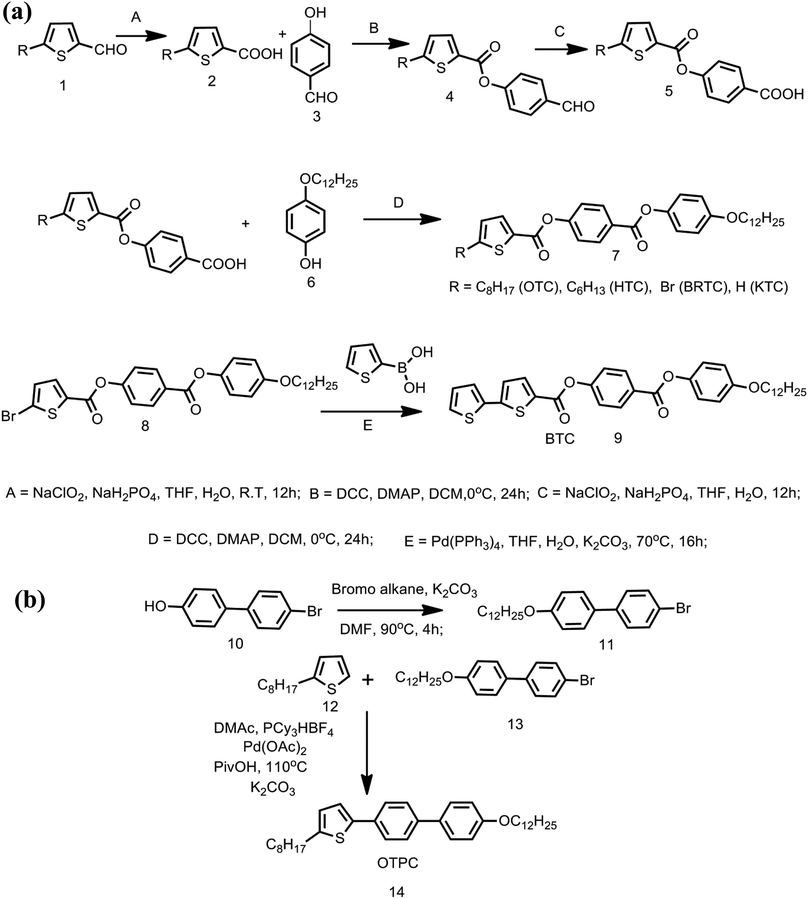
Effect of alkyl chain and linking units on mesophase transitions and molecular order of rod-like thiophene mesogens: 13C NMR investigation†
Y.
Santhosh Kumar Reddy
a,
Nitin P.
Lobo
b and
T.
Narasimhaswamy
 *a
*a
aPolymer Science & Technology, CSIR-Central Leather Research Institute, Adyar, Chennai 600020, India. E-mail: tnswamy99@hotmail.com
bInorganic & Physical Chemistry, CSIR-Central Leather Research Institute, Adyar, Chennai 600020, India
First published on 29th November 2017
Abstract
Thermotropic liquid crystals with π-conjugated cores are increasingly witnessed due to their promising optoelectronic and optophotonic properties. As π-conjugated mesogens are often realized by direct linking of the rings of the core, the mesophase transitions are usually modulated by inserting the required alkyl/alkoxy chains either at a terminal or lateral location. In this work, five mesogens in which thiophene and two phenyl rings are common in the core unit with terminal alkyl/alkoxy chains are investigated to probe the influence of (a) a terminal alkyl chain on the mesophase characteristics, (b) the linking units on the mesophase range and (c) the disparity of thiophene orientational constraints. The mesophase properties reveal that whenever the alkyl chain is located at one end of the molecule, polymesomorphism is observed. The XRD studies reveal layer ordering typical of smectic mesophases with lower temperature and higher order phases, namely SmB and CrE phases. A detailed 13C NMR study of the mesogens in the liquid crystalline phase indicates that the order parameters are governed by the nature of the substitution of thiophene and its linkage with two phenyl rings. Importantly, the 13C NMR data of the terminal chains in the liquid crystalline phase are compared with the recently reported thiophene mesogen in which the n-hexyl chain is located at the lateral position and completely different orientation characteristics were found for the terminal versus lateral chains.
1. Introduction
Thermotropic liquid crystals based on a rod-like core are continuing to lure the attention of investigators because of their structural simplicity and well-known structure–property correlation.1–4 The recent past has witnessed increasing interest in π-conjugated mesogens in which the core is often rod-like in shape.5–8 These molecules are generally synthesized by directly linking the carbocyclic or heterocyclic aromatic rings with suitable terminal chains.9–11 Because of the direct link of the rings of the core, the π-conjugated mesogens conceivably exhibit interesting opto-electronic and opto-photonic properties.12–14 Even though a direct link between the rings of the core favors excellent π-conjugation, due to inherent rigidity of the core unit, the melting and clearing transitions are generally found to be high.15–17 To modulate the mesophase transitions of such mesogens, either normal or branched alkyl chains are introduced at different locations of the molecules.18–24 For instance, in the case of thiophene-based π-conjugated systems, the alkyl chains are often inserted at the 3-position of the thiophene ring so that the molecules show solubility in common solvents at room temperature.25,26 In low molar mass and oligomeric π-conjugated mesogens, the alkyl chains are placed at the terminal position not only to achieve solubility without disrupting the π-conjugation but also to enhance the aspect ratio of the molecule.27,28 Recent research results also emphasized the role of alkyl chains in influencing the property characteristics as well as the morphology of materials.29,30 In other words, depending on the position of the alkyl chain, i.e., the terminal versus lateral location, the mesophase properties dramatically differ.23,31 For instance, placing the alkyl chain at the terminal location of mesogens may favor layer ordering while its location at the lateral position would disrupt the layer ordering.32,33 It is generally noticed that mesogens with two terminal chains which can be independently varied in length can exhibit tilted phases if the lengths are similar.19–22 On the other hand, orthogonal phases such as SmB, crystal B and crystal E are found if the shorter chains are at the terminal location.19–22 In order to understand the influence of alkyl chains on the mesophase properties as well as orientational constraints of thiophene mesogens, hot-stage optical polarizing microscopy (HOPM), differential scanning calorimetry (DSC) and 2D solid state 13C NMR investigations are carried out respectively.In recent years, solid state 13C NMR studies of liquid crystals have been increasingly witnessed owing to the accessibility of anisotropic information in the form of alignment-induced chemical shifts, as well as 13C–1H dipolar couplings that can be extracted from 1D and 2D experiments in the mesophase.34–37 Furthermore, the extracted anisotropic information of the different moieties of the mesogens, i.e. the constituent units of the core as well as the terminal/lateral chains, provide order parameter values which are a direct consequence of the dynamics of the corresponding moieties.38,39 By comparing the orientational order of the constituent rings of the core unit, the molecular shape or topology can be extracted. As a result, a wide range of topologically different mesogens have been subjected to 13C NMR investigation in the mesophase to map the orientational constraints. It is also noticed that on the replacement of the phenyl ring with a thiophene ring in the core, profound changes in molecular shape or topology results which is well reflected through the order parameter values.40,41 Hence, in the present work, 13C NMR is employed for understanding the orientational constraints of phenyl as well as thiophene rings of rod-like mesogens with terminal alkyl/alkoxy chains. Furthermore, the orientational constraints of terminal alkyl chains determined from 13C NMR data are compared with the recently reported mesogen in which the alkyl chain is located on the thiophene at the 3-position i.e. at a lateral location.23 In other words, the influence of orientational constraints on terminal versus lateral location of the alkyl chain is evaluated. For better comprehension, for all the mesogens examined, the core is designed to have two phenyl rings with an additional thiophene ring as part of the mesogenic core. The main focus of the study is to address (i) the influence of the terminal alkyl chain on the mesophase characteristics, (ii) the effect of the linking unit on the mesophase range and (iii) the variation of thiophene orientational constraints with respect to the molecular structure. Towards this goal, the synthesized mesogens are investigated by HOPM, DSC and powder X-ray diffraction (XRD) for phase assignment as well as transition temperatures and by 13C NMR to find the orientational constraints.
2. Experimental section
Materials
5-Hexylthiophene-2-carbaldehyde, 2-hexyl thiophene, 5-bromothiophene-2-carboxaldehyde, 4-(dodecyloxy) phenol, 4-hydroxybenzaldehyde, thiophene 2-boronic acid, potassium carbonate, dichloromethane, tetrakis(triphenylphosphine) palladium (0), tetrahydrofuran, dicyclohexyl carbodiimide, 4-dimethylamino pyridine, phosphorous oxychloride, 4'-bromobiphenyl-4-ol, and 1-bromododecane were purchased from Sigma-Aldrich, USA and used without further purification. Dimethyl formamide, chloroform, potassium hydroxide, sodium chlorite, sodium dihydrogen orthophosphate dihydrate, anhydrous sodium sulfate, acetonitrile, dichloromethane, hexane, ethyl acetate and silica gel (100–200 mesh) were obtained from Merck, India, and 5-octyl thiophene-2-carbaldehyde was obtained from Avra Synthesis, India, and used as received.Instrumental details
Both 1H and 13C NMR spectra were run on a 400 MHz Bruker Avance-III HD spectrometer at room temperature using CDCl3 as a solvent and tetramethylsilane (TMS) as an internal reference. The resonance frequencies of 1H and 13C were 400.23 and 100.64 MHz respectively. The optical polarizing micrographs were taken using a Carl Zeiss axiocam MRC5 Polarizing microscope equipped with a Linkam THMS heating stage with a TMS 94 temperature programmer. The samples were placed between 12 mm glass coverslips and transferred to the heating stage and heated at a programmed heating rate. The photomicrographs were taken using an imager A2M digital camera. The differential scanning calorimetry traces were recorded using a DSC Q 200 instrument at a heating rate of 10 °C per minute in a nitrogen atmosphere. The samples were subjected to two heating and two cooling cycles. The data obtained from the second heating and cooling cycle were considered for discussion. High-Resolution Mass Spectra (HRMS) of the mesogens were recorded using a MICROMASS-Q-TOF mass spectrometer (Fig. S1–S3, ESI†).The synthetic details of the intermediates and final mesogens are given in the ESI.†
Solid state NMR measurements
All the solid-state NMR experiments were conducted under static conditions on a Bruker Avance III HD 400 WB NMR spectrometer (9.4 T) at a frequency of 400.07 MHz for 1H and 100.61 MHz for 13C. A 5 mm double resonance Bruker VTN probe with a horizontal solenoid coil was used for the measurements. At room temperature, the powdered samples were packed in a 4 mm Zirconia rotor with a Zirconia cap and placed inside a 5 mm glass tube. In order to achieve the sample alignment, all the samples were gradually heated to their corresponding isotropic phase and later slowly cooled to the respective mesophases for the measurement. The cross-polarization (CP)42 sequence along with a linear ramp from 100–50% (1H channel) was employed to get 1D static 13C NMR spectra of the mesogens in their respective mesophases. Typically, a contact time τ of 3 ms, number of scans 128, recycle delay of 8 s and 62.5 kHz of radio frequency (RF) field strength on both the 1H and 13C channels during the period τ were used. High-resolution 2D separated local field (SLF)43,44 spectra were obtained by using the SAMPI-4 pulse scheme45 (Fig. S4, ESI†) and its utility for topologically different mesogens was highlighted in our earlier works.23,31,32,36–40,46 The SAMPI-4 experimental parameters for the five mesogens were as follows KTC/HTC/OTC/OTPC/BTC: CP contact time τ = 3 ms, t1 increments = 128, number of scans = 24/28/20/42/28, 1H 90° pulse length = 5 μs and recycle delay = 13 s/15 s/14 s/16 s/13 s (to avoid RF heating effects). The 2D data sets were zero-filled in both the t1 and t2 dimensions to form a 256 × 4096 data matrix. Phase-shifted sine bell multiplication was applied in both dimensions prior to Fourier transformation. For all the experiments, 30 kHz SPINAL-6447 proton decoupling was employed during carbon acquisition. The Bruker BVTB-3500 temperature control unit was used for temperature regulation and the 13C chemical shifts were referenced externally to adamantane methine resonance at 29.5 ppm.Computational details
The gas phase molecular geometries of the representative mesogens were optimized using the density functional theory (DFT)48 based Becke's three-parameter hybrid exchange functional and Lee–Yang–Parr correlation functional (B3LYP) method employing the 6-31G(d) basis set using the Gaussian 09W suite of programs.49Powder X-ray measurements
The powder XRD studies of the un-oriented samples in a Lindemann capillary with a diameter of 1 mm (Hampton Research, Aliso Viejo, CA, USA) were carried out using a PANalytical instrument (DY 1042-Empyrean) operating with a line focused Ni-filtered Cu-Kα (λ = 1.54 Å) beam and a linear detector (PIXcel 3D). The sample temperature was controlled with a precision of 0.1 °C using a heater and a temperature controller (Linkam).503. Results and discussion
Five mesogens in which thiophene is at one end of the core unit and a dodecyloxy chain at the other end are synthesized as per the strategy outlined in Schemes 1a and b and in the ESI.† The two phenyl ring based thiophene containing mesogens are synthesized from 5-substituted thiophene-2-carboxaldehyde. In the case of the mesogen with the bithiophene unit (BTC), the second thiophene is introduced by Suzuki coupling51–54 of the corresponding bromo mesogen with thiophene-2-boronic acid. For the case of OTPC, where no connecting units are present in the core, synthesis was conducted by direct arylation31,55,56 of 2-hexyl thiophene using 4-bromo-4′-(dodecyloxy)-1,1′-biphenyl employing palladium acetate as a catalyst. The main focus of the investigation is to find the influence of terminal chains on the mesophase transition temperatures as well as on the molecular order. Furthermore, the 13C NMR data of terminal chains in the liquid crystalline phase are compared with the recently reported thiophene mesogen in which the n-hexyl chain is located at the lateral position. It is anticipated that by comparing the ordering of the terminal chain on thiophene versus the chain at the lateral location of the reported mesogen, the utility of 13C NMR for understating the dynamics and orientation of the chains in mesogens can be correlated with the molecular structure (Fig. 1). Among the five mesogens i.e. KTC, HTC, OTC, OTPC and BTC, KTC with no substituent on the terminal thiophene serves as a benchmark mesogen for comparing the mesophase properties. The synthesized mesogens are initially characterized by solution 1D 1H and 13C NMR, and later 2D NMR experiments are performed to establish the structural integrity (Fig. S5–S50, ESI†).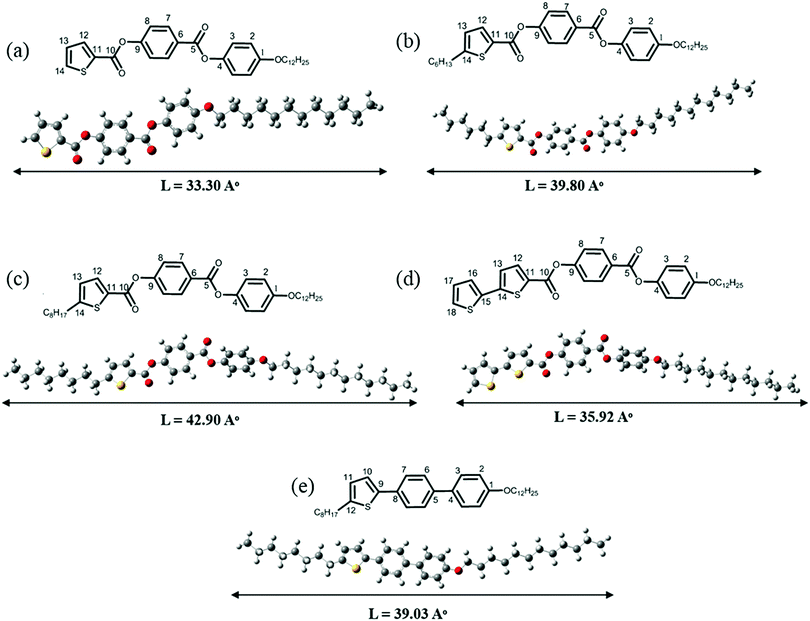 | ||
| Fig. 1 Planar and DFT energy optimized structures of mesogens. (a) KTC, (b) HTC, (c) OTC, (d) BTC and (e) OTPC. | ||
Mesophase identification by HOPM and DSC
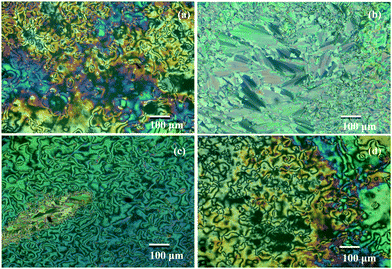
The mesophase assignment of the synthesized molecules is carried out by HOPM, where the formation of texture upon cooling the sample from the isotropic phase is observed (Fig. 2 and 3). In KTC, on cooling from the isotropic phase, birefringent threads (Fig. 2a) are observed at 118 °C. On further cooling, the threaded nematic texture57,58 characteristic of the nematic phase is noticed. The sample experienced crystallization at 85.8 °C. For HTC, the formation of birefringent droplets is seen at 116.8 °C, followed by a threaded nematic texture. At 73 °C transition bars (Fig. 2b) are noticed succeeded by a broken fan texture57,58 indicating the phase transition to the SmC phase. The crystallization of the sample, on the other hand, is noticed at 62.6 °C. For OTC also birefringent threads are witnessed at 125.4 °C on cooling the isotropic phase. Similar to HTC, the phase change at 102 °C is noticed where transition bars59 are observed (Fig. 2c). The appearance of a schlieren texture57,58 at 102.9 °C indicates the SmC phase. On further cooling, the sample crystallizes at 70.8 °C. For BTC, the appearance of the nematic phase is confirmed by noticing birefringent threads (Fig. 2d) at 188.6 °C and crystallization is noted at 109 °C. In the case of OTPC, upon cooling from the isotropic liquid, formation of broken fans characteristic of the SmC phase (Fig. 3a) is observed and on further cooling OTPC shows the SmB and CrE phases (Fig. 3b and c). The crystallization of OTPC is observed at 60 °C.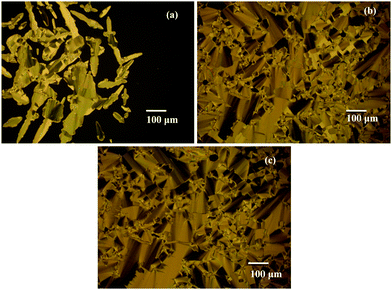 | ||
| Fig. 3 HOPM textures of OTPC on cooling from the isotropic phase. (a) SmC phase at 169 °C, (b) SmB phase at 164 °C, and (c) CrE phase at 160 °C. | ||
The HOPM assignments are further confirmed by DSC investigations where multiple peaks associated with the corresponding phase transitions are clearly noted (Fig. 4 and Tables 1 and 2). On cooling, the transition enthalpy values of the isotropic to nematic phase for the mesogens are in the range of 0.12 to 0.13 kcal mol−1, while that of isotropic to SmC phase is found to be 3.40 kcal mol−1. For OTC the nematic to SmC transition (98.5 °C) showed an enthalpy value of 0.27 kcal mol−1. For OTPC the SmC–SmB transition is observed at 165.6 °C showed an enthalpy value of 0.81 kcal mol−1. For all the mesogens, the enthalpy values of mesophase to crystallization varied in the range of 2–10 kcal mol−1. In the case of KTC only nematic phase is noticed (Fig. 4a). Furthermore, for HTC and OTC (cooling cycle) multiple peaks are noticed with characteristic transition enthalpies (Fig. 4b and c). For OTPC, the SmB to CrE transition which is further confirmed by X-ray diffraction exhibited an enthalpy of 0.75 kcal mol−1. XRD is also used for confirming the SmC–SmB transition. The HOPM and DSC investigations provide data which can be correlated with the molecular structure. The common feature of all the mesogens is the presence of two phenyl rings along with either one or two thiophene rings in the core. Since the alkyl chain is located at one end of the molecule for HTC, OTC and OTPC, polymesomorphism is observed. Furthermore, the presence of smectic phases in HTC, OTC, and OTPC could be attributed to a high aspect ratio of the molecule due to the presence of the alkoxy and alkyl chains at both ends of the molecule.19–22 As a result, the segregation of aliphatic chains from the rigid core units leads to nano segregation resulting in layer order.39,60–62
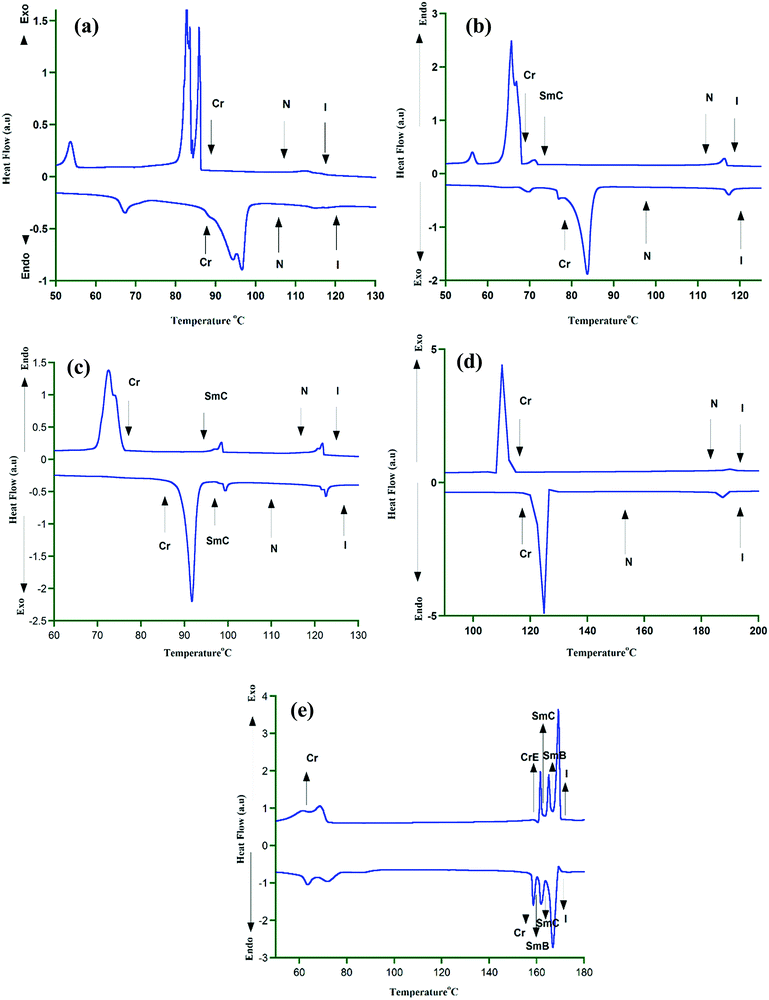 | ||
| Fig. 4 DSC scans of mesogens in heating and cooling cycles. (a) KTC, (b) HTC, (c) OTC, (d) BTC and (e) OTPC. | ||
| Code | Phase transition temperatures and enthalpy values (ΔH kcal mol−1) | ||
|---|---|---|---|
| Cr: crystallization; N: nematic; I: isotropic; SmC: smectic C. | |||
| KTC |
Cr–N 96.6 °C
(4.41) |
N–I 118.9 °C
(0.12) |
|
| HTC |
Cr–N 83.7 °C
(7.13) |
N–I 117.4 °C
(0.20) |
|
| OTC |
Cr–SmC 91.7 °C
(6.94) |
SmC–N 99.4 °C
(0.15) |
N–I 122.5 °C
(0.23) |
| OTPC |
Cr–SmB 162.3 °C
(0.71) |
SmB–SmC 165.6 °C
(0.81) |
SmC–I 170.5 °C
(3.49) |
| BTC |
Cr–N 126.3 °C
(12.81) |
N–I 189.7 °C
(0.20) |
|
| Code | Phase transition temperatures and enthalpy values (ΔH kcal mol−1) | |||
|---|---|---|---|---|
| [ ] indicates monotropic transition. Cr: crystallization; N: nematic; I: isotropic; SmC: smectic C. SmB: smectic B; CrE: crystal E. | ||||
| KTC |
I–N 116.1 °C
(0.12) |
N–Cr 85.8 °C
(1.57) |
||
| HTC |
I–N 116.3 °C
(0.24) |
N–[SmC] 71.2 °C
(0.24) |
[SmC]–Cr 65.6 °C
(9.40) |
|
| OTC |
I–N 121.8 °C
(0.30) |
N–SmC 98.5 °C
(0.27) |
SmC–Cr 72.5 °C
(7.20) |
|
| OTPC |
I–SmC 169.2 °C
(3.40) |
SmC–SmB 165.1 °C
(0.81) |
[SmB]–CrE 161.6 °C
(0.75) |
[CrE]–Cr 68.7 °C
(0.67) |
| BTC |
I–N 188.6 °C
(0.16) |
N–Cr 110.1 °C
(2.16) |
||
Powder XRD studies
The existence of smectic mesophases for OTC and OTPC is ascertained using the powder XRD technique. Fig. 5a shows the XRD scan of OTC in the smectic C phase in the temperature range of 85–95 °C. The XRD profile measured at 95 °C shows an intense sharp reflection at 2θ = 2.8° with a layer spacing (d) of 31.51 Å and a diffuse and broad peak in the wide angle region (4.54 Å). The intense peak in the small angle region is a characteristic of layer ordering commonly observed in smectic mesophases.63 The wide angle broad diffuse reflection, on the other hand, indicates the liquid-like nature of molecules within the layers.58 These observations further indicate the fluid nature of the smectic mesophase. To estimate the tilt angle of the molecules in the fluid smectic phase, the d/L ratio is calculated where L is the energy optimized molecular structure of OTC in the fully extended conformation. The molecular length of OTC is found to be L = 42.90 Å (Fig. 1c) and accordingly, the d/L ratio is 0.72 which suggests that the molecules are tilted in the layers. The estimated tilt angle based on the expression d = L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ is 43°, which is the value usually found for mesogens that show a nematic to smectic C phase.39,64 Thus, the XRD data are in agreement with the HOPM and DSC observations where the appearance of a smectic C phase is noted. Furthermore, the d spacing shows a decreasing trend in OTC with lowering of the temperature suggesting an increase in tilt angle in the SmC phase. The XRD patterns of OTPC measured in different smectic mesophases in the temperature range of 160–175 °C are shown in Fig. 5b. The XRD profile measured at 175 °C shows a sharp and intense signal in the small angle region with 2θ = 2.70° corresponding to a layer spacing (d) of 32.68 Å and a diffuse peak in the wide angle region at 4.58 Å. At 168 °C, the XRD pattern shows changes both in the small angle and wide angle regions. The position of the small angle reflection changed from (2θ) 2.70 to 2.51 while the wide angle peak split into two peaks. At 160 °C, in the wide angle region, multiple peaks are noted while the reflection in the small-angle region is unchanged (2θ = 2.51°). Accordingly, the phases have been identified as SmC, SmB and CrE31,58,65 respectively. The tilt angle in the SmC phase is 33.13° and is determined from d = L
θ is 43°, which is the value usually found for mesogens that show a nematic to smectic C phase.39,64 Thus, the XRD data are in agreement with the HOPM and DSC observations where the appearance of a smectic C phase is noted. Furthermore, the d spacing shows a decreasing trend in OTC with lowering of the temperature suggesting an increase in tilt angle in the SmC phase. The XRD patterns of OTPC measured in different smectic mesophases in the temperature range of 160–175 °C are shown in Fig. 5b. The XRD profile measured at 175 °C shows a sharp and intense signal in the small angle region with 2θ = 2.70° corresponding to a layer spacing (d) of 32.68 Å and a diffuse peak in the wide angle region at 4.58 Å. At 168 °C, the XRD pattern shows changes both in the small angle and wide angle regions. The position of the small angle reflection changed from (2θ) 2.70 to 2.51 while the wide angle peak split into two peaks. At 160 °C, in the wide angle region, multiple peaks are noted while the reflection in the small-angle region is unchanged (2θ = 2.51°). Accordingly, the phases have been identified as SmC, SmB and CrE31,58,65 respectively. The tilt angle in the SmC phase is 33.13° and is determined from d = L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ where L is the length of the molecule (39.03 Å) calculated from the energy optimized structure from DFT calculations (Fig. 1e). Thus, the smectic polymesopmorphism in OTPC is clearly established by XRD studies.
θ where L is the length of the molecule (39.03 Å) calculated from the energy optimized structure from DFT calculations (Fig. 1e). Thus, the smectic polymesopmorphism in OTPC is clearly established by XRD studies.
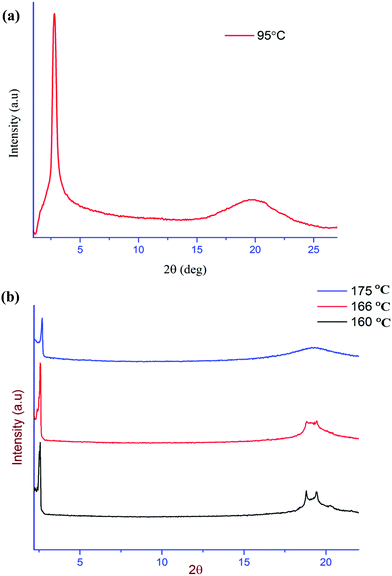 | ||
| Fig. 5 Powder X-ray diffraction profile of the mesogens. (a) OTC in the SmC phase at 95 °C and (b) OTPC at 160 °C, 166 °C, and 175 °C. | ||
Solid state 13C NMR investigations of the mesogens
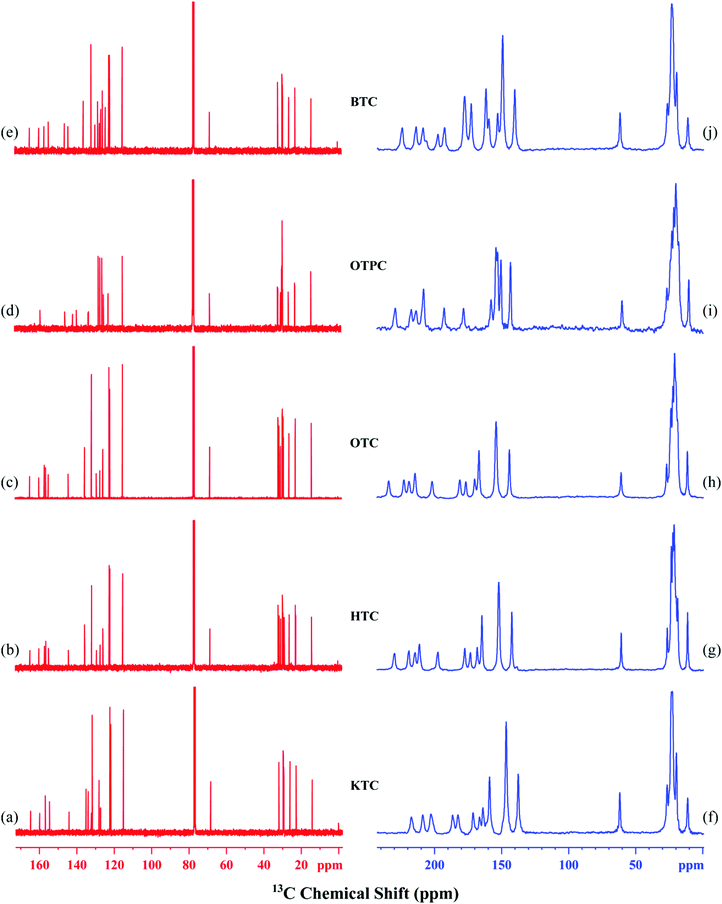
The synthesized mesogens are characterized by 13C NMR in the liquid crystalline phase to determine the orientational order parameters. The common structural feature of the mesogens is the presence of two phenyl rings in the core unit and also the location of thiophene at one end of the molecule. Thus, the determination of the order parameters of the synthesized mesogens would yield information that can be correlated with the individual molecular structure. Furthermore, the presence of flexible hexyl/octyl chains at one end of the mesogen significantly influences the mesophase sequence as well as the ordering of the thiophene rings. Hence, all the mesogens are examined by 1D and 2D 13C experiments in the mesophase to find the alignment-induced chemical shifts (δLC–δiso) as well as the 13C–1H dipolar couplings. For all the molecules, the solution 13C NMR is run in CDCl3 at room temperature and then the static 1D 13C NMR experiments in the liquid crystalline phase. Furthermore, 2D SAMPI-4 experiments were also performed to get the 13C–1H dipolar couplings and using them the orientational order parameters are computed.The solution 13C NMR spectra of the mesogens are shown in Fig. 6a–e. For KTC, HTC and OTC, the solution 13C NMR spectra show 14 lines for the core unit which include phenyl rings, ester carbonyls and a thiophene ring. The chemical shift assignment of the core unit carbons is attempted by comparing the spectra generated from chemsketch and also comparing the literature 13C NMR data.23,31,32,36–40Table 3 lists the chemical shift values of 14 carbons of the core unit of KTC, HTC and OTC. A close look at the chemical shift values indicates that the chemical shifts of C1–C10 are similar for the three mesogens while for C11–C14, a slight change is noticed. Furthermore, the change in the values is attributed to the presence of a hexyl/octyl chain in the HTC and OTC cases as opposed to KTC. For these two mesogens, however, the C11–C14 values are similar since the hexyl/octyl chain is part of the thiophene ring. For BTC and OTPC, not only the number of lines but also the chemical shifts of the core unit carbons are different. This is comprehensible since ester linking units are part of the core for BTC while in OTPC, the rings are connected directly without any linking units. Accordingly, BTC shows 18 lines while OTPC exhibited 12 lines for the core unit carbons. For all the mesogens the chemical shift range for the core unit carbons is from 114.0 to 165.0 ppm.
| C. N. | KTC | Soln | HTC | Soln | OTC | Soln | BTC | Soln | OTPC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soln | 98 °C (nematic) | 85 °C (smectic C) | 96 °C (smectic C) | 130 °C (nematic) | 166 °C (smectic C) | |||||||||||||||
| LC | AIS | DOFb | LC | AIS | DOFb | LC | AIS | DOFb | LC | AIS | DOFb | LC | AIS | DOFb | ||||||
| (ppm) | (ppm) | (ppm) | (kHz) | (ppm) | (ppm) | (ppm) | (kHz) | (ppm) | (ppm) | (ppm) | (kHz) | (ppm) | (ppm) | (ppm) | (kHz) | (ppm) | (ppm) | (ppm) | (kHz) | |
| a Soln: solution chemical shift, LC: mesophase chemical shift; AIS: alignment induced shift; DOF: dipolar oscillation frequencies. b The errors on the values of DOF are about ±0.040 and 0.055 kHz respectively, for protonated and non protonated carbons. | ||||||||||||||||||||
| 1 | 156.9 | 217.3 | 60.4 | 1.29 | 156.2 | 229.6 | 73.4 | 1.51 | 156.9 | 234.0 | 77.1 | 1.71 | 156.9 | 223.9 | 67.0 | 1.41 | 158.8 | 229.1 | 70.3 | 1.75 |
| 2 | 115.1 | 137.5 | 22.4 | 2.40 | 115.1 | 142.3 | 27.2 | 2.83 | 115.1 | 143.9 | 28.8 | 3.15 | 115.1 | 139.9 | 24.8 | 2.59 | 114.9 | 143.1 | 28.2 | 3.33 |
| 3 | 122.3 | 146.5 | 24.2 | 2.13 | 122.3 | 151.8 | 29.5 | 2.54 | 122.3 | 153.9 | 31.6 | 2.85 | 122.3 | 148.9 | 26.6 | 2.36 | 127.7 | 154.0 | 26.3 | 3.65 |
| 4 | 144.1 | 202.7 | 58.6 | 1.25 | 144.2 | 211.3 | 67.1 | 1.42 | 144.2 | 214.3 | 70.1 | 1.61 | 144.1 | 208.3 | 64.2 | 1.38 | 132.9 | 208.2 | 75.3 | 1.63 |
| 5 | 164.7 | 201.5 | 36.8 | 0.47 | 164.8 | 210.3 | 45.5 | 0.70 | 164.8 | 214.3 | 49.5 | 1.17 | 164.7 | 206.2 | 41.5 | 0.53 | 141.4 | 217.3 | 75.9 | 1.71 |
| 6 | 127.3 | 186.5 | 59.2 | 1.26 | 127.2 | 197.4 | 70.2 | 1.52 | 127.1 | 201.6 | 74.5 | 1.75 | 127.3 | 192.2 | 64.9 | 1.42 | 125.7 | 150.3 | 24.6 | 3.54 |
| 7 | 131.8 | 158.9 | 27.1 | 2.20 | 131.7 | 164.6 | 32.9 | 2.65 | 131.7 | 166.7 | 35.0 | 2.95 | 131.8 | 161.4 | 29.6 | 2.47 | 126.9 | 152.8 | 25.9 | 3.63 |
| 8 | 121.8 | 146.5 | 24.7 | 2.13 | 121.9 | 152.1 | 30.2 | 2.58 | 121.9 | 153.9 | 32.0 | 2.85 | 121.8 | 148.9 | 27.1 | 2.36 | 133.1 | 214.2 | 81.1 | 1.70 |
| 9 | 154.6 | 208.7 | 54.1 | 1.27 | 156.9 | 219.0 | 62.1 | 1.53 | 154.8 | 222.6 | 67.8 | 1.74 | 154.6 | 213.5 | 58.9 | 1.40 | 139.4 | 208.6 | 69.2 | 1.25 |
| 10 | 159.9 | 201.5 | 41.6 | 0.47 | 159.9 | 210.3 | 50.4 | 0.70 | 159.9 | 214.3 | 54.4 | 1.17 | 159.7 | 206.2 | 46.5 | 0.53 | 122.5 | 157.7 | 35.2 | 5.69 |
| 11 | 132.3 | 166.4 | 34.1 | 0.71 | 129.1 | 173.2 | 44.1 | 1.10 | 129.1 | 176.4 | 47.3 | 1.28 | 129.7 | 177.3 | 47.6 | 0.76 | 124.9 | 178.1 | 53.2 | 4.25 |
| 12 | 135.1 | 171.2 | 36.1 | 5.29 | 135.4 | 168.1 | 32.7 | 2.45 | 135.4 | 169.8 | 34.4 | 2.47 | 136.0 | 172.4 | 36.4 | 3.73 | 145.6 | 192.6 | 47.0 | 1.19 |
| 13 | 128.1 | 163.9 | 35.8 | 4.38 | 125.6 | 177.4 | 51.8 | 2.80 | 125.6 | 180.9 | 55.3 | 2.74 | 124.3 | 152.7 | 28.4 | 3.00 | ||||
| 14 | 134.0 | 182.5 | 48.5 | 7.20 | 154.8 | 214.3 | 59.5 | 1.51 | 156.2 | 218.4 | 62.2 | 1.61 | 136.1 | 172.4 | 36.3 | 0.98 | ||||
| 15 | 146.0 | 197.2 | 51.2 | 0.93 | ||||||||||||||||
| 16 | 125.6 | 177.3 | 51.7 | 2.10 | ||||||||||||||||
| 17 | 128.1 | 159.2 | 31.1 | 1.85 | ||||||||||||||||
| 18 | 126.6 | 177.3 | 50.7 | 8.90 | ||||||||||||||||
In the liquid crystalline phase, static 1D 13C NMR experiments are carried out to find the alignment-induced chemical shifts of all the mesogens (Fig. 6f–j). For assigning the chemical shifts of the core unit carbons of the mesogens, the literature data of structurally related mesogens are considered.23,31,32,36–40,66 For instance, 13C NMR data from our earlier work on thiophene-based mesogens as well as the data of 4-hexyloxy benzoic acid are utilized for assigning the core unit carbons.23,31,32,36,37,67,68 Further, 2D SAMPI 4 experiments that provide 13C–1H dipolar couplings are also employed for assigning the thiophene ring carbons. It is clear from Table 3 that the mesophase 13C NMR data of KTC, HTC and OTC mesogens are dissimilar from their respective solution NMR chemical shifts. For example, in solution, the chemical shift values of C1–C10 are exactly the same, while for C11–C14, minor variation is noticed. In the mesophase, on the other hand, the differences in the 13C chemical shifts between the core units of KTC, HTC and OTC are due to the fact that the mesophase transition temperatures are not similar. Furthermore, OTC exhibits the SmC phase as opposed to KTC and HTC which show the nematic phase. The calculated alignment induced chemical shift values of the mesogens are listed in Table 3.
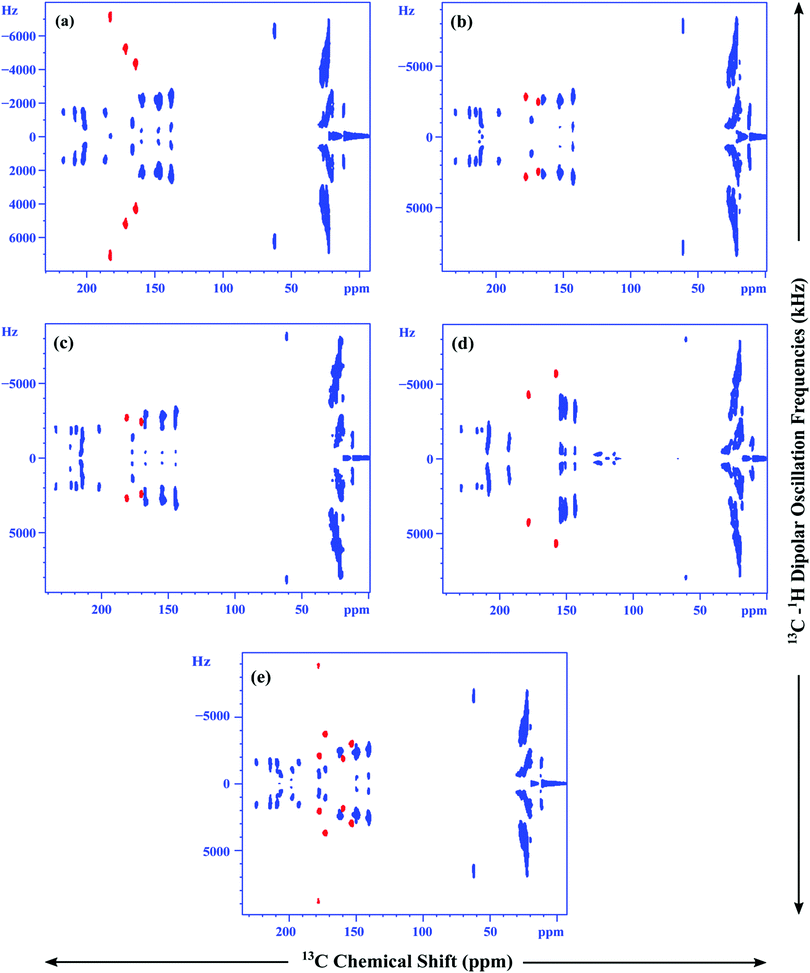
The 2D SAMPI-4 experiments for all the mesogens are performed in the liquid crystalline phase to find the 13C–1H dipolar couplings (Fig. 7). The 1D dipolar slices for aromatic resonances for each mesogen are shown in Fig. S51–S55, ESI.† From the structure point of view, the thiophene ring in KTC is mono substituted. For other cases i.e. HTC, OTC, BTC and OTPC, the thiophene ring is 2,5-disubstituted. This variation in thiophene substitution is clearly reflected in the 13C NMR data in the mesophase. In particular, the 13C–1H dipolar couplings of the thiophene ring show a characteristic trend. In HTC, due to the presence of three CH carbons in thiophene, the CH contours in 2D are clearly separated from the rest of the phenyl ring CH contours owing to the variation in the orientation of the phenyl rings from the thiophene ring with reference to the long axis. For other rings, owing to the 2,5-disubstitution nature of thiophene, only two CH carbons are noticed. The 2D spectra of these mesogens show 13C–1H dipolar couplings which are almost comparable in magnitude with the phenyl ring methine carbons. This particular feature, i.e., a large variation in the 13C–1H dipolar couplings from monosubstituted thiophene to 2,5-disubstituted thiophene is due to the change in thiophene ring orientation with respect to the location of the long axis.23,31 As a result, the 13C–1H dipolar couplings of KTC versus other mesogens dramatically vary as noticed in 2D SAMPI-4. BTC, by virtue of the presence of two thiophene rings, is different from the other mesogens. In the 2D spectrum, five CH contours of the bithiophene unit are expected as seen in the spectrum (Fig. 7). As stated earlier, for OTPC, the linking units such as ester groups are absent and all the rings are directly connected.
Generally, in SAMPI-4 experiment when carbons are coupled to more than one proton, the dipolar coupling information needs to be extracted from the experimental dipolar oscillation frequency.69,70 Considering the established procedure,23,31,32,36–40,46,66–68,71 the relationship between the main orientational order parameters and dipolar couplings for the phenyl or thiophene ring will be expressed by the following equation.72,73
DCH = K[Szz(3cos2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θz − 1)/2 + (Sxx − Syy) (cos2 θz − 1)/2 + (Sxx − Syy) (cos2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θx − cos2 θx − cos2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θy)/2 + Sxz(cos θy)/2 + Sxz(cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θx θx![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θz)] θz)] | (1) |
| Mesogen | Ring | CCH bond angles | S zz | S xx − Syy | Calculated dipolar oscillation frequencies (kHz) | RMSD (kHz) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| θ b | θ c | b | c | d | a | |||||
| a Ring I (KTC/HTC/OTC/BTC/OTPC): a = C1, b = C2, c = C3, d = C4. Ring II (KTC/HTC/OTC/BTC): a = C6, b = C7, c = C8, d = C9. Ring II (OTPC): a = C5, b = C6, c = C7, d = C8. Ring III (KTC/HTC/OTC/BTC): A = C11, B = C12, C = C13, D = C14. Ring III (OTPC): A = C9, B = C10, C = C11, D = C12. | ||||||||||
| KTC | I | 119.8 | 120.8 | 0.549 ± 0.022 | 0.056 ± 0.009 | 2.39 | 2.14 | 1.28 | 1.26 | 0.02 |
| II | 120.4 | 120.7 | 0.551 ± 0.024 | 0.050 ± 0.007 | 2.20 | 2.12 | 1.27 | 1.27 | 0.01 | |
| HTC | I | 119.6 | 120.5 | 0.638 ± 0.023 | 0.062 ± 0.009 | 2.82 | 2.56 | 1.48 | 1.46 | 0.04 |
| II | 120.5 | 120.7 | 0.650 ± 0.019 | 0.070 ± 0.006 | 2.65 | 2.59 | 1.53 | 1.52 | 0.01 | |
| OTC | I | 119.8 | 120.6 | 0.729 ± 0.020 | 0.068 ± 0.007 | 3.13 | 2.87 | 1.69 | 1.67 | 0.05 |
| II | 120.6 | 120.9 | 0.743 ± 0.023 | 0.076 ± 0.008 | 2.95 | 2.85 | 1.74 | 1.73 | 0.01 | |
| BTC | I | 119.8 | 120.6 | 0.601 ± 0.024 | 0.058 ± 0.009 | 2.59 | 2.37 | 1.39 | 1.37 | 0.02 |
| II | 120.4 | 120.8 | 0.607 ± 0.022 | 0.060 ± 0.008 | 2.47 | 2.36 | 1.42 | 1.41 | 0.01 | |
| OTPC | I | 119.5 | 118.6 | 0.748 ± 0.021 | 0.072 ± 0.008 | 3.33 | 3.65 | 1.68 | 1.72 | 0.03 |
| II | 119.1 | 118.8 | 0.757 ± 0.019 | 0.075 ± 0.007 | 3.54 | 3.65 | 1.72 | 1.72 | 0.01 | |
| Mesogen | Ring | β | CCH bond angles | S zz | S xx − Syy | Calculated dipolar oscillation frequencies (kHz) | RMSD (kHz) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| θ B | θ C | θ D | B | C | D | A | ||||||
| KTC | III | 0.5 | 106.9 | 40.0 | 32.2 | 0.510 ± 0.012 | 0.053 ± 0.004 | 5.29 | 4.39 | 7.20 | 0.72 | 0.01 |
| HTC | III | 17.0 | 122.5 | 58.5 | — | 0.753 ± 0.024 | 0.079 ± 0.009 | 2.42 | 2.79 | 1.45 | 1.20 | 0.06 |
| OTC | III | 17.0 | 122.7 | 58.0 | — | 0.830 ± 0.022 | 0.065 ± 0.008 | 2.47 | 2.74 | 1.61 | 1.28 | 0.02 |
| BTC | III | 7.5 | 114.6 | 45.6 | — | 0.550 ± 0.012 | 0.033 ± 0.006 | 3.74 | 2.99 | 0.92 | 0.82 | 0.04 |
| IV | 7.5 | 118.4 | 48.7 | 33.3 | 0.471 ± 0.010 | 0.008 ± 0.003 | 2.18 | 1.84 | 8.86 | 0.74 | 0.05 | |
| OTPC | III | 4.8 | 114.7 | 45.5 | — | 0.809 ± 0.010 | 0.078 ± 0.005 | 5.70 | 4.25 | 1.16 | 1.30 | 0.03 |
The 2D proton encoded local field (PELF)77–79 scheme (Fig. S57, ESI†) is employed for extracting the 13C–1H dipolar couplings of the terminal alkyl chains of the KTC, HTC and OTC mesogens as it provides better resolution for aliphatic carbons. The experimental details are provided in the ESI.† The 2D PELF spectra for the three mesogens are shown in Fig. S58, ESI† and the corresponding extracted 1D dipolar slices are plotted in Fig. S59–S61, ESI.† The 13C NMR data of the terminal alkyl chains for KTC, HTC and OTC are compared with a recently reported mesogen23 where the alkyl chain is located at the lateral position of the thiophene ring. In particular, the alignment-induced chemical shift values of carbons that are linked to the core unit versus the carbons of the chains located at the lateral position can provide qualitative information about the chain ordering. Table S1, ESI† lists the terminal chain carbon chemical shift values of the KTC and HTC mesogens in solution. The chemical shift assignment is completed by comparing the structurally similar compounds in the literature.31 For HTC, the n-hexyl chain carbon chemical shifts are identified by considering the HTC aliphatic region. In other words, the dodecyloxy chain is common for both the mesogens whereas the n-hexyl chain is present in only HTC. For the static 1D 13C NMR, again the chemical shift values of the terminal chain carbons of KTC are used for identifying the n-hexyl chain of HTC. In a recent work,23 we examined a π-conjugated thiophene based mesogen in which the n-hexyl chain is located at the lateral position. In this work, the n-hexyl chain carbons (HTC) in the liquid crystalline phase showed a different trend compared to the terminal chain carbons. In the reported mesogen, the hexyl chain is away from the long axis as it is positioned at the lateral location while the terminal chains are along the long axis. Accordingly, the n-hexyl chain carbons of the reported mesogen showed positive sign for alignment-induced chemical shifts whereas the terminal chain carbons of the same mesogen exhibited the usual negative sign. In the present work, the aliphatic carbons of the HTC mesogen exhibited alignment-induced chemical shifts with a negative sign. This is in sharp contrast to the reported π-conjugated mesogen where the n-hexyl chain carbons in the liquid crystalline phase showed positive sign for the alignment-induced chemical shift values. Interestingly, the solution 13C NMR values of the n-hexyl chain of the reported mesogen as well as the n-hexyl chain of HTC are comparable whereas the 13C chemical shifts in the liquid crystalline phase show a dramatically different trend. In HTC, as the n-hexyl chain is at the terminal location and in the liquid crystalline phase, it orients along the long axis. However, for the reported mesogen,23 the orientation of the hexyl chain which is at the lateral location is different. It is also clear from earlier studies that the methylene carbons that predominantly have trans conformation and orient with respect to the long axis show negative alignment-induced chemical shift values while those with cis conformation and away from the long axis exhibit a positive trend. Using the 13C–1H dipolar couplings the order parameters of the terminal chains of HTC, KTC and OTC are calculated. It is clear from Table S1, ESI† that the order parameter values follow an established style in concurrence with trends observed from alignment induced chemical shift values of terminal chain carbons.
The mesophase transition temperatures of mesogens provide scope for understanding the structure–property relationship. For structurally close mesogens i.e. KTC, HTC and OTC, the melting transition varies between 83–97 °C while the clearing temperatures are in the range of 116–123 °C. With the introduction of an alkyl chain i.e. the hexyl chain for HTC and octyl chain for OTC, an increase in the mesophase range is noticed. Furthermore, due to increase in the length of the molecule, the aspect ratio increases from KTC to OTC, and as a result, the tendency for layer ordering increases. In the case of KTC, only the nematic phase is noticed whereas for HTC and OTC, the smectic C phase is observed as monotropic and enantiotropic respectively in addition to the nematic phase. For the case of BTC, the melting and clearing temperatures are high due to the rigid bithiophene ring. Interestingly, despite increase in the length of the molecule, the smectic phase is not seen in BTC. For OTPC also, the melting and clearing temperatures are quite high similar to BTC but smectic polymesomorphism is noticed. Thus OTPC exhibits enantiotropic SmC, monotropic SmB and crystal E phases. The most interesting feature is that despite the very low mesophase range (8.5 °C), the appearance of smectic polymesomorphism could be attributed to the absence of flexible and polarizable ester units. The high melting and clearing temperatures are a clear indication of the overall rigidity of the mesogen due to the direct link between the phenyl as well as thiophene rings. Thus, insertion of bithiophene produces the highest mesophase range with an enantiotropic nematic mesophase.
4. Conclusion
Five mesogens in which thiophene is at one end of the core and a dodecyloxy chain at the other terminus were synthesized either by Suzuki coupling or direct arylation. The common feature of the synthesized mesogens was the presence of two phenyl rings and a thiophene ring in the core. The HOPM and DSC investigations revealed that mesogens with a terminal alkyl chain exhibited polymesomorphism. The appearance of smectic phases in HTC, OTC and OTPC was due to the high aspect ratio of the molecules since the alkoxy and alkyl chains at both ends result in segregation of the aliphatic chains from the rigid core units as supported by XRD investigation. The orientational order parameters of the constituent rings as well as the terminal alkyl chain were determined from 13C–1H dipolar couplings from 13C NMR studies. For KTC, the order parameters of both the phenyl rings were 0.55 whereas for the thiophene ring, the value was 0.51. In the case of HTC, the phenyl ring order parameter values were 0.638 (ring I) and 0.65 (ring II) and 0.753 for the thiophene ring. For OTC, the values were 0.729 and 0.743 for the phenyl rings and 0.83 for the thiophene ring. The notable feature of the 13C NMR study was about mesogens in which the alkyl chain was present on the terminal thiophene ring, which showed higher order parameters than the corresponding phenyl rings. If the alkyl chain, on the other hand, was absent or if thiophene was connected to other thiophene rings directly, the order parameter of the thiophene was lower than that of the corresponding phenyl rings. These variations were rationalized by considering the location of the long axis with respect to the substituent on the thiophene ring. Importantly, the 13C NMR data of the terminal chains in the liquid crystalline phase were compared with a thiophene mesogen in which the n-hexyl chain is located at the lateral position. The n-hexyl chain carbons of the reported mesogen showed positive sign for alignment-induced chemical shifts whereas the aliphatic carbons of the HTC mesogen exhibited a negative sign. Quite remarkably, the solution 13C NMR values of the n-hexyl chain of the reported mesogen as well as the n-hexyl chain of HTC are similar, whereas a distinct difference in the liquid crystalline phase was noticed. The stark contrasting trend was attributed to their location leading to different kinds of orientation with respect to the long axis. Thus, this study delivered interesting structure–property and orientational information on the core as well as the terminal chains of thiophene and phenyl ring based mesogens.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Dr B. Chandrasekaran, Director, CSIR-CLRI and Prof. K. V. Ramanathan, NMR Research Centre, Indian Institute of Science, Bangalore, India for their support and keen interest in this work. We thank Dr B. V. N. Phani Kumar and Mr R. Ravikanth Reddy, CSIR-CLRI for the help extended in carrying out the 2D solution NMR studies. We are grateful to Ms K. N. Vasudha, Raman Research Institute, Bangalore, India for the powder X-ray measurements. We also thank Dr R. Srinivasan, CSIR-CLRI for extending help in HRMS data interpretation. One of the authors Y. Santhosh Kumar Reddy thanks the Council of Scientific and Industrial Research (CSIR), New Delhi for the grant of a Senior Research Fellowship.References
- G. H. Brown, J. W. Doane and V. D. Neff, A Review of the Structure and Physical Properties of Liquid Crystals, CRC press, Cleveland, Ohio, 1971 Search PubMed.
- G. W. Gray, Molecular Structure and Properties of Liquid Crystals, Academic Press, New York, 1962 Search PubMed.
- R. Mandle, S. J. Cowling and J. W. Goodby, Phys. Chem. Chem. Phys., 2017, 19, 11429–11435 RSC.
- M. Funahashi and T. Kato, Liq. Cryst., 2015, 42, 909–917 CAS.
- Y. Shimizu, K. Oikawa, K. I. Nakayama and D. Guillond, J. Mater. Chem., 2007, 17, 4223–4229 RSC.
- A. Perez, J. L. Serrano, T. Sierra, A. Ballesteros, D. de Saa, R. Termine, U. K. Pandey and A. Golemme, New J. Chem., 2012, 36, 830–842 RSC.
- K. Yao, Y. Chen, L. Chen, D. Zha, F. Li, J. Pei, Z. Liu and W. Tian, J. Phys. Chem. C, 2010, 114, 18001–18011 CAS.
- J. Kim, J. Park, Y. J. Jung, E. C. Kim, T. Ahn, J. W. Ka and M. H. Yi, Liq. Cryst., 2011, 38, 589–599 CrossRef CAS.
- H. Gallardo, R. Cristiano, A. A. Vieira, R. A. W. N. Filho and R. M. Srivastava, Synthesis, 2008, 605–609 CrossRef CAS.
- T. Yasuda, K. Kishimoto and T. Kato, Chem. Commun., 2006, 3399–3401 RSC.
- C. Tschierske and G. Ungar, ChemPhysChem, 2016, 17, 9–26 CrossRef CAS PubMed.
- K. Akagi, in Handbook of Thiophene-based Materials; Applications in Organic Electronics and Photonics, ed. I. F. Perepichka, D. F. Perepichka, John Wiley & Sons, Ltd., Chichester, UK, 2009, ch. 12, p. 497 Search PubMed.
- P. Vlachos, B. Mansoor, M. P. Aldred, M. O’Neill and S. M. Kelly, Chem. Commun., 2005, 2921–2923 RSC.
- H. Iinoand and J. I. Hanna, Polym. J., 2017, 1, 23–30 Search PubMed.
- T. J. Dingemans, N. S. Murthy and E. T. Samulski, J. Phys. Chem. B, 2001, 105, 8845–8860 CrossRef CAS.
- N. A. Zafiropoulos, E. J. Choi, T. Dingemans, W. Lin and E. T. Samulski, Chem. Mater., 2008, 20, 3821–3831 CrossRef CAS.
- N. L. Campbell, W. L. Duffy, G. I. Thomas, J. H. Wild, S. M. Kelly, K. Bartle, M. O’Neill, V. Minter and R. P. Tuffin, J. Mater. Chem., 2002, 12, 2706–2721 RSC.
- M. O’Neill and S. M. Kelly, Adv. Mater., 2011, 23, 566–584 CrossRef PubMed.
- J. W. Goodby, in Handbook of Liquid Crystals: Smectic and Columnar Liquid Crystals, ed. J. W. Goodby, P. J. Collings, T. Kato, C. Tschierske, H. F. Gleeson and P. Raynes, Wiley-VCH, Weinheim, Germany, 2014, vol. 4, pp. 3–41 Search PubMed.
- J. W. Goodby, I. M. Saez, S. J. Cowling, J. S. Gasowska and R. A. MacDonald, et al. , Liq. Cryst., 2009, 36, 567–605 CrossRef CAS.
- J. W. Goodby, R. J. Mandle, E. J. Davis, T. Zhong and S. J. Cowling, Liq. Cryst., 2015, 42, 593–622 CAS.
- J. W. Goodby, Mol. Cryst. Liq. Cryst., 1981, 75, 179–199 CrossRef CAS.
- Y. Santhosh Kumar Reddy, N. P. Lobo, S. Sampath and T. Narasimhaswamy, J. Phys. Chem. C, 2016, 120, 17960–17971 Search PubMed.
- M. Funahashi, T. Ishii and A. Sonoda, ChemPhysChem, 2013, 14, 2750–2758 CrossRef CAS PubMed.
- C. Wang, H. Dong, W. Hu, Y. Liu and D. Zhu, Chem. Rev., 2011, 112, 2208–2267 CrossRef PubMed.
- H. Li, J. Choi and T. Nakanishi, Langmuir, 2013, 29, 5394–5406 CrossRef CAS PubMed.
- K. Oikawa, H. Monobe, J. Takahashi, K. Tsuchiya, B. Heinrich, D. Guillon and Y. Shimizu, Chem. Commun., 2005, 5337–5339 RSC.
- G. Shanker, M. Prehm, M. Nagaraj, J. K. Vij, M. Weyland, A. Eremin and C. Tschierske, ChemPhysChem, 2014, 15, 1323–1335 CrossRef CAS PubMed.
- T. Lei, J. Y. Wang and J. Pei, Chem. Mater., 2014, 26, 594–603 CrossRef CAS.
- J. Mei and Z. Bao, Chem. Mater., 2014, 26, 604–615 CrossRef CAS.
- B. Veeraprakash, N. P. Lobo, T. Narasimhaswamy and A. B. Mandal, Phys. Chem. Chem. Phys., 2015, 17, 19936–19947 RSC.
- B. Veeraprakash, N. P. Lobo and T. Narasimhaswamy, J. Phys. Chem. B, 2015, 119, 15063–15074 CrossRef CAS PubMed.
- H. Kelker and R. Hatz, Hand Book of Liquid Crystals, Verlag Chemie, Weinheim, 1980 Search PubMed.
- R. Y. Dong, Annu. Rep. NMR Spectrosc., 2016, 87, 41–174 CrossRef CAS.
- V. Domenici, M. Lelli, M. Cifelli, V. Hamplova, A. Marchetti and C. A. Veracini, ChemPhysChem, 2014, 15, 1485–1495 CrossRef CAS PubMed.
- K. Rajasekhar Reddy, E. Varathan, N. P. Lobo, S. Easwaramoorthi and T. Narasimhaswamy, J. Phys. Chem. C, 2016, 120, 22257–22269 Search PubMed.
- M. G. Reddy, N. P. Lobo and T. Narasimhaswamy, Liq. Cryst., 2016, 43, 896–909 CrossRef CAS.
- M. G. Reddy, N. P. Lobo, E. Varathan, S. Easwaramoorthi and T. Narasimhaswamy, RSC Adv., 2015, 5, 105066–105078 RSC.
- M. K. Reddy, E. Varathan, N. P. Lobo, B. B. Das, T. Narasimhaswamy and K. V. Ramanathan, J. Phys. Chem. C, 2014, 118, 15044–15053 CAS.
- K. Rajasekhar Reddy, N. P. Lobo and T. Narasimhaswamy, J. Phys. Chem. B, 2016, 120, 6897–6909 CrossRef PubMed.
- T. Narasimhaswamy, D. K. Lee, K. Yamamoto, N. Somanathan and A. Ramamoorthy, J. Am. Chem. Soc., 2005, 127, 6958–6959 CrossRef CAS PubMed.
- J. Schaefer and E. O. Stejskal, J. Am. Chem. Soc., 1976, 98, 1031–1032 CrossRef CAS.
- R. K. Hester, J. L. Ackerman, V. R. Cross and J. S. Waugh, Phys. Rev. Lett., 1975, 34, 993–995 CrossRef CAS.
- A. Ramamoorthy, Y. Wei and D. K. Lee, Annu. Rev. NMR Spectrosc., 2004, 52, 1–52 CrossRef.
- A. A. Nevzorov and S. J. Opella, J. Magn. Reson., 2007, 185, 59–70 CrossRef CAS PubMed.
- S. Kalaivani, T. Narasimhaswamy, B. B. Das, N. P. Lobo and K. V. Ramanathan, J. Phys. Chem. B, 2011, 115, 11554–11565 CrossRef CAS PubMed.
- B. M. Fung, A. K. Khitrin and K. Ermolaev, J. Magn. Reson., 2000, 142, 97–101 CrossRef CAS PubMed.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci and G. A. Petersson, et al., GAUSSIAN 09, revision A.02, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- S. Radhika, M. Monika, B. K. Sadashiva and A. Roy, Liq. Cryst., 2013, 40, 1282–1295 CrossRef CAS.
- J. Yin and S. L. Buchwald, J. Am. Chem. Soc., 2000, 122, 12051–12052 CrossRef CAS.
- G. A. Molander and N. Ellis, Acc. Chem. Res., 2007, 40, 275–286 CrossRef CAS PubMed.
- X. F. Wu, P. Anbarasan, H. Neumann and M. Beller, Angew. Chem., 2010, 49, 9047–9050 CrossRef CAS PubMed.
- J. P. Wolfe and S. L. Buchwald, Angew. Chem., 1999, 38, 2413–2416 CrossRef CAS.
- B. Liegault, D. Lapointe, L. Caron, A. Vlassova and K. Fagnou, J. Org. Chem., 2009, 74, 1826–1834 CrossRef CAS PubMed.
- D. J. Schipper and K. Fagnou, Chem. Mater., 2011, 23, 1594–1600 CrossRef CAS.
- I. Dierking, Textures of Liquid Crystals, Wiley-VCH, Weinheim, Germany, 2003 Search PubMed.
- G. W. Gray and J. W. Goodby, Smectic Liquid Crystals, Heyden, Philadelphia, 1984 Search PubMed.
- M. A. Guillevic, M. E. Light, S. J. Coles, T. Gelbrich, M. B. Hursthouse and D. W. Bruce, J. Chem. Soc., Dalton Trans., 2000, 9, 1437–1445 RSC.
- J. M. Seddon, in Hand Book of Liquid Crystals, ed. D. Demus, J. W. Goodby, G. W. Gray, H. W. Spiess and V. Vill, Wiley-VCH, Weinheim, Germany, 1998, vol. 1, pp. 635–679 Search PubMed.
- N. Kapernaum, C. S. Hartley, J. C. Roberts, F. Schoerg, D. Krueerke, R. P. Lemieux and F. Giesselmann, ChemPhysChem, 2010, 11, 2099–2107 CrossRef CAS PubMed.
- R. A. Reddy and C. Tschierske, J. Mater. Chem., 2006, 16, 907–961 RSC.
- R. W. Date, C. T. Imrie, G. R. Luckhurst and J. M. Seddon, Liq. Cryst., 1992, 12, 203–238 CrossRef CAS.
- M. F. Achard, S. Lecommandoux and F. Hardouin, Liq. Cryst., 1995, 19, 581–587 CrossRef CAS.
- S. Diele, S. Tosch, S. Mahnke and D. Demus, Cryst. Res. Technol., 1991, 26, 809–817 CrossRef CAS.
- M. Kesava Reddy, K. Subramanyam Reddy, K. Yoga, M. Prakash, T. Narasimhaswamy, A. B. Mandal, N. P. Lobo, K. V. Ramanathan, D. S. Shankar Rao and S. K. Prasad, J. Phys. Chem. B, 2013, 117, 5718–5729 CrossRef CAS PubMed.
- N. P. Lobo, M. Prakash, T. Narasimhaswamy and K. V. Ramanathan, J. Phys. Chem. A, 2012, 116, 7508–7515 CrossRef CAS PubMed.
- M. K. Reddy, K. S. Reddy, T. Narasimhaswamy, B. B. Das, N. P. Lobo and K. V. Ramanathan, New J. Chem., 2013, 37, 3195–3206 RSC.
- C. S. Nagaraja and K. V. Ramanathan, Liq. Cryst., 1999, 26, 17–21 CrossRef CAS.
- Z. Gan, J. Magn. Reson., 2000, 143, 136–143 CrossRef CAS PubMed.
- M. G. Reddy, E. Varathan, N. P. Lobo, S. Easwaramoorthi, T. Narasimhaswamy and A. B. Mandal, J. Phys. Chem. C, 2015, 119, 9477–9487 CAS.
- B. M. Fung, Prog. Nucl. Magn. Reson. Spectrosc., 2002, 41, 171–186 CrossRef CAS.
- J. W. Emsley and J. C. Lincon, NMR Spectroscopy Using Liquid Crystal Solvents, Pergamon, Oxford, 1975 Search PubMed.
- B. M. Fung, J. Afzal, T. L. Foss and M. H. Chau, J. Chem. Phys., 1986, 85, 4808–4814 CrossRef CAS.
- M. Concistre, G. De Luca, M. Longeri, G. Pileio and J. W. Emsley, ChemPhysChem, 2005, 6, 1483–1491 CrossRef CAS PubMed.
- N. P. Lobo, B. B. Das, T. Narasimhaswamy and K. V. Ramanathan, RSC Adv., 2014, 4, 33383–33390 RSC.
- M. Hong, A. Pines and S. Calderelli, J. Phys. Chem., 1996, 100, 14815–14822 CrossRef CAS.
- S. Calderelli, M. Hong, L. Emsley and A. Pines, J. Phys. Chem., 1996, 100, 18696–18701 CrossRef.
- R. Soong, P. E. S. Smith, J. Xu, K. Yamamoto, S. C. Im, L. Waskell and A. Ramamoorthy, J. Am. Chem. Soc., 2010, 132, 5779–5788 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: It contains synthetic details, HRMS data, figure of SAMPI-4 pulse sequence, solution 1D and 2D NMR spectra of mesogens, plots of SAMPI-4 1D dipolar slices, thiophene models for orientational order parameter computation, figure of PELF pulse sequence, PELF experimentsl details, data and spectra for aliphatic region of mesogens. See DOI: 10.1039/c7nj03525j |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2018 |