Quantum calculations of At-mediated halogen bonds: on the influence of relativistic effects†
Abstract
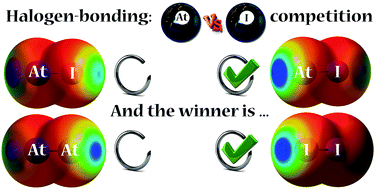
The infuence of relativistic effects, more specifically of spin–orbit coupling (SOC), on the geometric and energetic features of halogen bonds mediated through astatine (At) has been investigated through quantum chemistry calculations. For complexes between ammonia and diastatine or diiodine, the accounting of SOC results in stronger interaction energies with iodine, in contrast to scalar-relativistic calculations which predict astatine as a better halogen-bond (XB) donor. In AtI, where the competition is intramolecular, astatine always appears as the strongest XB donor. Whereas calculations in the absence of SOC predict that 15% of the XB complexes with toluene occur on the iodine atom, this population becomes three times lower when SOC is taken into account. The investigation of hypoastatous acid properties highlights a substantial decrease of the hydrogen-bond (HB) and XB interaction energies for AtOH when SOC is considered. The calculation of complementary electrostatic (electrostatic potential, VS,max) and charge transfer (local electrophilicity, ω+S,max) descriptors provide guidelines for the rationalization of these trends, underlining the significant role of SOC in astatine electronegativity. Finally, the SOC effects are shown in AtI and AtOH to be significantly transferred from astatine to its neighbouring atoms, resulting in stronger alterations of their XB or HB properties than on the XB donating ability of astatine itself.
- This article is part of the themed collection: The halogen bond: a new avenue in recognition and self-assembly


 Please wait while we load your content...
Please wait while we load your content...