Membrane activity profiling of small molecule B. subtilis growth inhibitors utilizing novel duel-dye fluorescence assay
Abstract
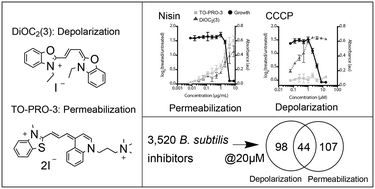
Small molecule disruption of the bacterial membrane is both a challenge and interest for drug development. While some avoid membrane activity due to toxicity issues, others are interested in leveraging the effects for new treatments. Existing assays are available for measuring disruption of membrane potential or membrane permeability, two key characteristics of the bacterial membrane, however they are limited in their ability to distinguish between these properties. Here, we demonstrate a high throughput assay for detection and characterization of membrane active compounds. The assay distinguishes the effect of small molecules on either the membrane potential or membrane permeability using the fluorescent dyes TO-PRO-3 iodide and DiOC2(3) without the need for secondary assays. We then applied this assay to a library of 3520 synthetic molecules previously shown to inhibit growth of B. subtilis in order to determine the frequency of membrane activity within such a biologically active library. From the library, we found 249 compounds that demonstrated significant membrane activity, suggesting that synthetic libraries of this kind do not contain a plurality of membrane active molecules.


 Please wait while we load your content...
Please wait while we load your content...