Halogen bonding and triiodide asymmetry in cocrystals of triphenylmethylphosphonium triiodide with organoiodines†
Abstract
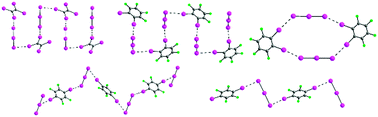
A family of cocrystals based on triphenylmethylphosphonium cations (PPh3Me+), triiodide anions (I3−), and organoiodine molecules tetraiodoethylene (TIE), 1,4-diiodotetrafluorobenzene (p-F4DIB), and 1,2-diiodotetrafluorobenzene (o-F4DIB) have been synthesized and structurally characterized. The triiodide anion has not been extensively studied previously for its halogen bonding tendencies, but it is intriguing for applications involving crystal engineering and structural design because of its inherent directionality. The present work begins to form a basis set of compounds to understand the role of halogen bonding with other intermolecular interactions, such as phenyl embraces, as complementary long-range interactions. In particular, these cocrystals feature type II I⋯I halogen bonding between the triiodide and organoiodine molecules, resulting in a variety of long range structural features including isolated halogen-bonded units, infinite chains, and three-dimensional frameworks. These networks are reinforced through edge → face and face⋯face phenyl embraces based on the packing of the cations. Asymmetry of the triiodide anions is observed, and is associated with the halogen bonding interactions that occur in the structures.
- This article is part of the themed collection: The halogen bond: a new avenue in recognition and self-assembly


 Please wait while we load your content...
Please wait while we load your content...