of the immense value of the information contained in raw (NMR) data triggers questions regarding intellectual property and data ownership. Notwithstanding the potential impact of the answers, which likely will vary by project, institution, and other factors, the body of evidence compiled in this review demonstrates that, at least from a scientific point-of-view, open sharing of raw data can generate an extraordinary amount of added scientific value. This benefit can apply to both, the sharing and the receiving scientists.
In the context of potential mutual benefit, the present findings provide support for the principles of Open Science, which seeks to enhance the accessibility of scientific research, data, and dissemination to the various levels of a society, including amateurs and professionals. While consideration of the benefit of access to shared resources vs. the desire of individual entities to profit is an open-ended discussion, the widely acknowledged complexity of research questions and endeavors, as well as global experience with multi-disciplinary research teams and approaches, indicate that availability and access to larger and more varied data sets bear major potential in advancing research outcomes.
11 Conflicts of interest
The authors declare the following competing financial interest(s): M. N. is founder of NMR Solutions Limited. Craig M. Williams is a consultant to EcoBiotics Ltd. The other authors declare no competing financial interest.12 Note added after first publication
This article replaces the version published on 13th July 2018, which contained errors in the reference details of ref. 83–85.13 Acknowledgements
The lead authors (JBM and GFP) and the CENAPT co-authors wish to acknowledge support through grant U41 AT008706 from NCCIH and ODS/NIH. We are also grateful to Ms. Sara Weber and Isoo Youn for valuable help with electronic literature management, as well as to Giulia K. Pauli for assistance with artwork. JBMcM and RGL and co-workers are grateful for support through the HiFAN grant U41 AT008718.14 References
- J. Bisson, C. Simmler, S.-N. Chen, J. B. Friesen, D. C. Lankin, J. B. McAlpine and G. F. Pauli, Nat. Prod. Rep., 2016, 33, 1028–1033 RSC.
- M. Elyashberg, A. J. Williams and K. Blinov, Contemporary Computer-Assisted Approaches to Molecular Structure Elucidation, The Royal Society of Chemistry, Cambridge, 2012 Search PubMed.
- A. V. Buevich and M. E. Elyashberg, J. Nat. Prod., 2016, 79, 3105–3116 CrossRef CAS.
- M. W. Lodewyk, C. Soldi, P. B. Jones, M. M. Olmstead, J. Rita, J. T. Shaw and D. J. Tantillo, J. Am. Chem. Soc., 2012, 134, 18550–18553 CrossRef CAS.
- S. Kuhn and N. E. Schlörer, Magn. Reson. Chem., 2015, 53, 582–589 CrossRef CAS.
- Anonymous, http://nmrshiftdb.org, accessed 6/21/2017, 2017.
- N. Haider and W. Robien, Nachr. Chem., 2016, 64, 196–198 CrossRef CAS.
- Anonymous, https://www.wsslabs.com, accessed 7/4/2017.
- Y. Binev, M. M. B. Marques and J. Aires-de-Sousa, J. Chem. Inf. Model., 2007, 47, 2089–2097 CrossRef CAS.
- Anonymous, http://www2.chemie.uni-erlangen.de/services/spinus/, accessed 6/1/2017.
- A. M. Castillo, A. Bernal, L. Patiny and J. Wist, Magn. Reson. Chem., 2015, 53, 603–611 CrossRef CAS.
- A. Bernal, A. M. Castillo, F. González, L. Patiny and J. Wist, J. Chem. Phys., 2015, 142, 074103 CrossRef.
- A. M. Castillo, A. Bernal, R. Dieden, L. Patiny and J. Wist, J. Cheminf., 2016, 8, 26 Search PubMed.
- Anonymous, http://www.acdlabs.com/products/com_iden/elucidation/struc_eluc/, accessed 8/1/2017.
- B. Plainchont and J.-M. Nuzillard, Magn. Reson. Chem., 2013, 51, 54–59 CrossRef CAS PubMed.
- G. E. Martin, B. D. Hilton and K. A. Blinov, Magn. Reson. Chem., 2011, 49, 248–252 CrossRef CAS.
- G. E. Martin, B. D. Hilton and K. A. Blinov, J. Nat. Prod., 2011, 74, 2400–2407 CrossRef CAS.
- E. Martineau, P. Giraudeau, I. Tea and S. Akoka, J. Pharm. Biomed. Anal., 2011, 54, 252–257 CrossRef CAS.
- K. C. Nicolaou and S. A. Snyder, Angew. Chem., Int. Ed., 2005, 44, 1012–1044 CrossRef CAS.
- W. Robien, in Progress in the Chemistry of Organic Natural Products 105, ed. A. D. Kinghorn, H. Falk, S. Gibbons and J. Kobayashi, Springer International Publishing, Cham, 2017, pp. 137–215, DOI:10.1007/978-3-319-49712-9_3.
- A. Latif, Y. Du, S. R. Dalal, M. L. Fernández-Murga, E. F. Merino, M. B. Cassera, M. Goetz and D. G. I. Kingston, Chem. Biodiversity, 2017, 14, e1700209 CrossRef.
- A. G. Kutateladze and D. M. Kuznetsov, J. Org. Chem., 2017, 82, 10795–10802 CrossRef CAS.
- A. G. Kutateladze and D. S. Reddy, J. Org. Chem., 2017, 82, 3368–3381 CrossRef CAS.
- D. S. Reddy and A. G. Kutateladze, Org. Lett., 2016, 18, 4860–4863 CrossRef CAS.
- H. E. Zimmerman and L. M. Tolbert, J. Am. Chem. Soc., 1975, 97, 5497–5507 CrossRef CAS.
- H. E. Zimmerman and T. R. Welter, J. Am. Chem. Soc., 1978, 100, 4131–4145 CrossRef CAS.
- D. J. Faulkner, Tetrahedron, 1977, 33, 1421–1443 CrossRef CAS.
- T. I. Williams, Robert Robinson, Chemist Extraordinary, Clarendon Press, Oxford, 1990 Search PubMed.
- G. F. Pauli, S.-N. Chen, C. Simmler, D. C. Lankin, T. Gödecke, B. U. Jaki, J. B. Friesen, J. B. McAlpine and J. G. Napolitano, J. Med. Chem., 2014, 57, 9220–9231 CrossRef CAS.
- B. K. Chhetri, S. Lavoie, A. M. Sweeney-Jones and J. Kubanek, Nat. Prod. Rep., 2018, 35 Search PubMed , in press.
- X.-Y. Wang, Y.-Q. Xu, S.-J. Lin, Z.-Z. Liu and J.-J. Zhong, Appl. Environ. Microbiol., 2011, 77, 6253–6257 CrossRef CAS.
- Y. Xu and J.-J. Zhong, Bioresour. Technol., 2011, 102, 9167–9174 CrossRef CAS.
- CN101880268 B, 2010.
- M. Chu, R. Mierzwa, L. Xu, L. He, J. Terracciano, M. Patel, W. Zhao, T. A. Black and T. M. Chan, J. Antibiot., 2002, 55, 215–218 CrossRef CAS.
- M. P. Singh, F. Kong, J. E. Janso, D. A. Arias, P. A. Suarez, V. S. Bernan, P. J. Petersen, W. J. Weiss, G. Carter and M. Greenstein, J. Antibiot., 2003, 56, 1033–1044 CrossRef CAS.
- F. Grundmann, V. Dill, A. Dowling, A. Thanwisai, E. Bode, N. Chantratita, R. Ffrench-Constant and H. B. Bode, Beilstein J. Org. Chem., 2012, 8, 749–752 CrossRef CAS.
- J. S. Bauer, M. G. K. Ghequire, M. Nett, M. Josten, H.-G. Sahl, R. De Mot and H. Gross, ChemBioChem, 2015, 16, 2491–2497 CrossRef CAS.
- R. J. R. Jaeger and P. Spiteller, J. Nat. Prod., 2010, 73, 1350–1354 CrossRef CAS.
- J. Zhang, Y. Jiang, Y. Cao, J. Liu, D. Zheng, X. Chen, L. Han, C. Jiang and X. Huang, J. Nat. Prod., 2013, 76, 2126–2130 CrossRef CAS.
- Y. Sugiyama, A. Oya, T. Kudo and A. Hirota, J. Antibiot., 2010, 63, 365–369 CrossRef CAS.
- H. J. Shin, H. S. Lee, J. S. Lee, J. Shin, M. A. Lee, H. S. Lee, Y. J. Lee, J. Yun and J. S. Kang, Mar. Drugs, 2014, 12, 3283–3291 CrossRef CAS.
- L. Chen, S.-B. Niu, L. Li, G. Ding, M. Yu, G.-s. Zhang, M.-h. Wang, L.-y. Li, T. Zhang, H.-M. Jia, H.-w. Zhang, H. Shang, X.-z. Liu and Z.-m. Zou, J. Nat. Prod., 2017, 80, 1944–1947 CrossRef CAS.
- J. Seixas de Melo, G. Quinteiro, J. Pina, S. Breda and R. Fausto, J. Mol. Struct., 2001, 565–566, 59–67 CrossRef CAS.
- A. Barroso-Bogeat, M. Alexandre-Franco, C. Fernández-González and V. Gómez-Serrano, Energy Fuels, 2014, 28, 4096–4103 CrossRef CAS.
- I. Djinni, A. Defant, M. Kecha and I. Mancini, Mar. Drugs, 2013, 11, 124–135 CrossRef CAS.
- E. Grienenberger, S. S. Kim, B. Lallemand, P. Geoffroy, D. Heintz, A. Souza Cde, T. Heitz, C. J. Douglas and M. Legrand, Plant Cell, 2010, 22, 4067–4083 CrossRef CAS.
- W. F. Reynolds and D. C. Burns, Annu. Rep. NMR Spectrosc., 2012, 76, 1–21 CrossRef CAS.
- K. A. Blinov, A. V. Buevich, R. T. Williamson and G. E. Martin, Org. Biomol. Chem., 2014, 12, 9481–9722 RSC.
- R. T. Williamson, A. V. Buevich, G. E. Martin and T. Parella, J. Org. Chem., 2014, 79, 3887–3894 CrossRef CAS.
- J. Saurí, N. Marcó, T. Williamson, G. E. Martin and T. Parella, J. Magn. Reson., 2015, 258, 25–32 CrossRef.
- R. C. Breton and W. F. Reynolds, Nat. Prod. Rep., 2013, 30, 501–524 RSC.
- M. Reibarkh, T. P. Wyche, J. Saurí, T. S. Bugni, G. E. Martin and R. T. Williamson, Magn. Reson. Chem., 2015, 53, 996–1002 CrossRef CAS.
- M. Morikawa, H. Daido, T. Takao, S. Murata, Y. Shimonishi and T. Imanaka, J. Bacteriol., 1993, 175, 6459–6466 CrossRef CAS.
- K. Washio, S. P. Lim, N. Roongsawang and M. Morikawa, Biosci., Biotechnol., Biochem., 2010, 74, 992–999 CrossRef CAS.
- A. Lange, H. Sun, J. Pilger, U. M. Reinscheid and H. Gross, ChemBioChem, 2012, 13, 2671–2675 CrossRef CAS PubMed.
- A. San Feliciano, M. Medarde, J. M. Miguel del Corral, A. Aramburu, M. Gordaliza and A. F. Barrero, Tetrahedron Lett., 1989, 30, 2851–2854 CrossRef CAS.
- J. M. Saya, K. Vos, R. A. Kleinnijenhuis, J. H. van Maarseveen, S. Ingemann and H. Hiemstra, Org. Lett., 2015, 17, 3892–3894 CrossRef CAS.
- B. Wang, Y. Xie, Q. Yang, G. Zhang and Z. Gu, Org. Lett., 2016, 18, 5388–5391 CrossRef CAS.
- G. F. Pauli, M. Niemitz, J. Bisson, M. W. Lodewyk, C. Soldi, J. T. Shaw, D. J. Tantillo, J. M. Saya, K. Vos, R. A. Kleinnijenhuis, H. Hiemstra, S.-N. Chen, J. B. McAlpine, D. C. Lankin and J. B. Friesen, J. Org. Chem., 2016, 81, 878–889 CrossRef CAS.
- J. G. Napolitano, D. C. Lankin, T. N. Graf, J. B. Friesen, S.-N. Chen, J. B. McAlpine, N. H. Oberlies and G. F. Pauli, J. Org. Chem., 2013, 78, 2827–2839 CrossRef CAS.
- W. Gao, J. G. Napolitano, D. C. Lankin, J.-Y. Kim, Y.-Y. Jin, H. Lee, J.-W. Suh, S.-N. Chen and G. F. Pauli, Magn. Reson. Chem., 2017, 55, 239–244 CrossRef CAS PubMed.
- G. F. Pauli, S.-N. Chen, D. C. Lankin, J. Bisson, R. Case, L. R. d. Chadwick, T. Gödecke, T. Inui, A. Krunic, B. U. Jaki, J. B. McAlpine, S. Mo, J. G. Napolitano, J. Orjala, J. Lehtivarjo, S.-P. Korhonen and M. Niemitz, J. Nat. Prod., 2014, 77, 1473–1487 CrossRef CAS PubMed.
- C. C. Gillispie, R. Fox and I. Grattan-Guinness, Pierre-Simon Laplace, 1749-1827: a life in exact science, Princeton University Press, Princeton, N.J., 1997 Search PubMed.
- R. A. Medina, D. E. Goeger, P. Hills, S. L. Mooberry, N. Huang, L. I. Romero, E. Ortega-Barría, W. H. Gerwick and K. L. McPhail, J. Am. Chem. Soc., 2008, 130, 6324–6325 CrossRef CAS.
- I. Coin, R. Dölling, E. Krause, M. Bienert, M. Beyermann, C. D. Sferdean and L. A. Carpino, J. Org. Chem., 2006, 71, 6171–6177 CrossRef CAS.
- G. Yao, Z. Pan, C. Wu, W. Wang, L. Fang and W. Su, J. Am. Chem. Soc., 2015, 137, 13488–13491 CrossRef CAS.
- K. M. Snyder, J. Sikorska, T. Ye, L. Fang, W. Su, R. G. Carter, K. L. McPhail and P. H. Y. Cheong, Org. Biomol. Chem., 2016, 14, 5826–5831 RSC.
- W. He, H.-B. Qiu, Y.-J. Chen, J. Xi and Z.-J. Yao, Tetrahedron Lett., 2014, 55, 6109–6112 CrossRef CAS.
- R. Nabika, T. L. Suyama, A. M. Hau, R. Misu, H. Ohno, J. E. Ishmael, K. L. McPhail, S. Oishi and N. Fujii, Bioorg. Med. Chem. Lett., 2015, 25, 302–306 CrossRef CAS.
- J. D. Serrill, X. Wan, A. M. Hau, H. S. Jang, D. J. Coleman, A. K. Indra, A. W. G. Alani, K. L. McPhail and J. E. Ishmael, Invest. New Drugs, 2016, 34, 24–40 CrossRef CAS.
- L. R. de Carvalho, M. T. Fujii, N. F. Roque and J. H. G. Lago, Phytochemistry, 2006, 67, 1331–1335 CrossRef CAS.
- M. T. Crimmins and C. O. Hughes, Org. Lett., 2012, 14, 2168–2171 CrossRef CAS.
- O. A. Mukhina, H. Koshino, M. T. Crimmins and A. G. Kutateladze, Tetrahedron Lett., 2015, 56, 4900–4903 CrossRef CAS.
- J. Yang, J. Tummatorn, R. Slegeris, S. F. Tlais and G. B. Dudley, Org. Lett., 2011, 13, 2065–2067 CrossRef CAS.
- J. C. Sheehan, The enchanted ring: the untold story of penicillin, MIT Press, Cambridge, Mass., 1982 Search PubMed.
- P. D. Brown and A. L. Lawrence, Nat. Prod. Rep., 2017, 34, 1193–1202 RSC.
- Z. F. Geng, K. Yang, Y. P. Li, S. S. Guo, C. X. You, W. J. Zhang, Z. Zhang and S. S. Du, Nat. Prod. Res., 2017, 31, 791–796 CrossRef CAS.
- J.-J. Chen, W.-J. Lin, C.-H. Liao and P.-C. Shieh, J. Nat. Prod., 2007, 70, 989–992 CrossRef CAS.
- N. Braga, F. Rodrigues and M. B. P. P. Oliveira, Nat. Prod. Res., 2015, 29, 1–18 CrossRef CAS.
- A. Z. Abdel Azeiz, D. K. Hanafi and S. E. Hasanein, Nat. Prod. Res., 2016, 30, 1816–1823 CrossRef CAS.
- R. Sun, X. Zheng, X. Wang, L.-Z. Dang, Z.-S. Yang, S.-L. Luo, K.-Q. Zhang and G.-H. Li, Phytochem. Lett., 2011, 4, 320–322 CrossRef CAS.
- A. I. Savchenko and C. M. Williams, Eur. J. Org. Chem., 2013, 2013, 7263–7265 CrossRef CAS.
- J. Bredt, J. Houben and P. Levy, Ber. Dtsch. Chem. Ges., 1902, 35, 1286–1292 CrossRef CAS.
- J. Bredt, J. Houben, P. Levy and S. Link, Justus Liebigs Ann. Chem., 1913, 395, 26–63 CrossRef CAS.
- J. Bredt, H. Thouet and J. Schmitz, Justus Liebigs Ann. Chem., 1924, 437, 1–13 CrossRef CAS.
- F. S. Fawcett, Chem. Rev., 1950, 47, 219–274 CrossRef CAS.
- G. Köbrich, Angew. Chem., Int. Ed., 1973, 12, 464–473 CrossRef.
- G. L. Buchanan, Chem. Soc. Rev., 1974, 3, 41–63 RSC.
- R. Keese, Angew. Chem., Int. Ed., 1975, 14, 528–538 CrossRef.
- P. M. Warner, Chem. Rev., 1989, 89, 1067–1093 CrossRef CAS.
- R. A. Abramovitch, Reactive Intermediates, Springer US, Boston, MA, 1983 Search PubMed.
- J. Y. W. Mak, R. H. Pouwer and C. M. Williams, Angew. Chem., Int. Ed., 2014, 53, 13664–13688 CrossRef CAS PubMed.
- Y. Cong, J.-G. Guo and J. Liu, Helv. Chim. Acta, 2013, 96, 345–349 CrossRef CAS.
- L. A. Maslovskaya, A. I. Savchenko, E. H. Krenske, C. J. Pierce, V. A. Gordon, P. W. Reddell, P. G. Parsons and C. M. Williams, Angew. Chem., Int. Ed., 2014, 53, 7006–7009 CrossRef CAS.
- E. H. Krenske and C. M. Williams, Angew. Chem., Int. Ed., 2015, 54, 10608–10612 CrossRef CAS.
- A. G. Kutateladze and O. A. Mukhina, J. Org. Chem., 2014, 79, 8397–8406 CrossRef CAS.
- A. G. Kutateladze and O. A. Mukhina, J. Org. Chem., 2015, 80, 5218–5225 CrossRef CAS.
- A. G. Kutateladze and O. A. Mukhina, J. Org. Chem., 2015, 80, 10838–10848 CrossRef CAS.
- Y.-M. Hsu, F.-R. Chang, I. W. Lo, K.-H. Lai, M. El-Shazly, T.-Y. Wu, Y.-C. Du, T.-L. Hwang, Y.-B. Cheng and Y.-C. Wu, J. Nat. Prod., 2016, 79, 2674–2680 CrossRef CAS.
- D. Yang, J. Wang, W. Li, W. Dong, W. Mei and H. Dai, Phytochem. Lett., 2016, 17, 94–99 CrossRef CAS.
- J.-K. Woo, C.-K. Kim, C.-H. Ahn, D.-C. Oh, K.-B. Oh and J. Shin, J. Nat. Prod., 2015, 78, 218–224 CrossRef CAS PubMed.
- M. E. Flores-Giubi, M. J. Durán-Peña, J. M. Botubol-Ares, F. Escobar-Montaño, D. Zorrilla, A. J. Macías-Sánchez and R. Hernández-Galán, J. Nat. Prod., 2017, 80, 2161–2165 CrossRef CAS PubMed.
- S. K. Graham, L. K. Lambert, G. K. Pierens, J. N. A. Hooper and M. J. Garson, Aust. J. Chem., 2010, 63, 867–872 CrossRef CAS.
- I. C. Piña, J. T. Gautschi, G.-Y.-S. Wang, M. L. Sanders, F. J. Schmitz, D. France, S. Cornell-Kennon, L. C. Sambucetti, S. W. Remiszewski, L. B. Perez, K. W. Bair and P. Crews, J. Org. Chem., 2003, 68, 3866–3873 CrossRef PubMed.
- P. B. Shinde, Y. M. Lee, H. T. Dang, J. Hong, C.-O. Lee and J. H. Jung, Bioorg. Med. Chem. Lett., 2008, 18, 6414–6418 CrossRef CAS.
- M. W. B. McCulloch, G. S. Coombs, N. Banerjee, T. S. Bugni, K. M. Cannon, M. K. Harper, C. A. Veltri, D. M. Virshup and C. M. Ireland, Bioorg. Med. Chem., 2009, 17, 2189–2198 CrossRef CAS.
- M. J. Garson, W. Hehre, G. K. Pierens and Suciati, Molecules, 2017, 22, 521 CrossRef.
- Suciati, J. A. Fraser, L. K. Lambert, G. K. Pierens, P. V. Bernhardt and M. J. Garson, J. Nat. Prod., 2013, 76, 1432–1440 CrossRef CAS.
- A. M. Sarotti and S. C. Pellegrinet, J. Org. Chem., 2009, 74, 7254–7260 CrossRef CAS.
- D. Alfonso, T. Colman-Saizarbitoria, G.-X. Zhao, G. Shi, Q. Ye, J. T. Schwedler and J. L. McLaughlin, Tetrahedron, 1996, 52, 4215–4224 CrossRef CAS.
- C.-Y. Choo, N. Abdullah and M. Diederich, Phytochem. Rev., 2014, 13, 835–851 CrossRef CAS.
- N. Li, Z. Shi, Y. Tang, J. Chen and X. Li, Beilstein J. Org. Chem., 2008, 4, 48 Search PubMed.
- S. Takahashi, D. Satoh, M. Hayashi, K. Takahashi, K. Yamaguchi, T. Nakamura and H. Koshino, J. Org. Chem., 2016, 81, 11222–11234 CrossRef CAS PubMed.
- S. Takahashi, N. Ogawa, H. Koshino and T. Nakata, Org. Lett., 2005, 7, 2783–2786 CrossRef CAS PubMed.
- S. Takahashi, R. Takahashi, Y. Hongo, H. Koshino, K. Yamaguchi and T. Miyagi, J. Org. Chem., 2009, 74, 6382–6385 CrossRef CAS PubMed.
- S. Koichi, M. Arisaka, H. Koshino, A. Aoki, S. Iwata, T. Uno and H. Satoh, J. Chem. Inf. Model., 2014, 54, 1027–1035 CrossRef CAS.
- L.-Q. Wang, N. Nakamura, M. R. Meselhy, M. Hattori, W.-M. Zhao, K.-F. Cheng, R.-Z. Yang and G.-W. Qin, Chem. Pharm. Bull., 2000, 48, 1109–1113 CrossRef CAS.
- G. Brader, S. Vajrodaya, H. Greger, M. Bacher, H. Kalchhauser and O. Hofer, J. Nat. Prod., 1998, 61, 1482–1490 CrossRef CAS.
- M. Bacher, O. Hofer, G. Brader, S. Vajrodaya and H. Greger, Phytochemistry, 1999, 52, 253–263 CrossRef CAS.
- L. Chen and W. Robien, Chemom. Intell. Lab. Syst., 1993, 19, 217–223 CrossRef CAS.
- W. Robien, in Progress in the Chemistry of Organic Natural Products, Springer, Cham, 2017, vol. 105, pp. 137–215 Search PubMed.
- C. Seger, O. Hofer and H. Greger, Monatshefte für Chemie/Chemical Monthly, 2000, 131, 1161–1165 CrossRef CAS.
- S. Kim, Y.-W. Chin, B.-N. Su, S. Riswan, L. B. S. Kardono, J. J. Afriastini, H. Chai, N. R. Farnsworth, G. A. Cordell, S. M. Swanson and A. D. Kinghorn, J. Nat. Prod., 2006, 69, 1769–1775 CrossRef CAS.
- A. A. Salim, A. D. Pawlus, H.-B. Chai, N. R. Farnsworth, A. Douglas Kinghorn and E. J. Carcache-Blanco, Bioorg. Med. Chem. Lett., 2007, 17, 109–112 CrossRef CAS.
- J. Phongmaykin, T. Kumamoto, T. Ishikawa, E. Saifah and R. Suttisri, Nat. Prod. Res., 2011, 25, 1621–1628 CrossRef CAS.
- C. Seger, T. Pacher, H. Greger, E. Saifah and O. Hofer, Monatshefte für Chemie/Chemical Monthly, 2002, 133, 97–100 CrossRef CAS.
- S. Hinterberger, O. Hofer and H. Greger, Tetrahedron, 1994, 50, 6279–6286 CrossRef CAS.
- H. Oberacher and K. Arnhard, TrAC, Trends Anal. Chem., 2016, 84, 94–105 CrossRef CAS.
- A. V. Ewing and S. G. Kazarian, Analyst, 2017, 142, 257–272 RSC.
- Ø. M. Andersen and K. R. Markham, Flavonoids: chemistry, biochemistry, and applications, CRC, Taylor & Francis, Boca Raton, FL, 2006 Search PubMed.
- W. Gaffield, R. E. Lundin, B. Gentili and R. M. Horowitz, Bioorg. Chem., 1975, 4, 259–269 CrossRef CAS.
- C. O. Miles and L. Main, J. Chem. Soc., Perkin Trans. 2, 1989, 1623–1632, 10.1039/P29890001623.
- F. Maltese, C. Erkelens, F. v. d. Kooy, Y. H. Choi and R. Verpoorte, Food Chem., 2009, 116, 575–579 CrossRef CAS.
- S. Rayyan, T. Fossen and Ø. M. Andersen, J. Agric. Food Chem., 2010, 58, 7211–7217 CrossRef CAS PubMed.
- R. G. Linington, B. R. Clark, E. E. Trimble, A. Almanza, L.-D. Ureña, D. E. Kyle and W. H. Gerwick, J. Nat. Prod., 2009, 72, 14–17 CrossRef CAS.
- K. Taori, Y. Liu, V. J. Paul and H. Luesch, ChemBioChem, 2009, 10, 1634–1639 CrossRef CAS.
- B. Miller, A. J. Friedman, H. Choi, J. Hogan, J. A. McCammon, V. Hook and W. H. Gerwick, J. Nat. Prod., 2014, 77, 92–99 CrossRef CAS.
- T. Conroy, J. T. Guo, R. G. Linington, N. H. Hunt and R. J. Payne, Chem.–Eur. J., 2011, 17, 13544–13552 CrossRef CAS PubMed.
- T. Conroy, J. T. Guo, N. H. Hunt and R. J. Payne, Org. Lett., 2010, 12, 5576–5579 CrossRef CAS PubMed.
- S. C. Stolze, E. Deu, F. Kaschani, N. Li, B. I. Florea, K. H. Richau, T. Colby, R. A. L. van der Hoorn, H. S. Overkleeft, M. Bogyo and M. Kaiser, Chem. Biol., 2012, 19, 1546–1555 CrossRef CAS PubMed.
- G. Platzer, M. Okon and L. P. McIntosh, J. Biomol. NMR, 2014, 60, 109–129 CrossRef CAS.
- H. Miyataka, T. Ozaki and S. Himeno, Biol. Pharm. Bull., 2007, 30, 667–670 CrossRef CAS.
- M. G. Reinecke, H. W. Johnson and J. F. Sebastian, J. Am. Chem. Soc., 1969, 91, 3817–3822 CrossRef CAS.
- K. Bingol and R. Brüschweiler, Anal. Chem., 2011, 83, 7412–7417 CrossRef CAS.
- F. Zhang and R. Brüschweiler, ChemPhysChem, 2004, 5, 794–796 CrossRef CAS PubMed.
- B. Bernet and A. Vasella, Helv. Chim. Acta, 2000, 83, 995–1021 CrossRef CAS.
- J. Kroon, L. M. J. Kroon-Batenburg, B. R. Leeflang and J. F. G. Vliegenthart, J. Mol. Struct., 1994, 322, 27–31 CrossRef CAS.
- E. Arunan, G. R. Desiraju, R. A. Klein, J. Sadlej, S. Scheiner, I. Alkorta, D. C. Clary, R. H. Crabtree, J. J. Dannenberg, P. Hobza, H. G. Kjaergaard, A. C. Legon, B. Mennucci and D. J. Nesbitt, Pure Appl. Chem., 2011, 83, 1637–1641 CAS.
- E. Arunan, G. R. Desiraju, R. A. Klein, J. Sadlej, S. Scheiner, I. Alkorta, D. C. Clary, R. H. Crabtree, J. J. Dannenberg, P. Hobza, H. G. Kjaergaard, A. C. Legon, B. Mennucci and D. J. Nesbitt, Pure Appl. Chem., 2011, 83, 1619–1636 CAS.
- S. J. Grabowski, Hydrogen Bonding—New Insights, Springer, Netherlands, 2006 Search PubMed.
- K. Imai and E. Ōsawa, Magn. Reson. Chem., 1990, 28, 668–674 CrossRef CAS.
- C. A. G. Haasnoot, F. A. A. M. de Leeuw and C. Altona, Tetrahedron, 1980, 36, 2783–2792 CrossRef CAS.
- Y.-Y. Fan, H. Zhang, Y. Zhou, H.-B. Liu, W. Tang, B. Zhou, J.-P. Zuo and J.-M. Yue, J. Am. Chem. Soc., 2015, 137, 138–141 CrossRef CAS.
- L. A. Maslovskaya, A. I. Savchenko, E. H. Krenske, V. A. Gordon, P. W. Reddell, C. J. Pierce, P. G. Parsons and C. M. Williams, Chem. Commun., 2014, 50, 12315–12317 RSC.
- L. A. Maslovskaya, A. I. Savchenko, V. A. Gordon, P. W. Reddell, C. J. Pierce, P. G. Parsons and C. M. Williams, Eur. J. Org. Chem., 2016, 2016, 1673–1677 CrossRef CAS.
- L. A. Maslovskaya, A. I. Savchenko, V. A. Gordon, P. W. Reddell, C. J. Pierce, P. G. Parsons and C. M. Williams, Chem.–Eur. J., 2017, 23, 537–540 CrossRef CAS PubMed.
- R. S. Thombal and V. H. Jadhav, Org. Biomol. Chem., 2015, 13, 9485–9491 RSC.
- T. J. V. Berg, D. M. Pinkerton and C. M. Williams, Org. Biomol. Chem., 2017, 15, 7102–7105 RSC.
- B.-G. Wang, J. B. Gloer, N.-Y. Ji and J.-C. Zhao, Chem. Rev., 2013, 113, 3632–3685 CrossRef CAS PubMed.
- J. G. Hall and J. A. Reiss, Aust. J. Chem., 1986, 39, 1401–1409 CrossRef CAS.
- N.-Y. Ji, X.-M. Li, K. Li and B.-G. Wang, J. Nat. Prod., 2007, 70, 1499–1502 CrossRef CAS.
- N.-Y. Ji, X.-M. Li, K. Li and B.-G. Wang, J. Nat. Prod., 2010, 73, 1192 CrossRef CAS.
- H. M. Sheldrake, C. Jamieson and J. W. Burton, Angew. Chem., Int. Ed., 2006, 45, 7199–7202 CrossRef CAS.
- S. G. Smith, R. S. Paton, J. W. Burton and J. M. Goodman, J. Org. Chem., 2008, 73, 4053–4062 CrossRef CAS PubMed.
- D. A. Dias and S. Urban, Phytochemistry, 2011, 72, 2081–2089 CrossRef CAS PubMed.
- B. S. Dyson, J. W. Burton, T.-i. Sohn, B. Kim, H. Bae and D. Kim, J. Am. Chem. Soc., 2012, 134, 11781–11790 CrossRef CAS PubMed.
- I. K. Kim, M. R. Brennan and K. L. Erickson, Tetrahedron Lett., 1989, 30, 1757–1760 CrossRef CAS.
- H. Kikuchi, T. Suzuki, E. Kurosawa and M. Suzuki, Bull. Chem. Soc. Jpn., 1991, 64, 1763–1775 CrossRef CAS.
- K. A. Rees, C. Bermudez, D. J. Edwards, A. G. Elliott, J. E. Ripen, C. Seta, J. X. Huang, M. A. Cooper, J. A. Fraser, T. C. Yeo and M. S. Butler, J. Nat. Prod., 2015, 78, 2141–2144 CrossRef CAS PubMed.
- X. Tang, J. Li, N. Millán-Aguiñaga, J. J. Zhang, E. C. O'Neill, J. A. Ugalde, P. R. Jensen, S. M. Mantovani and B. S. Moore, ACS Chem. Biol., 2015, 10, 2841–2849 CrossRef CAS PubMed.
- X. Tang, J. Li and B. S. Moore, ChemBioChem, 2017, 18, 1072–1076 CrossRef CAS.
- J. Li, X. Tang, T. Awakawa and B. S. Moore, Angew. Chem., Int. Ed., 2017, 56, 12234–12239 CrossRef CAS PubMed.
- T. J. Schmidt, W. Peters, F. R. Fronczek and N. H. Fischer, J. Nat. Prod., 1997, 60, 783–787 CrossRef CAS.
- T. J. Schmidt, H. M. Schmidt, E. Müller, W. Peters, F. R. Fronczek, A. Truesdale and N. H. Fischer, J. Nat. Prod., 1998, 61, 230–236 CrossRef CAS.
- T. J. Schmidt, J. Nat. Prod., 1999, 62, 684–687 CrossRef CAS PubMed.
- T. J. Schmidt, E. Müller and F. R. Fronczek, J. Nat. Prod., 2001, 64, 411–414 CrossRef CAS.
- I. Kouno, N. Kawano and C.-S. Yang, J. Chem. Soc., Perkin Trans. 1, 1988, 0, 1537–1539 RSC.
- I. Kouno, H. Irie and N. Kawano, J. Chem. Soc., Perkin Trans. 1, 1984, 0, 2511–2515 RSC.
- T. Kuriyama, T. J. Schmidt, E. Okuyama and Y. Ozoe, Bioorg. Med. Chem., 2002, 10, 1873–1881 CrossRef CAS.
- G. Pan, Z. Xu, Z. Guo, Hindra, M. Ma, D. Yang, H. Zhou, Y. Gansemans, X. Zhu, Y. Huang, L.-X. Zhao, Y. Jiang, J. Cheng, F. Van Nieuwerburgh, J.-W. Suh, Y. Duan and B. Shen, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 111131–111140 CrossRef.
- M. Hara, I. Takahashi, M. Yoshida, K. Asano, I. Kawamoto, M. Morimoto and H. Nakano, J. Antibiot., 1989, 42, 333–335 CrossRef CAS.
- S.-X. Huang, B.-S. Yun, M. Ma, H. S. Basu, D. R. Church, G. Ingenhorst, Y. Huang, D. Yang, J. R. Lohman, G.-L. Tang, J. Ju, T. Liu, G. Wilding and B. Shen, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 8278–8283 CrossRef CAS PubMed.
- J.-W. Nam, R. S. Phansalkar, D. C. Lankin, J. B. McAlpine, A. A. Leme-Kraus, C. M. P. Vidal, L.-S. Gan, A. Bedran-Russo, S.-N. Chen and G. F. Pauli, J. Org. Chem., 2017, 82, 1316–1329 CrossRef CAS PubMed.
- J.-W. Nam, R. S. Phansalkar, D. C. Lankin, J. Bisson, J. B. McAlpine, A. A. Leme, C. M. P. Vidal, B. Ramirez, M. Niemitz, A. Bedran-Russo, S.-N. Chen and G. F. Pauli, J. Org. Chem., 2015, 80, 7495–7507 CrossRef CAS PubMed.
- J. G. Napolitano, D. C. Lankin, J. B. McAlpine, M. Niemitz, S.-P. Korhonen, S.-N. Chen and G. F. Pauli, J. Org. Chem., 2013, 78, 9963–9968 CrossRef CAS.
- S. P. Gaudêncio and F. Pereira, Nat. Prod. Rep., 2015, 32, 779–810 RSC.
- C. R. Pye, M. J. Bertin, R. S. Lokey, W. H. Gerwick and R. G. Linington, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 5601–5606 CrossRef CAS.
- Dictionary of Natural Products 26.1, http://dnp.chemnetbase.com/faces/chemical/ChemicalSearch.xhtml, accessed 11/04/2017.
- M. Wang, J. J. Carver, V. V. Phelan, L. M. Sanchez, N. Garg, Y. Peng, D. D. Nguyen, J. Watrous, C. A. Kapono, T. Luzzatto-Knaan, C. Porto, A. Bouslimani, A. V. Melnik, M. J. Meehan, W.-T. Liu, M. Crüsemann, P. D. Boudreau, E. Esquenazi, M. Sandoval-Calderón, R. D. Kersten, L. A. Pace, R. A. Quinn, K. R. Duncan, C.-C. Hsu, D. J. Floros, R. G. Gavilan, K. Kleigrewe, T. Northen, R. J. Dutton, D. Parrot, E. E. Carlson, B. Aigle, C. F. Michelsen, L. Jelsbak, C. Sohlenkamp, P. Pevzner, A. Edlund, J. McLean, J. Piel, B. T. Murphy, L. Gerwick, C.-C. Liaw, Y.-L. Yang, H.-U. Humpf, M. Maansson, R. A. Keyzers, A. C. Sims, A. R. Johnson, A. M. Sidebottom, B. E. Sedio, A. Klitgaard, C. B. Larson, P. CAB, D. Torres-Mendoza, D. J. Gonzalez, D. B. Silva, L. M. Marques, D. P. Demarque, E. Pociute, E. C. O'Neill, E. Briand, E. J. N. Helfrich, E. A. Granatosky, E. Glukhov, F. Ryffel, H. Houson, H. Mohimani, J. J. Kharbush, Y. Zeng, J. A. Vorholt, K. L. Kurita, P. Charusanti, K. L. McPhail, K. F. Nielsen, L. Vuong, M. Elfeki, M. F. Traxler, N. Engene, N. Koyama, O. B. Vining, R. Baric, R. R. Silva, S. J. Mascuch, S. Tomasi, S. Jenkins, V. Macherla, T. Hoffman, V. Agarwal, P. G. Williams, J. Dai, R. Neupane, J. Gurr, A. M. C. Rodríguez, A. Lamsa, C. Zhang, K. Dorrestein, B. M. Duggan, J. Almaliti, P.-M. Allard, P. Phapale, L.-F. Nothias, T. Alexandrov, M. Litaudon, J.-L. Wolfender, J. E. Kyle, T. O. Metz, T. Peryea, D.-T. Nguyen, D. VanLeer, P. Shinn, A. Jadhav, R. Müller, K. M. Waters, W. Shi, X. Liu, L. Zhang, R. Knight, P. R. Jensen, B. Ø. Palsson, K. Pogliano, R. G. Linington, M. Gutiérrez, N. P. Lopes, W. H. Gerwick, B. S. Moore, P. C. Dorrestein and N. Bandeira, Nat. Biotechnol., 2016, 34, nbt3597 CrossRef PubMed.
- K. F. Nielsen, M. Månsson, C. Rank, J. C. Frisvad and T. O. Larsen, J. Nat. Prod., 2011, 74, 2338–2348 CrossRef CAS PubMed.
- G. A. Nagana Gowda and D. Raftery, Anal. Chem., 2017, 89, 490–510 CrossRef CAS PubMed.
- D. Rovnyak, M. Sarcone and Z. Jiang, Magn. Reson. Chem., 2011, 49, 483–491 CrossRef CAS.
- Y. Shrot and L. Frydman, J. Magn. Reson., 2011, 209, 352–358 CrossRef CAS PubMed.
- D. Schulze-Sünninghausen, J. Becker, M. R. M. Koos and B. Luy, J. Magn. Reson., 2017, 281, 151–161 CrossRef PubMed.
- S. Chopra, R. Hadsell and Y. LeCun, 2005 IEEE Computer Society Conference on Computer Vision and Pattern Recognition (CVPR'05), 2005 IEEE Computer Society Conference on Computer Vision and Pattern Recognition (CVPR'05), 2005 Search PubMed.
- C. Zhang, Y. Idelbayev, N. Roberts, Y. Tao, Y. Nannapaneni, B. M. Duggan, J. Min, E. C. Lin, E. C. Gerwick, G. W. Cottrell and W. H. Gerwick, Sci. Rep., 2017, 7, 14243 CrossRef PubMed.
- P. D. Boudreau, T. Byrum, W.-T. Liu, P. C. Dorrestein and W. H. Gerwick, J. Nat. Prod., 2012, 75, 1560–1570 CrossRef CAS.
- E. Mevers, W.-T. Liu, N. Engene, H. Mohimani, T. Byrum, P. A. Pevzner, P. C. Dorrestein, C. Spadafora and W. H. Gerwick, J. Nat. Prod., 2011, 74, 928–936 CrossRef CAS PubMed.
- T. Grkovic, R. H. Pouwer, M.-L. Vial, L. Gambini, A. Noël, J. N. A. Hooper, S. A. Wood, G. D. Mellick and R. J. Quinn, Angew. Chem., Int. Ed., 2014, 53, 6070–6074 CrossRef CAS PubMed.
- T. Grkovic, R. H. Pouwer, Q. Wang, G. P. Guymer, J. Holst and R. J. Quinn, J. Nat. Prod., 2015, 78, 1215–1220 CrossRef CAS PubMed.
- C. A. Romero, T. Grkovic, J. Han, L. Zhang, J. R. J. French, D. I. Kurtboke and R. J. Quinn, RSC Adv., 2015, 5, 104524–104534 RSC.
- R. J. Quinn, A. R. Carroll, N. B. Pham, P. Baron, M. E. Palframan, L. Suraweera, G. K. Pierens and S. Muresan, J. Nat. Prod., 2008, 71, 464–468 CrossRef CAS.
- D. Camp, R. A. Davis, M. Campitelli, J. Ebdon and R. J. Quinn, J. Nat. Prod., 2012, 75, 72–81 CrossRef CAS PubMed.
- Y. Dashti, T. Grkovic, U. R. Abdelmohsen, U. Hentschel and R. J. Quinn, J. Nat. Prod., 2017, 80, 828–836 CrossRef CAS PubMed.
- G. Lewin, N. Kunesch, J. Poisson and T. Sévenet, J. Indian Chem. Soc., 1978, 55, 1096–1098 CAS.
- G. Adams and A. B. Smith, in The Alkaloids, ed. H.-J. Knölker, New York, 2016, vol. 76, pp. 171–257 Search PubMed.
- J. M. Smith, J. Moreno, B. W. Boal and N. K. Garg, Angew. Chem., Int. Ed., 2015, 54, 400–412 CAS.
- P. Tane, M. Tene and O. Sterner, Bull. Chem. Soc. Ethiop., 2002, 16, 165–168 CAS.
- X.-W. Yang, X.-J. Qin, Y.-L. Zhao, P. K. Lunga, X.-N. Li, S.-Z. Jiang, G.-G. Cheng, Y.-P. Liu and X.-D. Luo, Tetrahedron Lett., 2014, 55, 4593–4596 CrossRef CAS.
- X.-J. Qin, Y.-L. Zhao, C.-W. Song, B. Wang, Y.-Y. Chen, L. Liu, Q. Li, D. Li, Y.-P. Liu and X.-D. Luo, Nat. Prod. Bioprospect., 2015, 5, 185–193 CrossRef CAS PubMed.
- X.-W. Yang, X.-D. Luo, P. K. Lunga, Y.-L. Zhao, X.-J. Qin, Y.-Y. Chen, L. Liu, X.-N. Li and Y.-P. Liu, Tetrahedron, 2015, 71, 3694–3698 CrossRef CAS.
- R. Antonio and G.-R. Silvina, Curr. Med. Chem., 2003, 10, 1891–1915 CrossRef.
- Y. Li, S. Zhu, J. Li and A. Li, J. Am. Chem. Soc., 2016, 138, 3982–3985 CrossRef CAS.
- G. Lewin and J. Poisson, Bull. Soc. Chim. Fr., 1980, II-400–II-404 Search PubMed.
- A. E. Fox Ramos, C. Alcover, L. Evanno, A. Maciuk, M. Litaudon, C. Duplais, G. Bernadat, J.-F. Gallard, J.-C. Jullian, E. Mouray, P. Grellier, P. M. Loiseau, S. Pomel, E. Poupon, P. Champy and M. A. Beniddir, J. Nat. Prod., 2017, 80, 1007–1014 CrossRef CAS.
- M. A. Beniddir, G. Genta-Jouve and G. Lewin, J. Nat. Prod., 2018, 81, 1075–1078 CrossRef CAS.
- P. Crews, Y. Kakou and E. Quinoa, J. Am. Chem. Soc., 1988, 110, 4365–4368 CrossRef CAS.
- Discovery & Development Services | DTP, https://dtp.cancer.gov/discovery_development/nci-60/.
- G. N. Gomes, R. Dasari, S. Chandra, R. Kiss and A. Kornienko, Mar. Drugs, 2016, 14 CrossRef.
- R. N. Sonnenschein, T. A. Johnson, K. Tenney, F. A. Valeriote and P. Crews, J. Nat. Prod., 2006, 69, 145–147 CrossRef CAS.
- L. Wang and K. J. Hale, Org. Lett., 2015, 17, 4200–4203 CrossRef CAS PubMed.
- N. Lorig-Roach, P. C. Still, D. Coppage, J. E. Compton, M. S. Crews, G. Navarro, K. Tenney and P. Crews, J. Nat. Prod., 2017, 80, 2304–2310 CrossRef CAS PubMed.
- T. F. Molinski and B. I. Morinaka, Tetrahedron, 2012, 68, 9307–9343 CrossRef CAS PubMed.
- N. Sheppard and J. J. Turner, Proc. R. Soc. London, Ser. A, 1959, 252, 506–519 CAS.
- H. Luesch, R. Pangilinan, W. Y. Yoshida, R. E. Moore and V. J. Paul, J. Nat. Prod., 2001, 64, 304–307 CrossRef CAS.
- Q.-Y. Chai, Z. Yang, H.-W. Lin and B.-N. Han, Mar. Drugs, 2016, 14 CrossRef CAS PubMed.
- S. Ómarsdóttir, X. Wang, H.-B. Liu, B. M. Duggan and T. F. Molinski, J. Org. Chem., 2018 Search PubMed , submitted.
- D. Liang, M. Wang, B. Bekturhun, B. Xiong and Q. Liu, Adv. Synth. Catal., 2010, 352, 1593–1599 CrossRef CAS.
- P. B. Oelrichs, M. W. Hill, P. J. Vallely, J. K. MacLeod and T. F. Molinski, Phytochemistry, 1983, 22, 531–534 CrossRef CAS.
- M. C. Wani, H. L. Taylor, M. E. Wall, P. Coggon and A. T. McPhail, J. Am. Chem. Soc., 1971, 93, 2325–2327 CrossRef CAS.
- D. S. Dalisay, B. I. Morinaka, C. K. Skepper and T. F. Molinski, J. Am. Chem. Soc., 2009, 131, 7552–7553 CrossRef CAS PubMed.
- T. F. Molinski, J. Ko, K. A. Reynolds, S. C. Lievens and K. R. Skarda, J. Nat. Prod., 2011, 74, 882–887 CrossRef CAS PubMed.
- T. F. Molinski and C. M. Ireland, J. Org. Chem., 1988, 53, 2103–2105 CrossRef CAS.
- C. K. Skepper and T. F. Molinski, J. Org. Chem., 2008, 73, 2592–2597 CrossRef CAS.
- V. R. Espejo, X.-B. Li and J. D. Rainier, J. Am. Chem. Soc., 2010, 132, 8282–8284 CrossRef CAS PubMed.
- N. Marcó, A. A. Souza, P. Nolis, R. R. Gil and T. Parella, J. Magn. Reson., 2017, 276, 37–42 CrossRef PubMed.
- D. Schulze-Sünninghausen, J. Becker and B. Luy, J. Am. Chem. Soc., 2014, 136, 1242–1245 CrossRef.
- Ē. Kupče and T. D. W. Claridge, Angew. Chem., Int. Ed., 2017, 56, 11779–11783 CrossRef.
- K. Kazimierczuk and V. Y. Orekhov, Angew. Chem., Int. Ed., 2011, 50, 5556–5559 CrossRef CAS.
- S. A. Bradley, T. A. Smitka, D. J. Russell and K. Krishnamurthy, Curr. Metabolomics, 2015, 3, 21–31 CrossRef CAS.
- T. C. Lima, A. D. C. Santos, D. T. M. Costa, R. J. Souza, A. Barison, M. Steindel and M. W. Biavatti, Rev. Bras. Farmacogn., 2015, 25, 7–10 CrossRef CAS.
- T. C. Lima, R. J. Souza, A. D. C. Santos, M. H. Moraes, N. E. Biondo, A. Barison, M. Steindel and M. W. Biavatti, Nat. Prod. Res., 2016, 30, 551–557 CrossRef CAS PubMed.
- A. P. D. Pedroso, S. C. Santos, A. A. Steil, F. Deschamps, A. Barison, F. Campos and M. W. Biavatti, Rev. Bras. Farmacogn., 2008, 18, 63–69 CrossRef CAS.
- S. K. S. Amoah, E. Kouloura, L. M. Dutra, A. Barison, L. M. Wildner, M. L. Bazzo, M. Halabalaki, L. A. Skaltsounis and M. W. Biavatti, Phytochem. Lett., 2015, 13, 267–274 CrossRef CAS.
- L. A. L. da Silva, L. G. Faqueti, F. H. Reginatto, A. D. C. dos Santos, A. Barison and M. W. Biavatti, Rev. Bras. Farmacogn., 2015, 25, 375–381 CrossRef.
- H. Buskuhl, R. A. Freitas, F. D. Monache, A. Barison, F. R. Campos, Y. E. Corilo, M. N. Eberlin and M. W. Biavatti, J. Braz. Chem. Soc., 2009, 20, 1327–1333 CrossRef CAS.
- L. A. E. Pollo, C. F. Bosi, A. S. Leite, C. Rigotto, J. Kratz, C. M. O. Simões, D. E. P. Fonseca, D. Coimbra, G. Caramori, A. Nepel, F. R. Campos, A. Barison and M. W. Biavatti, Phytochemistry, 2013, 95, 375–383 CrossRef CAS PubMed.
- F. M. M. Ocampos, L. R. A. Menezes, L. M. Dutra, M. F. C. Santos, S. Ali and A. Barison, in eMagRes, Wiley Online Library, 2017, vol. 6, pp. 325–342 Search PubMed.
- W. E. Maas, F. H. Laukien and D. G. Cory, J. Am. Chem. Soc., 1996, 118, 13085–13086 CrossRef CAS.
- A. D. C. Santos, F. A. Fonseca, L. M. Lião, G. B. Alcantara and A. Barison, TrAC, Trends Anal. Chem., 2015, 73, 10–18 CrossRef CAS.
- M.-L. Kondracki and M. Guyot, Tetrahedron, 1989, 45, 1995–2004 CrossRef CAS.
- J. Rodríguez, E. Quiñoá, R. Riguera, B. M. Peters, L. M. Abrell and P. Crews, Tetrahedron, 1992, 48, 6667–6680 CrossRef.
- X. Zhang, H.-Y. Xu, A.-M. Huang, L. Wang, Q. Wang, P.-Y. Cao and P.-M. Yang, Chem. Pharm. Bull., 2016, 64, 1036–1042 CrossRef CAS PubMed.
- T. Oda, W. Wang, K. Ukai, T. Nakazawa and M. Mochizuki, Mar. Drugs, 2007, 5, 151–156 CrossRef CAS PubMed.
- T. Oda, W. Wang, A. Fujita, M. Mochizuki, K. Ukai and M. Namikoshi, J. Nat. Med., 2007, 61, 434–437 CrossRef CAS.
- H. M. Nguyen, T. Ito, N. N. Win, T. Kodama, V. Q. Hung, H. T. Nguyen and H. Morita, Phytochem. Lett., 2016, 17, 288–292 CrossRef CAS.
- J. Li, B.-B. Gu, F. Sun, J.-R. Xu, W.-H. Jiao, H.-B. Yu, B.-N. Han, F. Yang, X.-C. Zhang and H.-W. Lin, J. Nat. Prod., 2017, 80, 1436–1445 CrossRef CAS PubMed.
- S. Aoki, D. Kong, K. Matsui, R. Rachmat and M. Kobayashi, Chem. Pharm. Bull., 2004, 52, 935–937 CrossRef CAS PubMed.
- T. Ling, E. Poupon, E. J. Rueden, S. H. Kim and E. A. Theodorakis, J. Am. Chem. Soc., 2002, 124, 12261–12267 CrossRef CAS PubMed.
- R. Berger, G. Resnati, P. Metrangolo, E. Weber and J. Hulliger, Chem. Soc. Rev., 2011, 40, 3496–3508 RSC.
- S. Senaweera and J. D. Weaver, J. Am. Chem. Soc., 2016, 138, 2520–2523 CrossRef CAS PubMed.
- S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC.
- J. R. Dolbier, Guide to Fluorine NMR for Organic Chemists, John Wiley, New York, 2016 Search PubMed.
- M. Gerken, J. A. Boatz, A. Kornath, R. Haiges, S. Schneider, T. Schroer and K. O. Christe, J. Fluorine Chem., 2002, 116, 49–58 CrossRef CAS.
- J. Burdon, A. C. Childs, I. W. Parsons and J. C. Tatlow, J. Chem. Soc., Chem. Commun., 1982, 534–535, 10.1039/c39820000534.
- M. Muir and J. Baker, J. Fluorine Chem., 2005, 126, 727–738 CrossRef CAS.
- S. Boecker, S. Zobel, V. Meyer and R. D. Süssmuth, Fungal Genet. Biol., 2016, 89, 89–101 CrossRef CAS PubMed.
- R. J. M. Goss, S. Shankar and A. A. Fayad, Nat. Prod. Rep., 2012, 29, 870–889 RSC.
- B. E. Evans, K. E. Rittle, M. G. Bock, R. M. DiPardo, R. M. Freidinger, W. L. Whitter, G. F. Lundell, D. F. Veber, P. S. Anderson, R. S. L. Chang, V. J. Lotti, D. J. Cerino, T. B. Chen, P. J. Kling, K. A. Kunkel, J. P. Springer and J. Hirshfield, J. Med. Chem., 1988, 31, 2235–2246 CrossRef CAS.
- R. McDaniel, A. Thamchaipenet, C. Gustafsson, H. Fu, M. Betlach, M. Betlach and G. Ashley, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 1846–1851 CrossRef CAS.
- R. J. M. Goss, S. Lanceron, A. Deb Roy, S. Sprague, M. Nur-e-Alam, D. L. Hughes, B. Wilkinson and S. J. Moss, ChemBioChem, 2010, 11, 698–702 CrossRef CAS PubMed.
- M. C. Walker, B. W. Thuronyi, L. K. Charkoudian, B. Lowry, C. Khosla and M. C. Y. Chang, Science, 2013, 341, 1089 CrossRef CAS PubMed.
- M. C. Walker and M. C. Y. Chang, Chem. Soc. Rev., 2014, 43, 6527–6536 RSC.
- I. Ojima, J. Org. Chem., 2013, 78, 6358–6383 CrossRef CAS.
- I. Ojima, ChemBioChem, 2004, 5, 628–635 CrossRef CAS PubMed.
- D. O'Hagan and H. Deng, Chem. Rev., 2015, 115, 634–649 CrossRef PubMed.
- T. Fujiwara and D. O'Hagan, J. Flourine Chem., 2014, 167, 16–29 CrossRef CAS.
- H. Chen, S. Viel, F. Ziarelli and L. Peng, Chem. Soc. Rev., 2013, 42, 7971–7982 RSC.
- E. N. G. Marsh and Y. Suzuki, ACS Chem. Biol., 2014, 9, 1242–1250 CrossRef CAS PubMed.
- J. Rivera-Chávez, H. A. Raja, T. N. Graf, J. E. Burdette, C. J. Pearce and N. H. Oberlies, J. Nat. Prod., 2017, 80, 1883–1892 CrossRef PubMed.
- K. Takesako, S. Mizutani, H. Sakakibara, M. Endo, Y. Yoshikawa, T. Masuda, E. Sono-Koyama and I. Kato, J. Antibiot., 1996, 49, 676–681 CrossRef CAS.
- Y. Igarashi, Y. Yabuta, A. Sekine, K. Fujii, K.-I. Harada, T. Oikawa, M. Sato, T. Furumai and T. Oki, J. Antibiot., 2004, 57, 748–754 CrossRef CAS.
- A. S. Eustáquio, D. O'Hagan and B. S. Moore, J. Nat. Prod., 2010, 73, 378–382 CrossRef.
- H. Deng, D. O'Hagan and C. Schaffrath, Nat. Prod. Rep., 2004, 21, 773–784 RSC.
- W. R. Dolbier, Guide to Fluorine NMR for Organic Chemists, John Wiley & Sons, Inc., 2016 Search PubMed.
- N. D. Paguigan, M. H. Al-Huniti, H. A. Raja, A. Czarnecki, J. E. Burdette, M. González-Medina, J. L. Medina-Franco, S. J. Polyak, C. J. Pearce, M. P. Croatt and N. H. Oberlies, Bioorg. Med. Chem., 2017, 25, 5238–5246 CrossRef CAS PubMed.
- D. O'Hagan and D. B. Harper, J. Fluorine Chem., 1999, 100, 127–133 CrossRef.
- D. B. Harper and D. O'Hagan, Nat. Prod. Rep., 1994, 11, 123–133 RSC.
- N. Jaivel, C. Uvarani, R. Rajesh, D. Velmurugan and P. Marimuthu, J. Nat. Prod., 2014, 77, 2–8 CrossRef CAS PubMed.
- M. S. Ayoup, D. B. Cordes, A. M. Z. Slawin and D. O'Hagan, J. Nat. Prod., 2014, 77, 1249–1251 CrossRef CAS PubMed.
- H. Aldemir, S. V. Kohlhepp, T. Gulder and T. A. M. Gulder, J. Nat. Prod., 2014, 77, 2331–2334 CrossRef CAS PubMed.
- E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, J. Med. Chem., 2015, 58, 8315–8359 CrossRef CAS PubMed.
- L. Hunter, Beilstein J. Org. Chem., 2010, 6, 38 Search PubMed.
- D. A. Bock, C. W. Lehmann and B. List, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 20636–20641 CrossRef CAS PubMed.
- S. K. Holmgren, L. E. Bretscher, K. M. Taylor and R. T. Raines, Chem. Biol., 1999, 6, 63–70 CrossRef CAS PubMed.
- C. Loenarz, J. Mecinović, R. Chowdhury, L. A. McNeill, E. Flashman and C. J. Schofield, Angew. Chem., Int. Ed., 2009, 48, 1784–1787 CrossRef CAS PubMed.
- D. Q. J. Yap, R. Cheerlavancha, R. Lowe, S. Wang and L. Hunter, Aust. J. Chem., 2015, 68, 44–49 CrossRef CAS.
- L. Demange, A. Ménez and C. Dugave, Tetrahedron Lett., 1998, 39, 1169–1172 CrossRef CAS.
- C. Thibaudeau, J. Plavec and J. Chattopadhyaya, J. Org. Chem., 1998, 63, 4967–4984 CrossRef CAS.
- A. Mariotti, Nature, 1984, 311, 251 CrossRef CAS.
- G. E. Martin and C. E. Hadden, J. Nat. Prod., 2000, 63, 543–585 CrossRef CAS.
- G. E. Martin, P. A. Irish, B. D. Hilton, K. A. Blinov and A. J. Williams, Magn. Reson. Chem., 2007, 45, 624–627 CrossRef CAS PubMed.
- R. Marek, A. Lycka, E. Kolehmainen, E. Sievänen and J. Tousek, Curr. Org. Chem., 2007, 11, 1154–1205 CrossRef CAS.
- C. E. Hadden, G. E. Martin and V. V. Krishnamurthy, Magn. Reson. Chem., 2000, 38, 143–147 CrossRef CAS.
- M. Kline and S. Cheatham, Magn. Reson. Chem., 2003, 41, 307–314 CrossRef CAS.
- J. Berg, Science, 2018, 359, 9 CrossRef CAS PubMed.
- D. S. Wishart, C. G. Bigam, J. Yao, F. Abildgaard, H. J. Dyson, E. Oldfield, J. L. Markley and B. D. Sykes, J. Biomol. NMR, 1995, 6, 135–140 CrossRef CAS.
- G. J. Martin, M. L. Martin and J.-P. Gouesnard, 15N-NMR Spectroscopy, Springer Verlag, Berlin Heidelberg, 1981 Search PubMed.
- E. Martin Gary and A. J. Williams, in Annual Reports on NMR Spectroscopy, ed. G. A. Webb, Academic Press, 2015, pp. 1–76 Search PubMed.
- E. Fattorusso and O. Taglialatela-Scafati, Modern Alkaloids: Structure, Isolation, Synthesis and Biology, Wiley-VCH, Weinheim, 2008 Search PubMed.
- S. Mo, A. Krunic, G. Chlipala and J. Orjala, J. Nat. Prod., 2009, 72, 894–899 CrossRef CAS PubMed.
- H. Zhang, S. Neal and D. S. Wishart, J. Biomol. NMR, 2003, 173–195 CrossRef.
- L. D. Quin and A. J. Williams, Practical Interpretation of P-31 NMR Spectra and Computer Assisted Structure Verification, Advanced Chemistry Development, Inc., Toronto, 2004 Search PubMed.
- J. C. Tebby, CRC Handbook of Phosphorus-31 Nuclear Magnetic Resonance Data, CRC Press, Inc., Boca Raton, FL, 1991 Search PubMed.
- D. G. Gorenstein, Phosphorus-31 NMR - Principles and Applications, Academic Press, Inc., Orlando, FL, 1984 Search PubMed.
- M. M. Crutchfield, C. H. Dungan, J. H. Letcher, V. Mark and J. R. Van Wazer, P-31 Nuclear Magnetic Resonance, John Wiley & Sons., Inc., Hoboken, NJ, 1967 Search PubMed.
- C. Brevard and P. Granger, Handbook of High Resolution Multinuclear NMR, John Wiley & Sons., Inc., Hoboken, NJ, 1981 Search PubMed.
- W. W. Metcalf and W. A. van der Donk, Annu. Rev. Biochem., 2009, 78, 65–94 CrossRef CAS PubMed.
- H. Seto and T. Kuzuyama, Nat. Prod. Rep., 1999, 16, 589–596 RSC.
- S. C. Fields, Tetrahedron, 1999, 55, 12237–12273 CrossRef CAS.
- M. C. Chi and G. P. Horsman, J. Antibiot., 2015, 68, 752 CrossRef CAS PubMed.
- H. Inoue, H. Nakayama and M. Tsuhako, Phosphorus Res. Bull., 2002, 14, 1–8 CrossRef CAS.
- Z. Huang, K.-K. A. Wang and W. A. van der Donk, Chem. Sci., 2016, 7, 5219–5223 RSC.
- D. Hendlin, E. O. Stapley, M. Jackson, H. Wallick, A. K. Miller, F. J. Wolf, T. W. Miller, L. Chaiet, F. M. Kahan, E. L. Foltz, H. B. Woodruff, J. M. Mata, S. Hernandez and S. Mochales, Science, 1969, 166, 122 CrossRef CAS PubMed.
- H. Jiang, H. Chen, N. Cai, J. Zou and X. Ju, Magn. Reson. Chem., 2015, 53, 454–459 CrossRef CAS PubMed.
- A. Granata and D. S. Argyropoulos, J. Agric. Food Chem., 1995, 43, 1538–1544 CrossRef CAS.
- C. Crestini, H. Lange and G. Bianchetti, J. Nat. Prod., 2016, 79, 2287–2295 CrossRef CAS PubMed.
- S.-T. D. Hsu, E. Breukink, E. Tischenko, M. A. G. Lutters, B. de Kruijff, R. Kaptein, A. M. J. J. Bonvin and N. A. J. van Nuland, Nat. Struct. Mol. Biol., 2004, 11, 963–967 CrossRef CAS PubMed.
- C. F. Taylor, D. Field, S.-A. Sansone, J. Aerts, R. Apweiler, M. Ashburner, C. A. Ball, P.-A. Binz, M. Bogue, T. Booth, A. Brazma, R. R. Brinkman, A. M. Clark, E. W. Deutsch, O. Fiehn, J. Fostel, P. Ghazal, F. Gibson, T. Gray, G. Grimes, J. M. Hancock, N. W. Hardy, H. Hermjakob, R. K. Julian Jr, M. Kane, C. Kettner, C. Kinsinger, E. Kolker, M. Kuiper, N. L. Novère, J. Leebens-Mack, S. E. Lewis, P. Lord, A.-M. Mallon, N. Marthandan, H. Masuya, R. McNally, A. Mehrle, N. Morrison, S. Orchard, J. Quackenbush, J. M. Reecy, D. G. Robertson, P. Rocca-Serra, H. Rodriguez, H. Rosenfelder, J. Santoyo-Lopez, R. H. Scheuermann, D. Schober, B. Smith, J. Snape, C. J. Stoekert Jr, K. Tipton, P. Sterk, A. Untergasser, J. Vandesompele and S. Wiemann, Nat. Biotechnol., 2008, 26, 889 CrossRef CAS PubMed.
- H.-D. Yoo, S.-J. Nam, Y.-W. Chin and M.-S. Kim, Arch. Pharmacal Res., 2016, 39, 143–153 CrossRef CAS PubMed.
- T. L. Suyama, W. H. Gerwick and K. L. McPhail, Bioorg. Med. Chem., 2011, 19, 6675–6701 CrossRef CAS PubMed.
- H. Tanaka, T. Tanaka and H. Etoh, Phytochemistry, 1997, 45, 205–207 CrossRef CAS.
- H. Tanaka, H. Etoh, H. Shimizu, T. Makita and Y. Tateishi, Planta Med., 2000, 66, 578–579 CrossRef CAS PubMed.
- H. Tanaka, H. Etoh, H. Shimizu, T. Oh-Uchi, Y. Terada and Y. Tateishi, Planta Med., 2001, 67, 871–873 CrossRef CAS PubMed.
- S. Grabley, P. Hammann, W. Raether, J. Wink and A. Zeeck, J. Antibiot., 1990, 43, 639–647 CrossRef CAS.
- D. Samain, J. C. Cook and K. L. Rinehart, J. Am. Chem. Soc., 1982, 104, 4129–4141 CrossRef CAS.
- K. Fukushima, T. Arai, S. Iwasaki, M. Namikoshi and S. Okuda, J. Antibiot., 1982, 35, 1480–1494 CrossRef CAS PubMed.
- S. Iwasaki, M. Namikoshi, K. Sasaki, M. Yano, K. Fukushima, S. Nozoe and S. Okuda, Chem. Pharm. Bull., 1982, 30, 1669–1673 CrossRef CAS.
- S. Iwasaki, M. Namikoshi, K. Sasaki, K. Fukushima and S. Okuda, Chem. Pharm. Bull., 1982, 30, 4006–4014 CrossRef CAS.
- K. Takesako and T. Beppu, J. Antibiot., 1984, 37, 1170–1186 CrossRef CAS.
- T. Arai, J. Uno, I. Horimi and K. Fukushima, J. Antibiot., 1984, 37, 103–109 CrossRef CAS PubMed.
- H. Koshino, K. Kobinata, J. Uzawa, M. Uramoto, K. Isono and H. Osada, Tetrahedron, 1993, 49, 8827–8836 CrossRef CAS.
- D. Fréchet, M. Danzer, F. Debu, B. M. du Sorbier, D. Reisdorf, C. Snozzi and M. Vuilhorgne, Tetrahedron, 1991, 47, 61–70 CrossRef.
- S. Kumazawa, Y. Asami, K. Awane, H. Ohtani, C. Fukuchi, T. Mikawa and T. Hayase, J. Antibiot., 1994, 47, 688–696 CrossRef CAS.
- H. Stephan, C. Kempter, J. W. Metzger, G. Jung, O. Potterat, C. Pfefferle and H. P. Fiedler, J. Antibiot., 1996, 49, 765–769 CrossRef CAS PubMed.
- J. Frank, G. Dékány, I. Pelczer and J. W. ApSimon, Tetrahedron Lett., 1987, 28, 2759–2762 CrossRef CAS.
- A. Bakiri, B. Plainchont, V. de Paulo Emerenciano, R. Reynaud, J. Hubert, J.-H. Renault and J.-M. Nuzillard, Mol. Inf., 2017, 36, 1700027 CrossRef PubMed.
- R. Laatikainen, M. Niemitz, W. J. Malaisse, M. Biesemans and R. Willem, Magn. Reson. Med., 1996, 36, 359–365 CrossRef CAS PubMed.
- R. Laatikainen, M. Niemitz, U. Weber, J. Sundelin, T. Hassinen and J. Vepsalainen, J. Magn. Reson., Ser. A, 1996, 120, 1–10 CrossRef CAS.
- E. Kolehmainen, K. Laihia, R. Laatikainen, J. Vepsalainen, M. Niemitz and R. Suontamo, Magn. Reson. Chem., 1997, 35, 463–467 CrossRef CAS.
- R. Laatikainen, M. Tiainen, S.-P. Korhonen and M. Niemitz, in Encyclopedia of Magnetic Resonance, ed. R. K. Harris and R. E. Wasylishen, John Wiley & Sons, Ltd, Chicester, 2011, DOI:10.1002/9780470034590.emrstm1226.
- V. V. Mihaleva, S.-P. Korhonen, J. van Duynhoven, M. Niemitz, J. Vervoort and D. M. Jacobs, Anal. Bioanal. Chem., 2014, 406, 3091–3102 CrossRef CAS PubMed.
- J. Hubert, J.-M. Nuzillard, S. Purson, M. Hamzaoui, N. Borie, R. Reynaud and J.-H. Renault, Anal. Chem., 2014, 86, 2955–2962 CrossRef CAS PubMed.
- K. Haug, R. M. Salek, P. Conesa, J. Hastings, P. de Matos, M. Rijnbeek, T. Mahendraker, M. Williams, S. Neumann, P. Rocca-Serra, E. Maguire, A. González-Beltrán, S.-A. Sansone, J. L. Griffin and C. Steinbeck, Nucleic Acids Res., 2013, 41, D781–D786 CrossRef CAS PubMed.
- M. Sud, E. Fahy, D. Cotter, K. Azam, I. Vadivelu, C. Burant, A. Edison, O. Fiehn, R. Higashi, K. S. Nair, S. Sumner and S. Subramaniam, Nucleic Acids Res., 2016, 44, D463–D470 CrossRef CAS PubMed.
- J. A. Vizcaíno, E. W. Deutsch, R. Wang, A. Csordas, F. Reisinger, D. Ríos, J. A. Dianes, Z. Sun, T. Farrah, N. Bandeira, P.-A. Binz, I. Xenarios, M. Eisenacher, G. Mayer, L. Gatto, A. Campos, R. J. Chalkley, H.-J. Kraus, J. P. Albar, S. Martinez-Bartolomé, R. Apweiler, G. S. Omenn, L. Martens, A. R. Jones and H. Hermjakob, Nat. Biotechnol., 2014, 32, 223–226 CrossRef PubMed.
- M. D. Wilkinson, M. Dumontier, I. J. Aalbersberg, G. Appleton, M. Axton, A. Baak, N. Blomberg, J.-W. Boiten, L. B. d. S. Santos, P. E. Bourne, J. Bouwman, A. J. Brookes, T. Clark, M. Crosas, I. Dillo, O. Dumon, S. Edmunds, C. T. Evelo, R. Finkers, A. Gonzalez-Beltran, A. J. G. Gray, P. Groth, C. Goble, J. S. Grethe, J. Heringa, P. A. C. t. Hoen, R. Hooft, T. Kuhn, R. Kok, J. Kok, S. J. Lusher, M. E. Martone, A. Mons, A. L. Packer, B. Persson, P. Rocca-Serra, M. Roos, R. v. Schaik, S.-A. Sansone, E. Schultes, T. Sengstag, T. Slater, G. Strawn, M. A. Swertz, M. Thompson, J. v. d. Lei, E. v. Mulligen, J. Velterop, A. Waagmeester, P. Wittenburg, K. Wolstencroft, J. Zhao and B. Mons, Sci. Data, 2016, 3, sdata201618 Search PubMed.
- S.-A. Sansone, T. Fan, R. Goodacre, J. L. Griffin, N. W. Hardy, R. Kaddurah-Daouk, B. S. Kristal, J. Lindon, P. Mendes, N. Morrison, B. Nikolau, D. Robertson, L. W. Sumner, C. Taylor, M. v. d. Werf, B. v. Ommen and O. Fiehn, Nat. Biotechnol., 2007, 25, 846–848 CAS.
- M. H. Medema, R. Kottmann, P. Yilmaz, M. Cummings, J. B. Biggins, K. Blin, I. de Bruijn, Y. H. Chooi, J. Claesen, R. C. Coates, P. Cruz-Morales, S. Duddela, S. Düsterhus, D. J. Edwards, D. P. Fewer, N. Garg, C. Geiger, J. P. Gomez-Escribano, A. Greule, M. Hadjithomas, A. S. Haines, E. J. N. Helfrich, M. L. Hillwig, K. Ishida, A. C. Jones, C. S. Jones, K. Jungmann, C. Kegler, H. U. Kim, P. Kötter, D. Krug, J. Masschelein, A. V. Melnik, S. M. Mantovani, E. A. Monroe, M. Moore, N. Moss, H.-W. Nützmann, G. Pan, A. Pati, D. Petras, F. J. Reen, F. Rosconi, Z. Rui, Z. Tian, N. J. Tobias, Y. Tsunematsu, P. Wiemann, E. Wyckoff, X. Yan, G. Yim, F. Yu, Y. Xie, B. Aigle, A. K. Apel, C. J. Balibar, E. P. Balskus, F. Barona-Gómez, A. Bechthold, H. B. Bode, R. Borriss, S. F. Brady, A. A. Brakhage, P. Caffrey, Y.-Q. Cheng, J. Clardy, R. J. Cox, R. De Mot, S. Donadio, M. S. Donia, W. A. van der Donk, P. C. Dorrestein, S. Doyle, A. J. M. Driessen, M. Ehling-Schulz, K.-D. Entian, M. A. Fischbach, L. Gerwick, W. H. Gerwick, H. Gross, B. Gust, C. Hertweck, M. Höfte, S. E. Jensen, J. Ju, L. Katz, L. Kaysser, J. L. Klassen, N. P. Keller, J. Kormanec, O. P. Kuipers, T. Kuzuyama, N. C. Kyrpides, H.-J. Kwon, S. Lautru, R. Lavigne, C. Y. Lee, B. Linquan, X. Liu, W. Liu, A. Luzhetskyy, T. Mahmud, Y. Mast, C. Méndez, M. Metsä-Ketelä, J. Micklefield, D. A. Mitchell, B. S. Moore, L. M. Moreira, R. Müller, B. A. Neilan, M. Nett, J. Nielsen, F. O'Gara, H. Oikawa, A. Osbourn, M. S. Osburne, B. Ostash, S. M. Payne, J.-L. Pernodet, M. Petricek, J. Piel, O. Ploux, J. M. Raaijmakers, J. A. Salas, E. K. Schmitt, B. Scott, R. F. Seipke, B. Shen, D. H. Sherman, K. Sivonen, M. J. Smanski, M. Sosio, E. Stegmann, R. D. Süssmuth, K. Tahlan, C. M. Thomas, Y. Tang, A. W. Truman, M. Viaud, J. D. Walton, C. T. Walsh, T. Weber, G. P. van Wezel, B. Wilkinson, J. M. Willey, W. Wohlleben, G. D. Wright, N. Ziemert, C. Zhang, S. B. Zotchev, R. Breitling, E. Takano and F. O. Glöckner, Nat. Chem. Biol., 2015, 11, 625–631 CrossRef CAS PubMed.
- P. Rocca-Serra, R. M. Salek, M. Arita, E. Correa, S. Dayalan, A. Gonzalez-Beltran, T. Ebbels, R. Goodacre, J. Hastings, K. Haug, A. Koulman, M. Nikolski, M. Oresic, S.-A. Sansone, D. Schober, J. Smith, C. Steinbeck, M. R. Viant and S. Neumann, Metabolomics, 2016, 12, 14 CrossRef PubMed.
- R. M. Salek, S. Neumann, D. Schober, J. Hummel, K. Billiau, J. Kopka, E. Correa, T. Reijmers, A. Rosato, L. Tenori, P. Turano, S. Marin, C. Deborde, D. Jacob, D. Rolin, B. Dartigues, P. Conesa, K. Haug, P. Rocca-Serra, S. O'Hagan, J. Hao, M. van Vliet, M. Sysi-Aho, C. Ludwig, J. Bouwman, M. Cascante, T. Ebbels, J. L. Griffin, A. Moing, M. Nikolski, M. Oresic, S.-A. Sansone, M. R. Viant, R. Goodacre, U. L. Günther, T. Hankemeier, C. Luchinat, D. Walther and C. Steinbeck, Metabolomics, 2015, 11, 1587–1597 CrossRef CAS PubMed.
- D. Schober, D. Jacob, M. Wilson, J. A. Cruz, A. Marcu, J. R. Grant, A. Moing, C. Deborde, L. F. de Figueiredo, K. Haug, P. Rocca-Serra, J. M. Easton, T. M. D. Ebbels, J. Hao, C. Ludwig, U. L. Günther, A. Rosato, M. S. Klein, I. Lewis, C. Luchinat, A. R. Jones, A. Grauslys, M. Larralde, M. Yokochi, N. Kobayashi, A. Porzel, J. Griffin, M. R. Viant, D. S. Wishart, C. Steinbeck, R. M. Salek and S. Neumann, Anal. Chem., 2018, 90, 649–656 CrossRef CAS PubMed.
- P. Rocca-Serra, M. Brandizi, E. Maguire, N. Sklyar, C. Taylor, K. Begley, D. Field, S. Harris, W. Hide, O. Hofmann, S. Neumann, P. Sterk, W. Tong and S.-A. Sansone, Bioinformatics, 2010, 26, 2354–2356 CrossRef CAS PubMed.
- S. Beisken, M. Earll, C. Baxter, D. Portwood, Z. Ament, A. Kende, C. Hodgman, G. Seymour, R. Smith, P. Fraser, M. Seymour, R. M. Salek and C. Steinbeck, Sci. Data, 2014, 1, sdata201429 CrossRef PubMed.
- P.-M. Allard, J. Bisson, A. Azzollini, G. F. Pauli, G. A. Cordell and J.-L. Wolfender, Curr. Opin. Biotechnol., 2018, 54, 57–64 CrossRef CAS PubMed.
- D. Jeannerat, Magn. Reson. Chem., 2017, 55, 7–14 CrossRef CAS PubMed.
- M. Pupier, J. M. Nuzillard, J. Wist, E. Schlörer Nils, S. Kuhn, M. Erdelyi, C. Steinbeck, J. Williams Antony, C. Butts, D. W. Claridge Tim, B. Mikhova, W. Robien, H. Dashti, R. Eghbalnia Hamid, C. Farès, C. Adam, K. Pavel, F. Moriaud, M. Elyashberg, D. Argyropoulos, M. Pérez, P. Giraudeau, R. Gil Roberto, P. Trevorrow and D. Jeannerat, Magn. Reson. Chem., 2018, 56 DOI:10.1002/mrc.4737.
- The Editors, Magn. Reson. Chem., 2017, 55, 1057–1058 CrossRef PubMed.
- S. Blüml, A. S. Margol, R. Sposto, R. J. Kennedy, N. J. Robison, M. Vali, L. T. Hung, S. Muthugounder, J. L. Finlay, A. Erdreich-Epstein, F. H. Gilles, A. R. Judkins, M. D. Krieger, G. Dhall, M. D. Nelson and S. Asgharzadeh, Neuro-Oncology, 2016, 18, 126–131 CrossRef PubMed.
- A. Kovanlikaya, A. Panigrahy, M. D. Krieger, I. Gonzalez-Gomez, N. Ghugre, J. G. McComb, F. H. Gilles, M. D. Nelson and S. Blüml, Radiology, 2005, 236, 1020–1025 CrossRef PubMed.
- T. Leather, M. D. Jenkinson, K. Das and H. Poptani, Metabolites, 2017, 7 CrossRef CAS PubMed.
- C. Choi, S. K. Ganji, R. J. DeBerardinis, K. J. Hatanpaa, D. Rakheja, Z. Kovacs, X.-L. Yang, T. Mashimo, J. M. Raisanen, I. Marin-Valencia, J. M. Pascual, C. J. Madden, B. E. Mickey, C. R. Malloy, R. M. Bachoo and E. A. Maher, Nat. Med., 2012, 18, 624–629 CrossRef CAS PubMed.
- P. Alusta, I. Im, B. A. Pearce, R. D. Beger, R. M. Kretzer, D. A. Buzatu and J. G. Wilkes, J. Magn. Reson. Imaging, 2010, 32, 818–829 CrossRef PubMed.
- T. Tran, M. Csete, B. Ross, E. Geesaman, J. Wilkes and D. Buzatu, ISMRM 25th Annual Meeting & Exhibition, Honolulu, HI, 2017 Search PubMed.
- A. Jimeno Yepes and K. Verspoor, Database, 2014, 2014, bau003 CrossRef PubMed.
- A. D. Rodríguez, E. González and S. D. Huang, J. Org. Chem., 1998, 63, 7083–7091 CrossRef.
- T. J. Heckrodt and J. Mulzer, J. Am. Chem. Soc., 2003, 125, 4680–4681 CrossRef CAS PubMed.
- G. Zanoni and M. Franzini, Angew. Chem., 2004, 116, 4942–4946 CrossRef.
- S. D. Rychnovsky, Org. Lett., 2006, 8, 2895–2898 CrossRef CAS PubMed.
- N. Grimblat and A. M. Sarotti, Chem.–Eur. J., 2016, 22, 12246–12261 CrossRef CAS PubMed.
- S. Di Micco, M. G. Chini, R. Riccio and G. Bifulco, Eur. J. Org. Chem., 2010, 2010, 1411–1434 CrossRef.
- M. W. Lodewyk, M. R. Siebert and D. J. Tantillo, Chem. Rev., 2012, 112, 1839–1862 CrossRef CAS PubMed.
- P. H. Willoughby, M. J. Jansma and T. R. Hoye, Nat. Protoc., 2014, 9, 643 CrossRef CAS PubMed.
- Committee for Scientific and Technological Policy (CSTP), Business Models for Sustainable Research Data Repositories, OECD, 2017.
- J. L. Pons, T. E. Malliavin, D. Tramesel and M. A. Delsuc, Bioinformatics, 2004, 20, 3707–3709 CrossRef CAS PubMed.
- D. S. Wishart, T. Jewison, A. C. Guo, M. Wilson, C. Knox, Y. Liu, Y. Djoumbou, R. Mandal, F. Aziat and E. Dong, Nucleic Acids Res., 2013, 41, D801–D807 CrossRef CAS PubMed.
- E. L. Ulrich, H. Akutsu, J. F. Doreleijers, Y. Harano, Y. E. Ioannidis, J. Lin, M. Livny, S. Mading, D. Maziuk, Z. Miller, E. Nakatani, C. F. Schulte, D. E. Tolmie, R. Kent Wenger, H. Yao and J. L. Markley, Nucleic Acids Res., 2008, 36, D402–D408 CrossRef CAS PubMed.
- S. J. Chalk, J. Cheminf., 2016, 8, 55 Search PubMed.
- C. Simmler, J. G. Napolitano, J. B. McAlpine, S.-N. Chen and G. F. Pauli, Curr. Opin. Biotechnol., 2014, 25, 51–59 CrossRef CAS PubMed.
- G. F. Pauli, B. U. Jaki, T. Gödecke and D. C. Lankin, J. Nat. Prod., 2012, 75, 834–851 CrossRef CAS PubMed.
- R. S. Phansalkar, C. Simmler, J. Bisson, S.-N. Chen, D. C. Lankin, J. B. McAlpine, M. Niemitz and G. F. Pauli, J. Nat. Prod., 2017, 80, 634–647 CrossRef CAS PubMed.
- P. K. Sharma, L. J. Romanczyk Jr, L. Kondaveti, B. Reddy, J. Arumugasamy, R. Lombardy, Y. Gou and H. Schroeter, Org. Lett., 2015, 17, 2306–2309 CrossRef CAS PubMed.
- C.-M. Wang, Y.-M. Hsu, Y.-L. Jhan, S.-J. Tsai, S.-X. Lin, C.-H. Su and C.-H. Chou, Molecules, 2015, 20, 12787–12803 CrossRef CAS PubMed.
- H. Zhang, Y. Yang and C. Ma, J. Agric. Food Chem., 2013, 61, 8814–8820 CrossRef CAS PubMed.
- J. E. Kim, S. S. Kim, C.-G. Hyun and N. H. Lee, Int. J. Pharmacol., 2012, 8, 410–415 CAS.
- X. Li, H. Jin, M. Yang, G. Chen, Y. Shen and W. Zhang, Chem. Nat. Compd., 2010, 46, 106–108 CrossRef CAS.
- H. Lou, Y. Yamazaki, T. Sasaki, M. Uchida, H. Tanaka and S. Oka, Phytochemistry, 1999, 51, 297–308 CrossRef CAS.
- G.-I. Nonaka, S. Morimoto, J.-E. Kinjo, T. Nohara and I. Nishioka, Chem. Pharm. Bull., 1987, 35, 149–155 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Original NMR data (FIDs) of many cases discussed in this review are made available at DOI: http://dx.doi.org/10.7910/DVN/WB0DHJ. See DOI: 10.1039/c7np00064b |
| This journal is © The Royal Society of Chemistry 2019 |