Design, synthesis and biological evaluation of tacrine-1,2,3-triazole derivatives as potent cholinesterase inhibitors†
Gaochan
Wu
 a,
Yun
Gao
a,
Dongwei
Kang
a,
Yun
Gao
a,
Dongwei
Kang
 a,
Boshi
Huang
a,
Boshi
Huang
 a,
Zhipeng
Huo
a,
Zhipeng
Huo
 a,
Huiqing
Liu
c,
Vasanthanathan
Poongavanam
a,
Huiqing
Liu
c,
Vasanthanathan
Poongavanam
 *b,
Peng
Zhan
*b,
Peng
Zhan
 *a and
Xinyong
Liu
*a
*a and
Xinyong
Liu
*a
aDepartment of Medicinal Chemistry, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, 44 West Culture Road, 250012 Jinan, Shandong, PR China. E-mail: zhanpeng1982@sdu.edu.cn; xinyongl@sdu.edu.cn
bDepartment of Physics, Chemistry, and Pharmacy, University of Southern Denmark, DK-5230 Odense M, Denmark. E-mail: nobelvasanth@gmail.com
cInstitute of Pharmacology, School of Medicine, Shandong University, 44 West Culture Road, 250012 Jinan, Shandong, PR China
First published on 1st December 2017
Abstract
We report herein the design and synthesis of a series of 11 novel tacrine-1,2,3-triazole derivatives via a Cu(I)-catalyzed alkyne–azide 1,3-dipolar cycloaddition (CuAAC) reaction. The newly synthesized compounds were evaluated for their inhibition activity against Electrophorus electricus acetylcholinesterase (AChE) and horse serum butyrylcholinesterase (BChE) as potential drug targets for Alzheimer's disease (AD). Among the designed compounds, compound 8a2 exhibited potent inhibition against AChE and BChE with IC50 values of 4.89 μM and 3.61 μM, respectively. Further structure–activity relationship (SAR) and molecular modeling studies may provide valuable insights into the design of better tacrine-triazole analogues with potential therapeutic applications for AD.
Introduction
Alzheimer's disease (AD) characterized by a progressive loss of memory and other cognitive function impairments is the most common form of senile dementia.1 Environmental, genetic and some endogenous factors play a role during the development of AD. A recent study estimates that over 36 million people are living with AD worldwide with an estimated global economic impact of approximately $605 billion in 2010;2 however, the figure has changed to $818 billion in 2015,3 which indicates an increase of 35%, since 2010. It has been predicted that if an efficient treatment isn't developed by 2050, the number of patients with AD will rise to 70 million.4 The impact of this pathology is tremendous for the patients and their families. According to the Alzheimer's Association,5 AD is the only one without a means of preventing, curing, or slowing its progression among the top 10 causes of death in the United States. Although there has been considerable improvement in the management of AD, due to its complex pathogenesis, a successful AD chemotherapy is still demanding and challenging for medicinal chemists. Given the background, several hypotheses have been proposed which include the cholinergic hypothesis.6–8 This hypothesis suggests that the loss of cholinergic activity is related to the severity of AD.7 Studies have shown that human acetylcholinesterase (hAChE) or ‘pseudocholinesterase’ (also called human butyrylcholinesterase (hBChE)) can quickly break down acetylcholine in the body, produce inactive acetic acid and choline, and thereby terminate nerve impulses,9,10 causing the loss of learning ability and cognition. Thus, reducing acetylcholinesterase (AChE) so as to increase the level of acetylcholine (ACh) could be beneficial to alleviating the symptoms of AD. Meanwhile, there was evidence showing that butyrylcholinesterase (BChE), a serine hydrolase related to AChE, catalyzed the hydrolysis of esters of choline, including ACh, and played important roles in cholinergic neurotransmission.11 That is to say, the inhibition of butyrylcholinesterase (BChE) can raise ACh levels. Consequently, dual inhibition of AChE/BChE may be helpful to improve AD symptoms without remarkable side effects.12 Both hAChE and hBChE structures are very well characterized from X-ray crystallography studies as being small and narrow gorge structures, which are mainly composed of the catalytically active site (CAS) at the bottom of the gorge and the peripheral anionic site (PAS) at the entrance of the gorge.13 In hAChE, the CAS region is mainly composed of Ser203, Glu334, Trp86, Pro446, Phe295, Phe297, Gly120, Gly121, Ala204 and His447 residues.14 Among them, a catalytic triad (Ser203, Glu334, and His447) is mainly involved in the hydrolysis of choline, and the PAS region is composed of Asp74, Tyr72, Tyr124, Trp286 and Arg298 residues, which stabilize the substrate binding. Although hAChE and hBChE have high homology (54% identity and 81% similarity), the hAChE catalytic gorge is lined with fourteen aromatic residues, e.g., Tyr72, Tyr124, Trp286, Phe295, Phe297, and Tyr337, whereas six of these positions were replaced by aliphatic residues (Asn68, Gln119, Ala277, Leu286, Val288, and Ala328) in hBChE.14 Importantly, π-stacking interaction is the main force in the substrate and inhibitor binding, e.g. tacrine's aromatic ring.So far, five cholinergic drugs have been approved for clinical use, namely tacrine, donepezil, rivastigmine, galantamine, and huperzine A, and many others are in various stages of clinical trials. Among them, tacrine, the first drug approved by FDA, which binds at the CAS region,15 is a potent non-selective inhibitor of both hAChE and hBChE and shows large modifiable chemical space. Nonetheless, tacrine suffers from severe side effects, including liver toxicity.16 On the other hand, quinoline derivatives (I–IV)17–20 were reported to possess the ability to specifically bind with the PAS and displayed favorable anti-AD activity (Fig. 1). The purpose of this study was to investigate the effect of dual binding site inhibitors on the PAS and CAS of ChE and to find more effective ChE inhibitors.
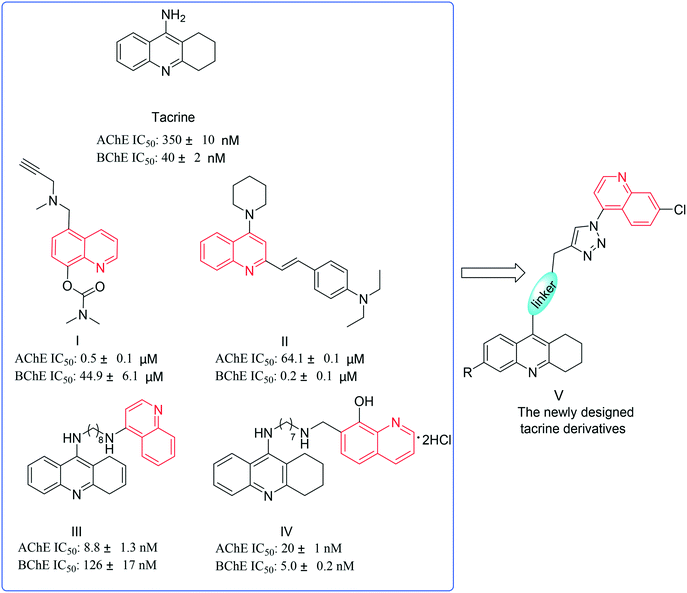 | ||
| Fig. 1 Tacrine and quinoline derivatives with favorable inhibitory activity and the newly designed tacrine derivatives. | ||
Meanwhile, conjugation of functional molecules through 1,2,3-triazole has received great attention in drug discovery for the reason that the 1,2,3-triazole structural motif is a neoclassical bioisostere of amide, which is a widely employed functional group in approved drugs.21 In general, 1,2,3-triazole has several advantages in drug design.22,23 For instance, the triazole has two H-bond acceptors, which is capable of interacting with the biomolecular targets through H-bonding, π–π stacking, and dipole interaction. Besides, the triazole ring exhibits better chemical stability in biological environments, and this improves the pharmacokinetic and toxicity properties. All these advantages make 1,2,3-triazole a remarkable fragment in drug design. Besides, the formation of 1,2,3-triazole compounds via a Cu(I)-catalyzed alkyne–azide 1,3-dipolar cycloaddition (CuAAC) reaction, often referred to as click chemistry, is widely used for the rapid assembly of heterocyclic molecules that may be subsequently used as candidate leads in drug development.24
Considering the above benefits and in continuation of our interest in searching for the anti-AChE and anti-BChE pharmacological effects of tacrine-based PAS–CAS dual binders, in the present study, we describe the synthesis and evaluation of a set of diverse tacrine-quinolines (see Fig. 1) as PAS–CAS dual inhibitors for electric eel AChE and horse serum BChE.
Results and discussion
Chemistry
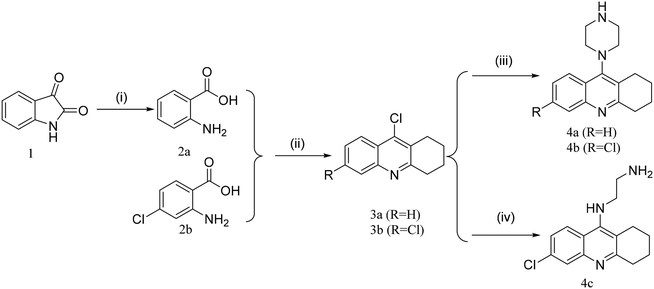
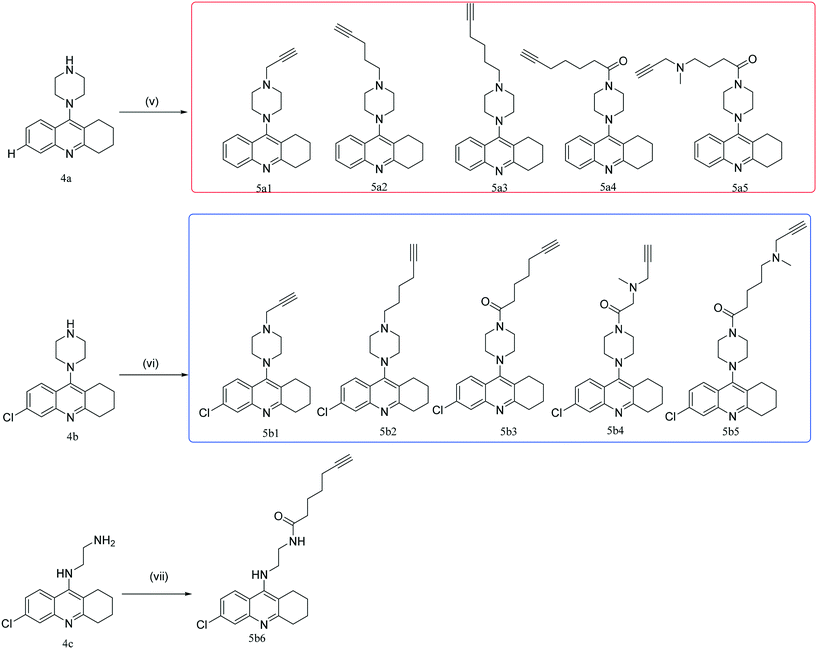
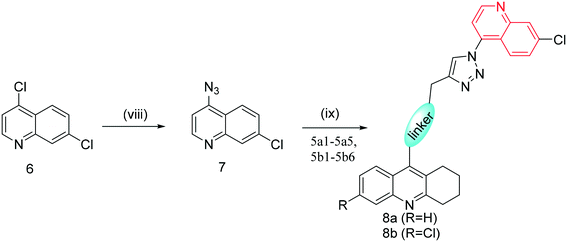
The synthetic route towards the target tacrine-1,2,3-triazole derivatives is outlined in Schemes 1–3. Initially, the commercially available starting material indoline-2,3-dione (1) yielded intermediate 2-aminobenzoic acid (2a) in the presence of sodium hydroxide and 30% hydrogen peroxide. Under the presence of POCl3, compound 2a (or 2b) reacted with cyclohexanone to obtain 9-chloro-1,2,3,4-tetrahydroacridine (3a) or 6,9-dichloro-1,2,3,4-tetrahydroacridine (3b), which were used to yield intermediate 4a (or 4b or 4c) by a nucleophilic substitution reaction. Then, 4a (or 4b or 4c) reacted with different fragments of alkynes giving the key intermediates 5a1–5a5 and 5b1–5b6.25–29 In the meantime, compound 6 was reacted with NaN3 in the presence of NaI to prepare compound 7, followed by its reaction with 5a1–5a5 and 5b1–5b6 to give the target products 8a and 8bvia the CuAAC reaction.30,31 The newly synthesized compounds were characterized by physicochemical and spectral means, and the MS and NMR spectral data were found to be in agreement with the assigned molecular structures.Cholinesterase inhibition evaluation
Based on the reported protocol,32–36 all the newly synthesized compounds (8a1–8a5, 8b1–8b6) were evaluated for their inhibitory activities toward electric eel AChE (Sigma-Aldrich, USA; EC: 3.1.1.7; product information file code: GRO, JWM, RBG, MAM 01/08-1, the download website is shown in the ESI†) and horse serum BChE (Sigma-Aldrich, USA; EC: 3.1.1.8; product information file code: TMG/AJH 10/07, the download website is shown in the ESI†). The authors declare that all procedures were performed strictly in compliance with Chinese relevant laws and institutional guidelines. Tacrine was selected as a reference drug. The initial in vitro screening was performed using a single concentration of 100 μM (see Table 1). Among all the tested compounds, compound 8a2 exhibited the most potent inhibitory activity against AChE and BChE with an inhibition ratio of 78.69% and 91.80% at 100 μM. In addition, compounds 8a3, 8a4, 8b2 and 8b3 showed inhibitory percentages for AChE more than 60% at 100 μM, which is comparable to that of 8a2. Interestingly, compound 8b6 had better selectivity for BChE and displayed an inhibitory percentage of 82.73% against BChE, which is about two times that for AChE (49.37%). After the preliminary screening, compounds 8a2–8a5, 8b2, 8b3, and 8b6 were chosen to determine their IC50 values for cholinesterase. Among them, 8a2 demonstrated the best anti-AChE (IC50 = 4.89 μM) and anti-BChE activity (IC50 = 3.61 μM). Besides, the inhibitory efficacy of 8a3, 8a4, 8b3, and 8b2 against AChE demonstrated IC50 values of 10, 11.07, 18.66 and 19.59 μM, respectively. As for the anti-BChE activity, 8b6 was inferior to 8a2, and its IC50 value was 6.06 μM, followed by 8a5 (IC50 = 61.13 μM) and 8a3 (IC50 = 66.68 μM). However, all the newly synthesized compounds showed weaker inhibitory activity compared to the reference drug tacrine.Preliminary structure–activity relationship (SAR) analysis indicated that the size and length of the linker between the piperazine and triazole motifs are major determinants for their inhibitory activity. An alkane chain with three carbons was the most active one (8a2); whether increasing or shortening the length of the carbon chain would decrease their inhibitory effect on AChE and BChE (inhibition activity: 8a2 > 8a3 > 8a1). Comparing series a with series b, the introduction of a chlorine atom at the C6 position of tacrine would decrease the inhibitory activity (inhibition activity: 8a1 > 8b1, 8a3 > 8b2, 8a4 > 8b3). The current SAR will provide additional information to help in the design of inhibitors with better potency.
Molecular modeling analysis
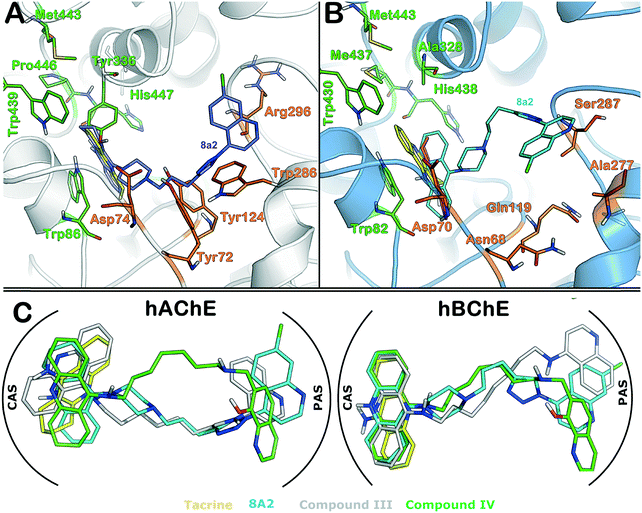
To provide better insight into the binding mode of compound 8a2 to hAChE and hBChE, molecular docking followed by MM-GBSA studies was performed. To this end, compound 8a2 was docked into the hAChE and hBChE ligand-binding site (see the Experimental section for details). The active site of hAChE has previously been well characterized as having a catalytic site (CAS) composed of Trp86, Glu334, Tyr336, Trp439, Met443, Pro446 and His447 residues and a peripheral site or anionic subsite (PAS) composed of Asp74, Tyr72, Tyr124, Trp286 and Arg298 residues. It is shown in the crystal structure of the hAChE complex with tacrine that the acridine ring is involved in the π–π interaction with the indole ring of Trp84. In addition to the acridine ring surrounded by hydrophobic residues, such as Phe330, Tyr334, Tyr442 and Trp434,37 the active site of hBChE is very similar to that of hAChE as having a similar set of residues in both CAS and PAS regions.38 In this study, the binding mode of compound 8a2 is compared in both hAChE and hBChE active sites with tacrine. The MM-GBSA method was employed to rank the best binding poses from the docking solutions, and the binding pose that showed the highest binding affinity in the MM-GBSA calculation for hAChE and hBChE was further discussed. It is clear from Fig. 2 that the binding mode of 8a2 is similar in both cases and that the acridine ring is involved in the π–π interaction with Trp86 (hAChE) and Trp82 (hBChE) as has previously been shown.37Moreover, the piperazine and triazole rings of compound 8a2 showed interactions with Tyr124 and Trp286 in hAChE. However, compound 8a2 binds slightly better in the hBChE structure, for instance, the quinoline ring strongly binds to Ser287 and Pro285 residues through π–π interactions, and importantly, the electronegative chlorine atom also shows a strong interaction with the neighboring residue e.g., Gln71. Besides, the tacrine structure was also prepared in the same manner as compound 8a2 and re-docked into hAChE and hBChE active sites to assess the docking protocol and compare the binding pose to the X-ray crystal structure. Overall, the docking simulation is able to reproduce the same binding pose as was observed in the X-ray crystallography studies with an RMSD of 0.05 Å (cf.Fig. 2 and ESI†). In addition to the comparison of 8a2 with tacrine, the binding mode of compounds III and IV (cf.Fig. 1) was compared to understand the impact of the linker fragment. Compounds III and IV were also docked in the same manner as tacrine into the hAChE and hBChE structures. As clearly seen from Fig. 2C, the binding mode of 8a2 was found to be similar to those of compounds III and IV (see the protein–ligand interaction map in the ESI†) but showed lesser potency as compared to tacrine or compound III/IV. The higher IC50 of 8a2 could be due to not only the rigid triazole and piperazine rings, but also its feature of having more polar atoms, which may lead to an unfavorable contribution to the hydrophobicity of active sites, particularly PAS as reported previously.17,18
Conclusion
To summarize, a concise and efficient synthetic approach has been established to readily access two novel series of tacrine-1,2,3-triazole derivatives in moderate to high yields. Subsequently, the synthesized compounds were screened for electric eel AChE and horse serum BChE inhibition. Among these compounds, compound 8a2 inhibited the AChE and horse serum BChE activity by more than 75% (IC50: 4.89 μM) and 90% (IC50: 3.61 μM) at a concentration of 100 μM, respectively. However, the cholinesterase inhibition activity of compound 8a2 is slightly lower as compared to that of the reference drug tacrine, with its unique binding mode at both CAS and PAS, making it a particularly promising lead for the development of new dual inhibitors of AChE and BChE. Further, molecular docking studies were also performed to understand its binding mode in the AChE and BChE active sites, particularly revealing the importance of quinoline substituents at the C-1 position of the 1,2,3-triazole ring involved in the π–π interaction with Trp286 (hAChE), Ser287 (hBChE) and Pro285 (hBChE) in the PAS region. Meanwhile, the acridine ring is involved in the π–π interaction with Trp86 (hAChE) and Trp82 (hBChE) in the CAS region, the same as the reference compound tacrine.Several works have reported the design of tacrine-based dimers with different linkers (e.g. disulfide bond, alkane, piperazine, amide, etc.), including dual binding site inhibitors on the PAS and CAS against AChE and BChE, which is a promising trend deserving to be explored and studied.39 In this work, we took advantage of CuAAC click chemistry in drug design and the unique physiochemical properties of 1,2,3-triazole, and thus, adapted a linker including the 1,2,3-triazole group introduced by the CuAAC reaction but maintained its unique binding mode like tacrine-based dimers. Although the most potent compound from this series is significantly weaker as compared to tacrine or previously known compounds, e.g., III and IV, a non-aliphatic linker design provides further opportunities to design a smarter linker which facilitates the binding of molecules to CAS and PAS. With this understanding, efforts are further expanded in our laboratory for further lead optimization of tacrine-based triazole analogues as potent cholinesterase inhibitors with the hope to develop promising candidates for AD chemotherapy.
Experimental section
General
All melting points of the compounds were determined using micro melting point apparatus and were uncorrected. 1H-NMR and 13C-NMR spectra were obtained via a Bruker Avance-400 NMR spectrometer in CDCl3. Chemical shifts were expressed in δ units and TMS was used as an internal reference. Mass spectrometry was conducted via a LC Autosampler Device: Standard G1313A instrument. Flash column chromatography was performed using a column packed with Silica Gel 60 (200–300 mesh). Meanwhile, TLC was conducted using Silica Gel GF254, and spots were visualized by irradiation with UV light (λ = 254 nm). Solvents were of reagent grade, which were purified and dried by standard methods when necessary. The reaction solutions were concentrated using a rotary evaporator under reduced pressure conditions.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 1
v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 60 mL). The resulting mixture was stirred at 80 °C for 8 h. After the reaction was cooled to room temperature, water (30 mL) was added to the reaction system. Then, the resulting mixture was extracted with ethyl acetate (3 × 40 mL). The combined organic phase was washed with saturated salt water (3 × 40 mL), dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give the corresponding crude target product, which was purified by flash column chromatography with petroleum ether/ethyl acetate to afford compound 7 as a white solid with a yield of 91%.
1, 60 mL). The resulting mixture was stirred at 80 °C for 8 h. After the reaction was cooled to room temperature, water (30 mL) was added to the reaction system. Then, the resulting mixture was extracted with ethyl acetate (3 × 40 mL). The combined organic phase was washed with saturated salt water (3 × 40 mL), dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give the corresponding crude target product, which was purified by flash column chromatography with petroleum ether/ethyl acetate to afford compound 7 as a white solid with a yield of 91%.
2-Aminobenzoic acid (2a, 1.0 g, 7.3 mmol) and cyclohexanone (0.9 g, 8.8 mmol) were mixed in a flask. Then, POCl3 (10 mL) was added dropwise into the flask at 0 °C. The above resulting mixture was refluxed at 100 °C for 3 h. Then, the mixture was quenched with ice water and neutralized to neutral pH with K2CO3 solution (1 mol L−1). Subsequently, the neutralized solution was extracted with ethyl acetate (3 × 15 mL), and the combined organic phase was washed with saturated salt water (3 × 15 mL), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure to give the corresponding crude target product. Finally, the crude product was purified by flash column chromatography with petroleum ether/ethyl acetate to afford the product compound 3a as a yellow solid. Yield: 88%. Notably, the synthetic procedure for compound 3b (yellow solid; yield: 81.5%) was also the same as that for compound 3a.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 1
v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The resulting mixture was stirred at room temperature for 5 h. Then, the reaction mixture was extracted with ethyl acetate (3 × 10 mL), and the combined organic phase was washed with saturated salt water (3 × 10 mL), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure to give the corresponding crude target product, which was purified by flash column chromatography to afford products 8a1–8a5 (or 8b1–8b6). Yield: 37–92%.
1). The resulting mixture was stirred at room temperature for 5 h. Then, the reaction mixture was extracted with ethyl acetate (3 × 10 mL), and the combined organic phase was washed with saturated salt water (3 × 10 mL), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure to give the corresponding crude target product, which was purified by flash column chromatography to afford products 8a1–8a5 (or 8b1–8b6). Yield: 37–92%.
Acetylcholinesterase and butyrylcholinesterase inhibition assay
The inhibition ability of tacrine derivatives 8a1–8a5 and 8b1–8b6 against electric eel acetylcholinesterase (eeAChE; Sigma-Aldrich, USA; Enzyme Information Download Website:https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Product_Information_Sheet/c3389pis.pdf) and horse serum butyrylcholinesterase (hsBChE; Sigma-Aldrich, USA; Enzyme Information Download Website: https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Datasheet/c1057dat.pdf) was tested using the 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) method with the related assay kit (Keygen, China). DTNB would generate 5-mercapto-2-nitrobenzoic acid – a detectable chromophore at the 405–412 nm range. The final concentrations (100 μM, 20 μM, 4 μM, 0.8 μM, 0.16 μM, and 0.032 μM) of screening compounds were prepared using DMSO. In 96-well plates, 20 μL eeAChE (or hsBChE, diluted to 0.5 U mL−1 using saline) was incubated with the above test compounds of different concentrations at room temperature for 15 min, followed by the addition of relative agents according to the kit instruction. Finally, the chromophore absorbance was examined at the wavelength of 405 nm via a microplate reader (Thermo MK3). The percentage of inhibition was calculated by the comparison of compound-treated to various control incubations.Molecular simulations
The 3D structures of 8a2 and tacrine were built with Maestro and preprocessed using the LigPrep module (v2.5)44 of the Schrödinger modeling software. Preprocessing includes energy minimization using the OPLS_2005 force field and determination of ionization states at pH = 7 (±2).45
Molecular docking simulation
The receptor grid generation module of Glide46 was used to define both peripheral sites (PASs) and catalytic sites (CASs) for the docking experiments in hAChE and hBChE. A set of PAS and CAS residues was used as the centroid of the grid box (of size 20 Å).40,41 Water molecules at the active site were deleted. Glide uses an in-build docking scoring function, resulting in a Glidescore (standard precision). The docking and scoring function parameters and settings used in this study are described in detail elsewhere.47 The best 25 docking solutions for each protein structure were selected for investigation, followed by MM-GBSA calculation.48–50 The Prime MM-GBSA method calculates the binding affinity of the ligand (ΔGbind) by energy minimization procedures. The binding energy of the ligand was extracted from an energy-optimized protein–ligand complex and Prime MM-GBSA using the VSGB 2.0 solvation (implicit) model.50,51 During binding affinity calculation, the program offers the option to treat the ligand and protein as flexible; in this study, a 5 Å region of the protein around the ligand was treated as flexible.Ethical statement
All procedures were conducted in strict accordance with Chinese relevant laws and institutional guidelines (School of Pharmaceutical Sciences, Shandong University). This study was performed in strict accordance with the NIH guidelines for the care and use of laboratory animals (NIH Publication No. 85-23 Rev. 1985) and was approved by the Institutional Animal Care and Use Committee of Shandong University (Shandong, China).Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
Financial support from the Key Research and Development Plan of Shandong Province (No. 2017CXGC1401) and the Major Project of Science and Technology of Shandong Province (No. 2015ZDJS04001) is gratefully acknowledged.References
- D. M. Walsh and D. J. Selkoe, Neuron, 2004, 44, 181–193 CrossRef CAS PubMed.
- A. Wimo, L. Jönsson, J. Bond, M. Prince and B. Winblad, Alzheimers Dement., 2013, 9, 1–11 CrossRef PubMed.
- A. Wimo, M. Guerchet, G. C. Ali, Y. T. Wu, A. M. Prina, B. Winblad, L. Jönsson, Z. Liu and M. Prince, Alzheimers Dement., 2016, 13, 1–7 Search PubMed.
- P. D. Sloane, S. Zimmerman, C. Suchindran, P. Reed, L. Wang, M. Boustani and S. Sudha, Annu. Rev. Public Health, 2002, 23, 213–231 CrossRef PubMed.
- X. Zha, D. Lamba, L. Zhang, Y. Lou, C. Xu, D. Kang, L. Chen, Y. Xu, L. Zhang, A. De Simone, S. Samez, A. Pesaresi, J. Stojan, M. G. Lopez, J. Egea, V. Andrisano and M. Bartolini, J. Med. Chem., 2016, 59, 114–131 CrossRef CAS PubMed.
- A. Enz, R. Amstutz, H. Boddeke, G. Gmelin and J. Malanowski, Prog. Brain Res., 1993, 98, 431–438 CAS.
- P. Davies and A. J. Maloney, Lancet, 1976, 2, 1403 CrossRef CAS.
- M. A. Babu, M. Lakshmi, P. Vasanthanathan and S. G. Kaskhedikar, Indian J. Pharm. Sci., 2005, 67, 1–10 CAS.
- M. Weinstock, CNS Drugs, 1999, 12, 307–323 CrossRef CAS.
- N. H. Greig, T. Utsuki, D. K. Ingram, Y. Wang, G. Pepeu, Q. S. Yu, K. Furukawa, K. Sambamurti, A. Brossi and D. K. Lahiri, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 17213–17218 CrossRef CAS PubMed.
- S. Darvesh, D. A. Hopkins and C. Geula, Nat. Rev. Neurosci., 2003, 4, 131–138 CrossRef CAS PubMed.
- R. M. Lane, S. G. Potkin and A. Enz, Int. J. Neuropsychopharmacol., 2006, 9, 101–124 CrossRef CAS PubMed.
- D. C. Vellom, Z. Radić, Y. Li, N. A. Pickering, S. Camp and P. Taylor, Biochemistry, 1993, 32, 12–17 CrossRef CAS PubMed.
- F. B. M. Isabel, P. Concepción, C. N. Eugenia, P. J. Antonio, U. Paola, G. P. Esther, L. Manuela, G. V. Mercedes and G. Antonio, ChemMedChem, 2010, 4, 828–841 Search PubMed.
- V. Tumiatti, A. Minarini, M. L. Bolognesi, A. Milelli, M. Rosini and C. Melchiorre, Curr. Med. Chem., 2010, 17, 1825–1838 CrossRef CAS PubMed.
- P. B. Watkins, H. J. Zimmerman, M. J. Knapp, S. I. Gracon and K. W. Lewis, JAMA, J. Am. Med. Assoc., 1994, 271, 992–998 CrossRef CAS PubMed.
- P. R. Carlier, E. S. Chow, Y. Han, J. Liu, Y. J. El and Y. P. Pang, J. Med. Chem., 1999, 42, 4225–4231 CrossRef CAS PubMed.
- M. I. Fernándezbachiller, C. Pérez, G. C. Gonzálezmuñoz, S. Conde, M. G. López, M. Villarroya, A. G. García and M. I. Rodríguezfranco, J. Med. Chem., 2010, 53, 4927–4937 CrossRef PubMed.
- H. Zheng, M. Fridkin and M. Youdim, Pharmaceuticals, 2014, 7, 113–115 CrossRef PubMed.
- X. Q. Wang, C. L. Xia, S. B. Chen, J. H. Tan, T. M. Ou, S. L. Huang, D. Li, L. Q. Gu and Z. S. Huang, Eur. J. Med. Chem., 2015, 89, 349–361 CrossRef CAS PubMed.
- S. G. Agalave, S. R. Maujan and V. S. Pore, Chem. – Asian J., 2011, 6, 2696–2718 CrossRef CAS PubMed.
- X. Wang, B. Huang, X. Liu and P. Zhan, Drug Discovery Today, 2016, 21, 118–132 CrossRef CAS PubMed.
- P. Gao, L. Sun, J. Zhou, X. Li, P. Zhan and X. Liu, Expert Opin. Drug Discovery, 2016, 11, 857–871 CrossRef CAS PubMed.
- C. Wang, D. Ikhlef, S. Kahlal, J. Y. Saillard and D. Astruc, Coord. Chem. Rev., 2016, 316, 1–20 CrossRef CAS.
- E. Ö. Özer, O. U. Tan, K. Ozadali, T. Küçükkılınç, A. Balkan and G. Uçar, Bioorg. Med. Chem. Lett., 2013, 23, 440 CrossRef PubMed.
- S. Ariyasu, A. Sawa, A. Morita, K. Hanaya, M. Hoshi, I. Takahashi, B. Wang and S. Aoki, Bioorg. Med. Chem., 2014, 22, 3891–3905 CrossRef CAS PubMed.
- L. Guetzoyan, F. Ramiandrasoa, H. Dorizon, C. Desprez, A. Bridoux, C. Rogier, B. Pradines and M. Perrée-Fauvet, Bioorg. Med. Chem., 2007, 15, 3278–3289 CrossRef CAS PubMed.
- R. Musiol, J. Jampilek, J. E. Nycz, M. Pesko, J. Carroll, K. Kralova, M. Vejsova, J. O'Mahony, A. Coffey and A. Mrozek, Molecules, 2010, 15, 288–304 CrossRef CAS PubMed.
- J. J. Bornstein, T. J. Eckroat, J. L. Houghton, C. K. Jones, K. D. Green and S. Garneau-Tsodikova, MedChemComm, 2011, 2, 406–412 RSC.
- J. A. González-Vera, E. Luković and B. Imperiali, J. Org. Chem., 2009, 74, 7309–7314 CrossRef PubMed.
- J. Zhao and Q. Zhu, Bioorg. Med. Chem. Lett., 2010, 20, 6222–6225 CrossRef PubMed.
- T. Mohamed and P. P. Rao, Bioorg. Med. Chem. Lett., 2010, 20, 3606–3609 CrossRef CAS PubMed.
- T. Mohamed, J. C. K. Yeung, M. S. Vasefi, M. A. Beazely and P. P. N. Rao, Bioorg. Med. Chem. Lett., 2012, 22, 4707–4712 CrossRef CAS PubMed.
- G. L. Ellman, K. D. Courtney, V. Jr. Andres and R. M. Feather-Stone, Biochem. Pharmacol., 1961, 7, 88–95 CrossRef CAS PubMed.
- G. Tin, T. Mohamed, N. Gondora, M. A. Beazely and P. P. N. Rao, MedChemComm, 2015, 6, 1930–1941 RSC.
- I. Khan, S. Bakht, A. Ibrar, S. Abbas, S. Hameed, J. White, U. Rana, S. Zaib, M. Shahid and J. Iqbal, RSC Adv., 2015, 5, 21249–21267 RSC.
- M. Harel, I. Schalk, L. Ehret-Sabatier, F. Bouet, M. Goeldner, C. Hirth, P. H. Axelsen, I. Silman and J. L. Sussman, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 9031–9035 CrossRef CAS.
- M. I. Fernandez-Bachiller, C. Perez, N. E. Campillo, J. A. Paez, G. C. Gonzalez-Munoz, P. Usan, E. Garcia-Palomero, M. G. Lopez, M. Villarroya, A. G. Garcia, A. Martinez and M. I. Rodriguez-Franco, ChemMedChem, 2009, 4, 828–841 CrossRef CAS PubMed.
- A. Milelli, S. A. De, N. Ticchi, H. H. Chen, N. Betari, V. Andrisano and V. Tumiatti, Curr. Med. Chem., 2017, 24 Search PubMed.
- G. Kryger, M. Harel, K. Giles, L. Toker, B. Velan, A. Lazar, C. Kronman, D. Barak, N. Ariel, A. Shafferman, I. Silman and J. L. Sussman, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2000, 56, 1385–1394 CrossRef CAS.
- Y. Nicolet, O. Lockridge, P. Masson, J. C. Fontecilla-Camps and F. Nachon, J. Biol. Chem., 2003, 278, 41141–41147 CrossRef CAS PubMed.
- Maestro, Schrödinger, LLC, New York, NY, 2015 Search PubMed.
- G. M. Sastry, M. Adzhigirey, T. Day, R. Annabhimoju and W. Sherman, J. Comput.-Aided Mol. Des., 2013, 27, 221–234 CrossRef PubMed.
- LigPrep, Schrödinger, LLC, New York, NY, 2015 Search PubMed.
- Epik, Schrödinger, LLC, New York, NY, 2015 Search PubMed.
- R. A. Friesner, J. L. Banks, R. B. Murphy, T. A. Halgren, J. J. Klicic, D. T. Mainz, M. P. Repasky, E. H. Knoll, M. Shelley, J. K. Perry, D. E. Shaw, P. Francis and P. S. Shenkin, J. Med. Chem., 2004, 47, 1739–1749 CrossRef CAS PubMed.
- V. Poongavanam and J. Kongsted, PLoS One, 2013, 8, e73478 CAS.
- Prime, Schrödinger, LLC, New York, NY, 2015 Search PubMed.
- S. Genheden and U. Ryde, Expert Opin. Drug Discovery, 2015, 10, 449–461 CrossRef CAS PubMed.
- P. A. Greenidge, C. Kramer, J. C. Mozziconacci and R. M. Wolf, J. Chem. Inf. Model., 2013, 53, 201–209 CrossRef CAS PubMed.
- J. Li, R. Abel, K. Zhu, Y. Cao, S. Zhao and R. A. Friesner, Proteins, 2011, 79, 2794–2812 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7md00457e |
| This journal is © The Royal Society of Chemistry 2018 |