Studies on drug release kinetics and antibacterial activity against drug-resistant bacteria of cefotaxime sodium loaded layered double hydroxide–fenugreek nanohybrid
Eswaravara Prasadarao
Komarala
a,
Sejal
Doshi
a,
Shankar
Thiyagarajan
a,
Mohammed
Aslam
b and
Dhirendra
Bahadur
 *a
*a
aDepartment of Metallurgical Engineering and Materials Science, Indian Institute of Technology Bombay, Mumbai, India. E-mail: dhirenb@iitb.ac.in; Fax: +91 22 2572 3480; Tel: +91 22 2576 7632
bDepartment of Physics, Indian Institute of Technology Bombay, Mumbai, India
First published on 14th November 2017
Abstract
In the current work, we report the loading of cefotaxime sodium on Mg–Al layered double hydroxide (Cefo-LDH) and the formation of a nanohybrid with a fenugreek polymer (CLF nanohybrid). This nanohybrid was synthesized through the method of anion-exchange followed by sonication. The as-synthesized CLF nanohybrid was thoroughly characterized by XRD, FTIR and zeta potential measurements, which revealed that the cefotaxime drug was bound to the LDH surface. The drug loading capacities of Cefo-LDH and the CLF nanohybrid were found to be 85.6 and 72.5 μg mg−1, respectively. The drug released from the CLF nanohybrid demonstrates a controlled and sustained profile at pH 7.3 over a period of 72 h. The mechanism of drug release is explained by the first-order and parabolic kinetic models. The kinetic models suggest that the release of cefotaxime is dependent on the dissolution and diffusion of drug molecules in the physiological medium. At a concentration up to 1 mg mL−1, both Cefo-LDH and the CLF nanohybrid are seen to be biocompatible with murine fibroblast (L929) cells. Furthermore, antibacterial activity studies against the cefotaxime drug-resistant Escherichia coli (E. coli) strain show about 98% cell mortality with 1 mg mL−1 of the nanohybrid loaded with cefotaxime.
Introduction
In the current scenario, the development of antibacterial agents against multidrug resistant bacteria is a great challenge and a concern for health globally. It is a common observation that with the use of conventional antibacterial agents, a high dose of antibiotics is required to combat bacterial infections. With every increase in dosage, these bacteria adapt themselves and evolve into drug-resistant strains that, in turn, become even more difficult to deal with. This leads to dreadful side effects and generates unbearable toxicity.1,2 This has motivated improvements to the conventional methods and exploration of new strategies to treat multidrug resistant bacteria. Several efforts have been made in this direction and recently it has been shown that functional nanomaterials can offer a solution to combat multidrug-resistant bacteria as an individual platform or as carriers of antibiotics.3–7In the past decade, it has been proven that layered double hydroxides (LDHs) are an efficient nanomaterial platform as safe drug vehicles for many antibiotics.8,9 LDHs are anionic clay materials containing brucite like positive layers compensated by intercalated anions and water molecules. The general chemical formula of LDHs is [MII1−xMIIIx(OH)2]·[An−x/n·mH2O], where MII and MIII are divalent and trivalent metal cations and An− are exchangeable intercalated anions.10 The biocompatible nature and high anion exchange capacity of LDHs facilitates their extensive usage as drug carriers.11 Earlier, several groups reported the intercalation and sustained release of antibiotic drugs such as amoxicillin, benzyl penicillin, norfloxacin, succinate and tetracycline, etc. using LDHs as drug carriers.12–15 Rives et al. reviewed the capability of intercalating various antibiotic and anticancer drugs and their controlled release.9 However, to the best of our knowledge, there is no report on the loading and sustained release of cefotaxime sodium antibiotic with LDH based systems.
Cefotaxime sodium (Cefo) is an antibiotic that belongs to the third-generation cephalosporin drugs (Fig. 1a). It has a strong antibacterial activity against various Gram-negative and Gram-positive bacteria.16 It has been used in a variety of clinical applications for the treatment of low respiratory tract, gynaecologic, skin, bone and joint infections.17 Depending on the severity of infections, the dosage of cefotaxime varies from 3–6 g per day. Such high doses may cause severe side effects such as hypersensitivity syndrome and drug fever.18 The goal of any antibiotic is to limit its required dosage to slightly above the bacterial minimum inhibition concentration and increase the circulation half-life in order to counter the multidrug resistant bacteria. However, the short half-life of cefotaxime and drug-resistance by β-lactamase bacterial strains limits its clinical success.19 The efficacy of cefotaxime can be enhanced by enabling sustained drug release and fouling the resistant bacteria by incorporating drug carriers in high molecular weight biodegradable polymers.20 Fenugreek gum (Fenu) is a naturally occurring polymer (Fig. 1b), and is used mostly as a gelling agent in the food industry.21 Apart from being more stable than most commercial polymers, it has several health benefits such as an antidiabetic effect, antioxidant potency and a digestive stimulant effect.22,23
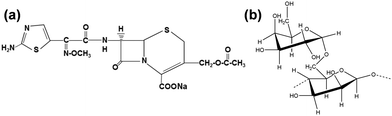 | ||
| Fig. 1 (a) Chemical structure of cefotaxime sodium and (b) primary structure of a fenugreek polymer. | ||
In the present study, we aimed to develop nanocarriers for cefotaxime for the treatment of drug-resistant bacteria. To this end, we synthesized a cefotaxime sodium loaded Mg–Al layered double hydroxide/fenugreek polymer (CLF) nanohybrid. We studied the loading and release of the cefotaxime drug with both LDH and the LDH–Fenu nanohybrid. The release kinetics were studied with first-order and parabolic diffusion kinetic models. Furthermore, we also investigated the antibacterial activity of this nanohybrid on the cefotaxime-sensitive E. coli 25922 ATCC (E. coli) strain and cefotaxime resistance extended spectrum beta-lactamase E. coli 949 (E. coli ESBL) strain.
Experimental section
Materials and reagents
Magnesium nitrate hexahydrate (Mg(NO3)2·6H2O, 99%), aluminium nitrate nonahydrate (Al(NO3)3·9H2O, 99%), and ammonium hydroxide (NH4OH) were procured from Sigma-Aldrich. Cefotaxime sodium injection (powder form) was purchased from Alkem Health Science, India. Fenugreek polymer was received from Chemtotal Labs Pvt. Ltd., Jodhpur, India and Milli-Q water (18.2 MΩ, pH 7.2) was taken from the Thermo Scientific Millipore system.Synthesis of Mg–Al LDH and the nanohybrid
Mg–Al LDH was synthesized using a method described in the literature, with minor modifications.24 In a typical synthesis, 40 mL aqueous solution of NH4OH was added to 250 mL solution containing Mg(NO3)2·6H2O and Al(NO3)3·9H2O in a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 under vigorous stirring and an N2 atmosphere. The pH of the solution was maintained at 10 using 1 M NaOH and the reaction was carried out for 1 h to obtain a white precipitate. The precipitate was then collected by centrifugation, washed with ammoniacal water 3–4 times and dried at 50 °C in vacuum.
1 under vigorous stirring and an N2 atmosphere. The pH of the solution was maintained at 10 using 1 M NaOH and the reaction was carried out for 1 h to obtain a white precipitate. The precipitate was then collected by centrifugation, washed with ammoniacal water 3–4 times and dried at 50 °C in vacuum.
750 mg of the obtained LDH was added to 100 mL aqueous solution containing 200 mg of cefotaxime sodium. The solution was stirred at room temperature for 24 h under an N2 atmosphere. Upon completion of the reaction, the product was washed with water 3–4 times and dried at 70 °C in vacuum to obtain cefotaxime sodium loaded LDH (Cefo-LDH).
Before preparing the nanohybrid, Cefo-LDH and fenugreek were dispersed in 50 mL of MilliQ water separately, to obtain a concentration of 1 mg mL−1. The solutions of Cefo-LDH and fenugreek were mixed and sonicated for 10 min. The product was separated by centrifugation and dried in a vacuum at room temperature. The obtained sample is addressed as the CLF nanohybrid hereafter.
Characterization techniques
X-ray diffraction (XRD) patterns of the samples were recorded on a Philips powder diffractometer PW3040/60 with CuKα (1.5406 Å) radiation. Fourier transform infrared (FTIR) spectra were obtained on a JASCO spectrometer (6100 type-A) instrument in the range of 400–4000 cm−1. The morphology of the as-synthesized nanohybrid was investigated using field emission gun transmission electron microscopy (FEG-TEM, JEOL JAM 2100F (200 kV)). The elemental analysis of the samples was obtained from an energy dispersive spectrum (EDS from FEG-SEM) and CHNS (ThermoFinnigan) analysis. The zeta potential and hydrodynamic diameter were measured using a ZetaPALS zeta potential analyzer (BI-200 Brookhaven Instruments Corporation) and dynamic light scattering (DLS; Beckman Coulter, Delsa™ Nano). Furthermore, antibacterial studies were carried out on the bacterial model E. coli 25922 ATCC (cefotaxime sensitive) and E. coli 949 ESBL (cefotaxime resistant) cells.Drug loading and release
Cefotaxime sodium (Cefo) was used to evaluate the potential of LDH as a delivery vehicle. The drug-loading efficiency of the as synthesized Cefo-loaded LDH and CLF nanohybrid were studied using UV-Vis spectra. The UV-Vis spectra of the supernatant (obtained after centrifugation of drug-loaded LDH) and 1 mL of pure Cefo (2 mg mL−1, for comparison) were then recorded. To determine the loading efficiency, the spectral intensities of the supernatants of Cefo loaded LDH were compared with that of the pure Cefo solution. The loading efficiency (w/w%) was calculated using the following relation: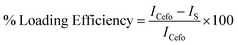 | (1) |
The drug release studies of Cefo-LDH and the CLF nanohybrid were carried out by monitoring the time dependent release of drug molecules in phosphate buffer. For the release study, 20 mg of the samples was dispersed in 30 mL of PBS solution at pH 7.3 under continuous stirring at 37 °C (to mimic the cellular environment). At fixed intervals of time, 1 mL PBS solution was withdrawn and analysed for the amount of Cefo released. The concentration gradient of the solution was maintained by replacing this with 1 mL of fresh PBS. The absorbance of the aliquot was then recorded. Furthermore, to understand the release kinetics, the drug release profiles were fitted according to the first-order and parabolic diffusion kinetic models.
Biocompatibility study
Prior to the antibacterial studies, the as-synthesized CLF nanohybrid and its constituents were tested for biocompatibility with L929 cells. In a typical SRB assay,25 cells (1 × 104) were incubated for 24 h in a 5% CO2 environment at 37 °C. Subsequently, 200 μL of different concentrations (serially diluted) of all samples (LDH, Fenu, Cefo, Cefo-LDH and the CLF nanohybrid), dispersed in an appropriate growth medium, were added to the cells and incubated under similar conditions. The cells were washed with phosphate buffered saline (PBS; pH 7.3) and further fixed with 10% trichloroacetic acid and incubated at 4 °C for 1 h. Then, the cells were stained with 0.4% SRB dissolved in 1% acetic acid and incubated in the dark. The optical density was measured using a multiwall plate reader at 560 nm. Cells without any material served as controls for the experiments and all the experiments were performed in triplicate.Antibacterial activity
Results and discussion
Characterization of the CLF nanohybrid
Fig. 2 shows XRD patterns of LDH, Cefo-LDH, and the CLF nanohybrid along with Cefo and Fenu. The XRD pattern of LDH (Fig. 2a) shows the diffraction pattern with the peaks corresponding to (003), (006), (012), (015), (018), (110) and (113) planes, characteristic of hydrotalcite-like compound with rhombohedral (R3m) symmetry. A d-spacing of 0.88 nm indicates that LDH has nitrates as intercalated anions.26 The presence of a peak positioned at 18.5° indicates an additional phase of Al(OH)3 along with LDH. An excess amount of precursors during the synthesis of LDH is responsible for the presence of Al(OH)3.27 Cefo-LDH and the CLF nanohybrid also show similar peaks of LDH (Fig. 2b and c), with a shift in the 2θ position towards a higher angle and also a change in the relative intensity and broadening of the diffraction peaks. This shift towards a higher angle indicates a slight decrease in the basal spacing in both Cefo-LDH (0.79 nm) and the nanohybrid (0.74 nm).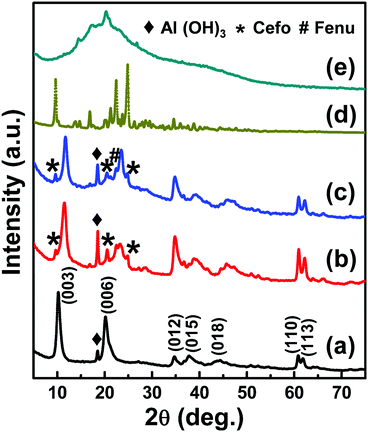 | ||
| Fig. 2 XRD Pattern of (a) LDH, (b) Cefo-LDH, (c) the CLF nanohybrid, (d) cefotaxime sodium and (e) the fenugreek polymer. | ||
The inter-planar distances and the lattice parameters of the primary peaks (003), (006) and (110) of LDH, Cefo-LDH and the CLF nanohybrid are compared in Table 1. Furthermore, the gallery height that gives an indication of the intercalated anion arrangement was measured by subtracting the thickness (0.48 nm)28 of the LDH layer from d003. The interlayer distance and the gallery height values in the case of Cefo-LDH and the nanohybrid were observed to be less compared with that of LDH. This decrease in lattice parameters may be attributed to (a) the removal of water molecules, (b) rearrangement of Mg/Al ions, (c) partial replacement of nitrate ions by the drug molecules and/or (d) the adsorption of Cefo drug molecules on the surface of LDH via hydrogen bonding.29,30 Hence, the lattice constant c [c = 3/2(d003 + 2d006)] corresponds to the Coulombic interaction between the cationic layers, and the anionic interlayer decreases for Cefo-LDH and the CLF nanohybrid compared with LDH. However, the constant a (a = 2d110, average cation–cation distance) is the same for all, which indicates that the composition in the layers remains unchanged.
| Sample | d 003 | d 006 | d 110 | h | a | c |
|---|---|---|---|---|---|---|
| LDH | 0.877 | 0.443 | 0.152 | 0.400 | 0.304 | 2.649 |
| Cefo-LDH | 0.793 | 0.434 | 0.152 | 0.313 | 0.304 | 2.492 |
| CLF nanohybrid | 0.741 | 0.430 | 0.152 | 0.261 | 0.304 | 2.402 |
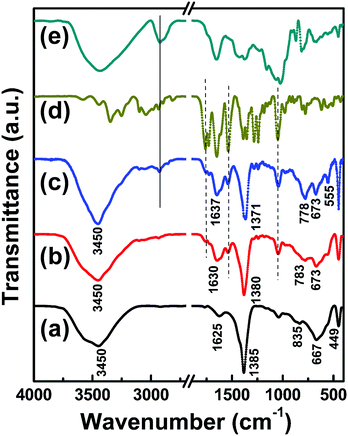
Fig. 3 shows the FT-IR spectra of LDH, Cefo-LDH and the CLF nanohybrid along with bare Cefo and fenugreek polymer ranging from 4000 to 400 cm−1. The spectrum of LDH (Fig. 3a) shows a broad peak centred at around 3450 cm−1 corresponding to O–H vibrations of water molecules and hydrogen-bonded –OH groups. The –OH bending is also evident from the vibration at 1625 cm−1. The intense absorption peak at 1385 cm−1 corresponds to the vibration mode of NO3− ions.31 The bonds associated with metal–oxygen (M–O) stretching and brucite lattice bendings are visible below 800 cm−1.32 In the case of Cefo-LDH (Fig. 3b), the O–H stretching at 3450 cm−1 remains the same while the vibrational band of NO3− ions is observed around 1380 cm−1 with a meagre shift. Similarly, in the case of the CLF nanohybrid (Fig. 3c), the peaks show a lower wavenumber shift. In particular, the peak at 835 cm−1 (corresponding to the M–O bonding in the LDH interlayer) of LDH has shifted to 783 cm−1 in the case of Cefo-LDH and to 778 cm−1 in the case of the CLF nanohybrid. This shift in the peak positions indicates that the metal–oxygen bonds change as the drug and polymer interact with the LDH phase. Also, the shift in the –OH vibrational band (at 1625 cm−1) towards a higher wavenumber indicates that the loading of the drug on LDH is via hydrogen bonding. The additional peaks observed in Cefo-LDH at 1760, 1530 and 1050 cm−1 correspond to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
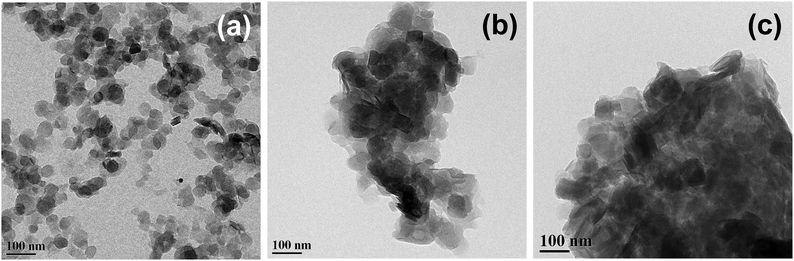
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–O stretchings of cefotaxime, respectively, and the peak at 2995 cm−1 in the CLF nanohybrid corresponds to –CH2 stretching of Fenu. Fig. 4 shows the morphology of LDH, Cefo-LDH and the CLF nanohybrid investigated by TEM. Fig. 4a shows a well separated disc shape of the LDH nanoparticles with an average diameter of 50–80 nm. Fig. 4b shows the agglomerated morphology of Cefo-LDH, which may be due to the interaction of the LDH nanoparticles with cefotaxime. A thin layer observed on the surface of LDH indicates the presence of drug on LDH. However, the CLF nanohybrid (Fig. 4c) shows more agglomeration and exhibits a compact structure after the interaction of Cefo-LDH with Fenu polymer. This kind of compact structure may be responsible for the slow release of drug from the nanohybrid (discussed later).
N and C–O stretchings of cefotaxime, respectively, and the peak at 2995 cm−1 in the CLF nanohybrid corresponds to –CH2 stretching of Fenu. Fig. 4 shows the morphology of LDH, Cefo-LDH and the CLF nanohybrid investigated by TEM. Fig. 4a shows a well separated disc shape of the LDH nanoparticles with an average diameter of 50–80 nm. Fig. 4b shows the agglomerated morphology of Cefo-LDH, which may be due to the interaction of the LDH nanoparticles with cefotaxime. A thin layer observed on the surface of LDH indicates the presence of drug on LDH. However, the CLF nanohybrid (Fig. 4c) shows more agglomeration and exhibits a compact structure after the interaction of Cefo-LDH with Fenu polymer. This kind of compact structure may be responsible for the slow release of drug from the nanohybrid (discussed later).
The atomic weight percentages of all the samples obtained from elemental analysis are summarized in Table 2. The Mg/Al ratio in LDH, Cefo-LDH and the CLF nanohybrid was maintained at ∼1.9 each. This indicates that the composition of brucite layers in these samples was the same as predicted from XRD analysis. The presence of nitrogen atoms in LDH indicates that the nitrate anions are intercalated in the brucite layers of LDH, which is in agreement with the XRD & FTIR spectra. The presence of C and S atoms in the EDS spectrum of Cefo-LDH corroborates the loading of cefotaxime on the surface of LDH. In the CLF nanohybrid, we observe higher atomic wt% of C and H compared with LDH and Cefo-LDH due to the presence of Fenu.
| Sample | Mg (wt%) | Al (wt%) | C (wt%) | H (wt%) | N (wt%) | S (wt%) |
|---|---|---|---|---|---|---|
| Cefotaxime | — | — | 38.11 | 3.15 | 13.73 | 10.58 |
| Fenugreek | — | — | 38.91 | 5.94 | 0.00 | 0.00 |
| LDH | 16.29 | 8.62 | 0.00 | 2.80 | 3.60 | 0.00 |
| Cefo-LDH | 12.85 | 6.76 | 7.02 | 3.75 | 3.14 | 3.36 |
| CLF nanohybrid | 4.06 | 2.14 | 18.04 | 4.40 | 2.27 | 1.29 |
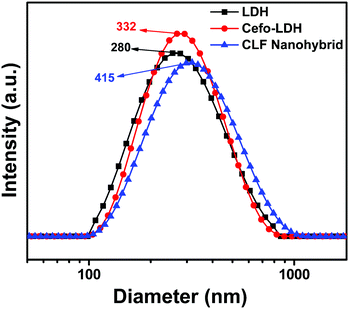
Furthermore, the aqueous stability of the samples was characterized by zeta potential measurement and a particle size analyser. The stable aqueous suspension of LDH, Cefo-LDH and the CLF nanohybrid shows a broad particle size distribution with average hydrodynamic diameters of 280, 332 and 415 nm, respectively (Fig. 5). The increase in the hydrodynamic diameter of Cefo-LDH and the nanohybrid is due to the attachment of cefotaxime and fenugreek on the surface of LDH. The surface charges of LDH, Cefo, Fenu, Cefo-LDH and the CLF nanohybrid dispersions were measured to be +38.0, −17.2, −28.3, 17.4 and −20.6 mV, respectively. The decreased zeta potential of Cefo-LDH with respect to LDH and the charge reversal of the CLF nanohybrid are attributed to the presence of cefotaxime and fenugreek. These values indicate that the attachment between the drug, polymer and LDH is not only via hydrogen bonding (as revealed in FTIR), but also through electrostatic interaction leading to the formation of nanohybrid.
Drug loading and release studies
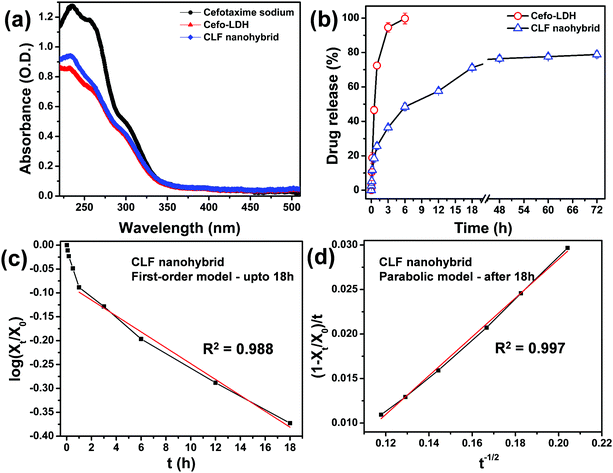
The drug loading and release properties of the as-prepared Cefo-LDH and CLF nanohybrid were investigated using UV-Vis spectroscopy. For the drug loading study, a pure cefotaxime spectrum was used for comparison, as shown in Fig. 6a. The loading amounts of cefotaxime onto Cefo-LDH and the CLF nanohybrid were calculated to be 32.1% and 27.2%, which are equivalent to 85.6 and 72.5 mg g−1, respectively. Fig. 6a shows that the absorption spectra of Cefo-LDH and the nanohybrid are similar to that of pure drug, which infers that there are no structural changes in cefotaxime after conjugation with LDH and the polymer. This structural integrity of the drug molecule is primarily responsible for its activity and thus it can be concluded that the conjugation of drug to the nanohybrid would not affect its antibacterial property in any manner. The decrease in the loading capacity of the nanohybrid might be due to the loss of loosely bound cefotaxime on LDH (Cefo-LDH) during the formation of the nanohybrid.Fig. 6b shows the cumulative release profile of cefotaxime from Cefo-LDH and the CLF nanohybrid under physiological conditions (pH, 7.3). The release profile of Cefo-LDH shows a burst release of Cefo from the surface of LDH with almost 100% of the drug release within 6 h. In contrast, in the CLF nanohybrid, about 15–20% of drug is released in the first hour, which could be the adsorbed drug molecules on the surface of the nanohybrid. The following 18 h shows continuous and increased drug release amounting to ∼70% and attains a plateau, thereafter. By the end of the study, i.e. 72 h, no significant difference is observed (compared with 18 h) and the total drug release amounts to ∼80% (Fig. 6b). This release profile suggests a sustained release pattern. The difference observed in the release behaviour could be attributed to the nature of binding of the Cefo molecules in Cefo-LDH and the CLF nanohybrid. In the case of Cefo-LDH, the drug molecules are weakly bound via hydrogen bonding on the surface of LDH, which results in its fast release. However, in the case of the CLF nanohybrid, the drug is encapsulated between the LDH surface and Fenu polymer leading to slower diffusion of the drug. Such a slow release of antibiotics has multiple advantages and is desirable in treating multidrug resistant bacteria and also effective in avoiding bacterial infections after surgery.18
To understand the drug release behavior of cefotaxime from the CLF nanohybrid, we used the following two kinetic models:33–35
(1) A first-order model, which determines the release behaviour of the systems for which the release rate depends on the amount of drug present in the system and is expressed as:
| log(Xt/X0) = −kdt | (2) |
(2) A parabolic diffusion model, which is used to describe the diffusion-controlled phenomena of drug from the nanohybrid and can be expressed as:
| (1 − Xt/X0)/t = kt−1/2 + a | (3) |
The drug release profile of Cefo from the CLF nanohybrid was fitted to the above equations and the linear correlation coefficient (R2) was evaluated. The profile follows the first-order model (R2 = 0.988, Fig. 6c) during the period of 1–18 h of release (loosely bound drug is released during the first hour) and the parabolic diffusion model (R2 = 0.997, Fig. 6d) afterwards. This can be attributed to two different mechanisms governing the release behaviour of drug from the CLF nanohybrid: (i) dissolution of Cefo from the surface of LDH in the first 18 h and (ii) diffusion of partially intercalated Cefo molecules from the brucite layers due to the swelling of LDH and Fenu polymer. The kinetics study of the CLF nanohybrid suggests that the weekly bound drug molecules via hydrogen bonding show continuous release up to 18 h via a dissolution process. The plateau obtained after 18 h can be attributed to the release of a small quantity of drug molecules, which are intercalated by partial replacement of nitrate ions within the interlayers of LDH and electrostatically interacted on the surface of LDH via a diffusion process.
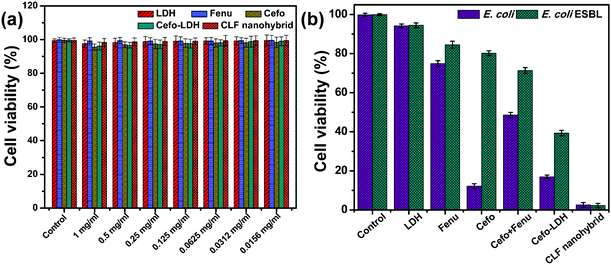
Biocompatibility and antibacterial activity studies
Prior to studying the antimicrobial activity, the biocompatibility of these materials was examined with L929 cells. The graphical representation in Fig. 7a confirms that LDH, Fenu, Cefo individually or as Cefo-LDH and the CLF nanohybrid are biocompatible with L929 cells. All samples up to a concentration of 1 mg mL−1 show >95% cell viability after treatment for 24 h. This observation suggests that all the samples can be used further in either in vivo or clinical settings.Furthermore, the antibacterial activity studies were carried out on E. coli and E. coli ESBL strains. Fig. 7b depicts the antibacterial activity of LDH, Fenu, Cefo, Cefo-Fenu mixture, Cefo-LDH and the CLF nanohybrid. LDH shows negligible cell death on any of the bacteria. Fenugreek polymer exhibits an antibacterial property up to a certain extent, but on comparison it is observed that Fenu shows almost similar antibacterial activity for E. coli sensitive (25.1 ± 1.5%) and resistant E. coli cells (15.5 ± 1.8%). On treating E. coli and E. coli ESBL with pure Cefo (1 mg mL−1), we observed cell viability of 12.2 ± 1.2% and 80.2 ± 1.2%, respectively, indicating the resistance of E. coli ESBL towards pure Cefo. In contrast, the mixture of Cefo and Fenu did not show much of an effect on bacterial cell lines. Intriguingly, only 2.6 ± 1.5% and 2.3 ± 1.1% of E. coli and E. coli EBSL, respectively, were viable upon treatment with the CLF nanohybrid (1 mg mL−1). These encouraging results could be explained by a better understanding of the mode of action of the drug and the cause of resistance in the bacteria.
Cefotaxime sodium belongs to a class of antibiotics that contains the same core 4-member “beta-lactam” ring. This ring is responsible for the activity of the drug as it binds to the cell wall transpeptidases (also known as Penicillin binding proteins) as shown in Fig. 8 and subsequently obstructs cell wall synthesis, causing the bacterial cell to die.36–38 The activity of this drug is largely attributed to the methoxy group on the side chain at the 7α position, which also provides resistance to hydrolysis by a variety of β-lactamases.39 Over a period of time, various strains of bacteria have developed resistance to these drug molecules to survive. One such strain is E. coli ESBL, which is taken as a model strain in our studies. This produces a special class of enzymes, Extended Spectrum Beta-Lactamases (ESBLs), which are primarily responsible for the hydrolysis of the β-lactam ring in the antibiotics. The activity of ESBLs is much extended compared with the regular β-lactamases and they are even capable of hydrolyzing cefotaxime sodium.40 Thus, to combat the resistant bacteria using Cefo-based treatments, we require a stealth carrier which disguises the drug molecule while entering the cell and only releases it to immediately bind with the penicillin binding proteins (Fig. 8). Our results thus show that the LDH–Fenu nanohybrid system is very efficient in loading the drug within its nanostructure, thereby successfully saving it from the action of ESBLs. From the above results, we conclude that Cefo was successfully delivered and exhibited ∼98% cell mortality, even to the resistant cells. This platform thus offers promising bacterial treatments even in the resistant strains.
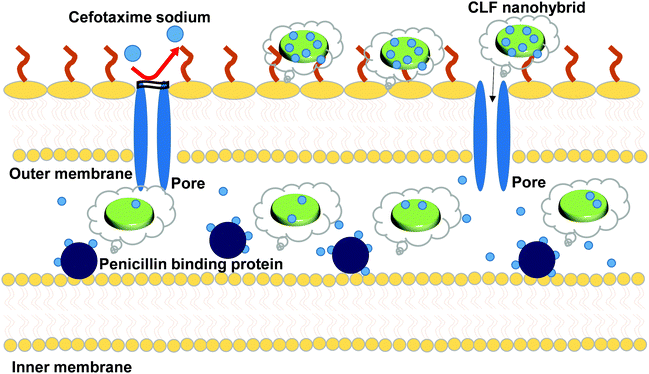 | ||
| Fig. 8 Schematic representation of the antibacterial activity of the CLF nanohybrid against E. coli 949 ESBL cefotaxime-resistant bacterial strains. | ||
Conclusions
A new nanohybrid structure of cefotaxime-loaded LDH–fenugreek nanohybrid was successfully synthesized. The XRD results suggest successful formation of LDH, Cefo-LDH, and the CLF nanohybrid. The XRD results also indicate that the drug molecules were attached to the surface and partially replaced intercalated anions. The hydrogen bonding and electrostatic interaction between cationic LDH and the anionic drug and polymer were confirmed by FTIR and zeta-potential studies. The drug release profiles suggest that there was a burst release of drug from Cefo-LDH, whereas in the case of the CLF nanohybrid, there was early fast release in the initial hour followed by sustained release up to 72 h, which attained saturation after ∼18 h. The understanding of the release kinetics shows that the cefotaxime release was both dissolution and diffusion dependent. The CLF nanohybrid showed biocompatibility with L929 cells up to 1 mg mL−1. Furthermore, about 98% of cell death was shown by the CLF nanohybrid on both cefotaxime-sensitive and -resistant E. coli. To summarize, the newly developed CLF nanohybrid platforms show sustained drug release and exhibit high antibacterial activity even against drug-resistant bacterial strains. These characteristics suggest that CLF nanohybrids can be used as an effective formulation for treating drug-resistant bacteria and other surgical infections.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful to Nanomission, the Department of Science and Technology, India, for their financial support and SAIF, IIT Bombay, for characterization facilities. The authors are also thankful to Mr Deepak Charan for providing fenugreek polymer.References
- M. J. Hajipour, K. M. Fromm, A. Akbar Ashkarran, D. Jimenez de Aberasturi, I. R. D. Larramendi, T. Rojo, V. Serpooshan, W. J. Parak and M. Mahmoudi, Trends Biotechnol., 2012, 30, 499–511 CrossRef CAS PubMed.
- M. E. Falagas and I. A. Bliziotis, Int. J. Antimicrob. Agents, 2007, 29, 630–636 CrossRef CAS PubMed.
- A. J. Huh and Y. J. Kwon, J. Controlled Release, 2011, 156, 128–145 CrossRef CAS PubMed.
- X. Li, S. M. Robinson, A. Gupta, K. Saha, Z. Jiang, D. F. Moyano, A. Sahar, M. A. Riley and V. M. Rotello, ACS Nano, 2014, 8, 10682–10686 CrossRef CAS PubMed.
- S. Z. H. Naqvi, U. Kiran, M. I. Ali, A. Jamal, A. Hameed, S. Ahmed and N. Ali, Int. J. Nanomed., 2013, 8, 3187–3195 CrossRef PubMed.
- M. K. Rai, S. D. Deshmukh, A. P. Ingle and A. K. Gade, J. Appl. Microbiol., 2012, 112, 841–852 CrossRef CAS PubMed.
- S. Siddhartha, B. Tanmay, R. Arnab, S. Gajendra, P. Ramachandrarao and D. Debabrata, Nanotechnology, 2007, 18, 225103 CrossRef.
- Y. Kuthati, R. K. Kankala and C.-H. Lee, Appl. Clay Sci., 2015, 112–113, 100–116 CrossRef CAS.
- V. Rives, M. del Arco and C. Martín, Appl. Clay Sci., 2014, 88–89, 239–269 CrossRef CAS.
- H. S. Panda, R. Srivastava and D. Bahadur, J. Phys. Chem. C, 2009, 113, 9560–9567 CAS.
- E. Ruiz-Hitzky, P. Aranda, M. Darder and G. Rytwo, J. Mater. Chem., 2010, 20, 9306–9321 RSC.
- M. Sui, Y. Zhou, L. Sheng and B. Duan, Chem. Eng. J., 2012, 210, 451–460 CrossRef CAS.
- J. Wang, Q. Liu, G. Zhang, Z. Li, P. Yang, X. Jing, M. Zhang, T. Liu and Z. Jiang, Solid State Sci., 2009, 11, 1597–1601 CrossRef CAS.
- Y. Wang and D. Zhang, Mater. Res. Bull., 2012, 47, 3185–3194 CrossRef CAS.
- Z. Xu, J. Fan, S. Zheng, F. Ma and D. Yin, J. Environ. Qual., 2009, 38 CAS.
- H. Zhang, J. Wang, Y. Chen and Q. Nie, J. Chem. Eng. Data, 2006, 51, 2239–2241 CrossRef CAS.
- L. O. Gentry, Am. J. Med., 1990, 88, S32–S37 CrossRef.
- W. B. Dale and M. H. Peter, Clin. Infect. Dis., 2004, 38, 1706–1715 CrossRef PubMed.
- J. K. Raddatz, B. E. Ostergaard and J. C. Rotschafer, Diagn. Microbiol. Infect. Dis., 1995, 22, 77–83 CrossRef CAS PubMed.
- W. B. Liechty, D. R. Kryscio, B. V. Slaughter and N. A. Peppas, Annu. Rev. Chem. Biomol. Eng., 2010, 1, 149–173 CrossRef CAS PubMed.
- N. D. Işıklı and E. Karababa, J. Food Eng., 2005, 69, 185–190 CrossRef.
- X. Huang, Y. Kakuda and W. Cui, Food Hydrocolloids, 2001, 15, 533–542 CrossRef CAS.
- K. Srinivasan, Food Rev. Int., 2006, 22, 203–224 CrossRef CAS.
- M. Chakraborty, S. Dasgupta, P. Bose, A. Misra, T. Kumar Mandal, M. Mitra, J. Chakraborty and D. Basu, J. Phys. Chem. Solids, 2011, 72, 779–783 CrossRef CAS.
- K. C. Barick, S. Singh, D. Bahadur, M. A. Lawande, D. P. Patkar and P. A. Hassan, J. Colloid Interface Sci., 2014, 418, 120–125 CrossRef CAS PubMed.
- S. Marappa, S. Radha and P. V. Kamath, Eur. J. Inorg. Chem., 2013, 2122–2128 CrossRef CAS.
- Z. P. Xu and H. C. Zeng, J. Phys. Chem. B, 2001, 105, 1743–1749 CrossRef CAS.
- S. Miyata and A. Okada, Clays Clay Miner., 1977, 25, 14–18 Search PubMed.
- R. Anbarasan, W. D. Lee and S. S. Im, Bull. Mater. Sci., 2005, 28, 145–149 CrossRef CAS.
- L. Zhang, J. Wang, J. Zhu, X. Zhang, K. San Hui and K. N. Hui, J. Mater. Chem. A, 2013, 1, 9046–9053 CAS.
- Y.-J. Lin, D.-Q. Li, D. G. Evans and X. Duan, Polym. Degrad. Stab., 2005, 88, 286–293 CrossRef CAS.
- C. Chen, P. Gunawan and R. Xu, J. Mater. Chem., 2011, 21, 1218–1225 RSC.
- P. K. Sahoo, H. S. Panda and D. Bahadur, Mater. Chem. Phys., 2013, 142, 106–112 CrossRef CAS.
- J.-H. Yang, Y.-S. Han, M. Park, T. Park, S.-J. Hwang and J.-H. Choy, Chem. Mater., 2007, 19, 2679–2685 CrossRef CAS.
- Z. Li, Langmuir, 1999, 15, 6438–6445 CrossRef CAS.
- D. Brown, Nat. Rev. Drug Discovery, 2015, 14, 821–832 CrossRef CAS PubMed.
- N. H. Georgopapadakou, Antimicrob. Agents Chemother., 1993, 37, 2045–2053 CrossRef CAS PubMed.
- D. H. Deck and L. G. Winston, in Basic and Clinical Pharmacology, ed. S. B. M. Bertram, G. Katzung, and A. J. Trevor, McGraw-Hill Medical, United states of America, 12th edn, 2012 Search PubMed.
- J. L. LeFrock, R. A. Prince and R. D. Left, Pharmacotherapy, 1982, 2, 174–184 CrossRef CAS PubMed.
- S. Shaikh, J. Fatima, S. Shakil, S. M. D. Rizvi and M. A. Kamal, Saudi J. Biol. Sci., 2015, 22, 90–101 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2018 |