Geo-material surface modification of microchips using layer-by-layer (LbL) assembly for subsurface energy and environmental applications
Abstract
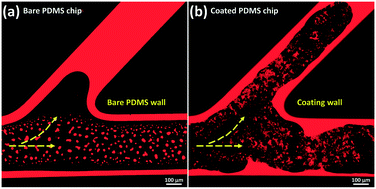
A key constraint in the application of microfluidic technology to subsurface flow and transport processes is the surface discrepancy between microchips and the actual rocks/soils. This research employs a novel layer-by-layer (LbL) assembly technology to produce rock-forming mineral coatings on microchip surfaces. The outcome of the work is a series of ‘surface-mimetic micro-reservoirs (SMMR)’ that represent multi-scales and multi-types of natural rocks/soils. For demonstration, the clay pores of sandstones and mudrocks are reconstructed by representatively coating montmorillonite and kaolinite in polydimethylsiloxane (PDMS) microchips in a wide range of channel sizes (width of 10–250 μm, depth of 40–100 μm) and on glass substrates. The morphological and structural properties of mineral coatings are characterized using a scanning electron microscope (SEM), optical microscope and profilometer. The coating stability is tested by dynamic flooding experiments. The surface wettability is characterized by measuring mineral oil–water contact angles. The results demonstrate the formation of nano- to micro-scale, fully-covered and stable mineral surfaces with varying wetting properties. There is an opportunity to use this work in the development of microfluidic technology-based applications for subsurface energy and environmental research.


 Please wait while we load your content...
Please wait while we load your content...