Excitation of H2O at the plasma/water interface by UV irradiation for the elevation of ammonia production
Abstract
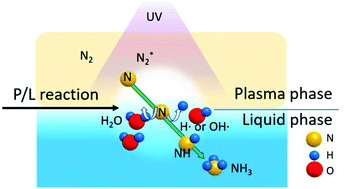
Ammonia is well known to be a very important chemical substance for human life. Simultaneously, the conventional ammonia production process needs pure nitrogen and pure hydrogen. Hydrogen has been produced from either liquid natural gas (LNG) or coal. In this study, water is used as a direct hydrogen source for ammonia production, thereby obviating the need for catalysts or water electrolysis. We have studied and developed a plasma/liquid interfacial reaction (P/L reaction) that can be used to produce ammonia from air (nitrogen) and water at ambient temperature and pressure, without any catalysts. In this study, the P/L reaction entails enhanced ultraviolet (UV) irradiation of the surface of the water phase. The nitrogen plasma/water interface reaction locus can produce ammonia. In contrast, the vacuum ultraviolet (VUV) irradiated interface reaction locus produces increased amounts of ammonia. In a spin trap electron spin resonance (st-ESR) experiment, large amounts of atomic H (H˙) were produced by UV irradiation, especially by VUV irradiation. The derived H˙ effectively enhanced the P/L reaction rate.
- This article is part of the themed collection: 2018 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...