Micelle-enabled clean and selective sulfonylation of polyfluoroarenes in water under mild conditions†
Abstract
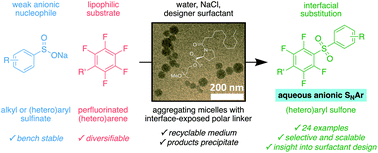
Proline-based designer surfactant FI-750-M has been demonstrated to enable selective nucleophilic aromatic substitution of polyfluoro(hetero)arenes by sulfinate salts in water under mild micellar conditions. Resultant sulfones were obtained from diverse substrates in good yields without side product formation and could usually be purified by simple filtration. The nature of micelles of FI-750-M and the solubility of coupling partners in different micellar regions has been supported by dynamic light scattering, cryo-TEM, and DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...