Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Application of pulsed electric fields to tomato fruit for enhancing the bioaccessibility of carotenoids in derived products
Sandra
González-Casado
,
Olga
Martín-Belloso
 ,
Pedro
Elez-Martínez
,
Pedro
Elez-Martínez
 and
Robert
Soliva-Fortuny
and
Robert
Soliva-Fortuny
 *
*
Department of Food Technology, Agrotecnio Center, University of Lleida, Av. Alcalde Rovira Roure 191, 25198 Lleida, Spain. E-mail: rsoliva@tecal.udl.cat; Fax: +34 973 702596; Tel: +34 973 702678
First published on 7th March 2018
Abstract
The application of pulsed electric fields (PEFs) to whole tomatoes is proposed as a pre-processing treatment to obtain purees with high health-related properties. Tomato fruit was subjected to different electric field strengths (0.4, 1.2 and 2 kV cm−1) and number of pulses (5, 18 and 30 pulses). Tomatoes were stored at 4 °C for 24 h after PEF processing and then ground and mixed with 5% olive oil. The resulting tomato-based product was subjected to in vitro gastrointestinal digestion. PEF treatments significantly increased the amount and bioaccessible fraction of carotenoids in the derived product. Treatments conducted at 2 kV cm−1 and 30 pulses led to the greatest increase in the concentration of any of the carotenoids studied in tomato-based products. The amount of carotenoids incorporated into the micellar phase was increased in the products obtained from PEF-treated tomatoes, especially after the application of 5 pulses at 2 kV cm−1. Under such treatment conditions, the bioaccessibility of lycopene, δ-carotene, β-carotene, γ-carotene and lutein was increased by 132%, 2%, 53%, 527% and 125%, respectively. Therefore, the application of PEFs as a pre-treatment could be considered as a promising technology to obtain tomato derivatives with high antioxidant potential.
1. Introduction
Several epidemiological studies have concluded that the increased consumption of tomatoes and their derivatives is associated with lower rates of age-related macular degeneration and cataract, better immune response, and a lower risk of cardiovascular disease and certain types of cancer.1 These beneficial properties of tomatoes are often related to the presence of high amount of carotenoids, which are lipophilic phytonutrients that are efficient singlet oxygen quenchers, and hence effective antioxidants.2,3Over the last few decades, pulsed electric fields (PEFs) have emerged as a non-thermal technology with several potential applications in food processing. During PEF treatments, food tissues are subjected to an external electrical field for a few microseconds, which induces local structural changes and eventually causes the breakdown of cell membranes.4 Based on this process, called electropermeabilization, PEFs can be exploited for different goals, e.g. the inactivation of microorganisms5 and quality-related enzymes6 and the improvement of both osmotic dehydration processes7 and extraction of intracellular metabolites.8 In addition, the use of PEF treatments has been recently proposed to induce stress reactions in metabolically active plants at the cellular level.9,10 These stress reactions are thought to activate a wide range of metabolic pathways that lead to the accumulation of secondary metabolites involved in the defense response of plants under both biotic and abiotic stress conditions.11,12 In a previous study, Vallverdú-Queralt et al. (2013)13 proposed the application of PEF treatments to increase the amount of carotenoids in tomato fruit as well as in tomato juices obtained from PEF-treated fruit. However, the effect of PEF processing on the bioaccessibility of carotenoids in fruit, vegetables and their derived products has been scarcely studied.
Bioaccessibility may be defined as the fraction of an ingested compound that is released from the food matrix during digestion thus becoming accessible for intestinal uptake.14 The bioaccessible fraction of bioactive compounds is more relevant than the total amount present in the original food.15 In this regard, the determination of bioaccessibility is accepted as an effective procedure to study the nutritional and functional potential of food products.16 As already reported by many authors, carotenoids’ bioaccessibility is influenced by several factors. The matrix in which the compound is embedded, the content of dietary fat and fibre, and the type and amount of carotenoid compounds as well as their particle size and distribution are among the most relevant factors.17 Carotenoids are naturally present in chromoplasts, which have been suggested to act as important physical structural barriers hindering the micellarization of these lipophilic compounds.18 Several studies have reported that processing operation that disrupts the food matrix may facilitate their release, transformation and absorption during digestion, thus increasing their bioaccessibility.3,19,20 Since PEF treatments produce an electric breakdown of the cell membranes, it is thought that this technology could favour the release of carotenoids from the food matrix. In this regard, Rodríguez-Roque et al. (2015)21 reported that the application of high intensity PEF treatments enhanced the bioaccessibility of some carotenoids in fruit-based beverages. To the best of our knowledge, no information is available regarding the bioaccessibility of carotenoids in a processed plant-based food product as affected by the application of PEF treatments to intact raw fruit. Therefore, the main objective of this work was to evaluate changes in the concentration and bioaccessible fraction of individual carotenoids in the derived products obtained from tomato fruit treated under different PEF conditions.
2. Materials and methods
2.1. Reagents
All digestive enzymes (α-amylase from porcine pancreas, pepsin from hog stomach, pancreatin from porcine pancreas, and bile extract porcine), magnesium hydroxide carbonate, calcium chloride dehydrate, magnesium chloride hexahydrate (99%), magnesium sulphate hexahydrate, sodium chloride, sodium bicarbonate and sodium phosphate were purchased from Sigma-Aldrich (St Louis, MO, USA). Potassium chloride was obtained from Panreac (Barcelona, Spain). Monopotassium phosphate was purchased from Acros Organics (New Jersey, USA). Butyl hydroxytoluene (BHT), hydrochloric acid and sodium hydroxide were acquired from Scharlau Chemie S.A. (Barcelona, Spain). Lycopene, γ-carotene, δ-carotene, β-carotene, lutein, phytofluene and phytoene were obtained from Carote-Nature (Ostermundigen, Switzerland).2.2. Tomato fruit
Tomatoes (Lycopersicum esculentum cv. Raf) were purchased from a local market (Lleida, Spain) at the turning stage (10–30% of the tomato surface showing red colour). They were stored at 12 °C until they reached the red-ripe stage, meaning that more than 90% of the surface had turned red.22 Before PEF treatments, tomatoes were rinsed with tap water and dried carefully with paper cloth.2.3. Pulsed electric field treatments
PEF treatments were carried out using a bench scale system (Physics International, San Leandro, CA, USA) which delivers monopolar exponential-wave pulses from a capacitor of 0.1 μF at a frequency of 0.1 Hz. PEF treatments were conducted at 20 °C in a treatment chamber consisting of a parallelepiped methacrylate container (200 × 80 mm) equipped with two parallel stainless steel electrodes separated by a gap of 10 cm. A batch of tomatoes (2 pieces of fruit; ca. 260 g per batch) were placed into the treatment chamber filled with tap water (conductivity of 0.03 S m−1). According to previous studies, different electric field strengths (0.4, 1.2 and 2 kV cm−1) and number of pulses (5, 18 and 30 pulses) were applied at a low frequency of 0.1 Hz. The specific energy input corresponding to each treatment was calculated according to Soliva-Fortuny et al. (2017)23 and is displayed in Table 1. Each PEF treatment was repeated twice. PEF-treated tomatoes were immediately stored at 4 °C for 24 h, as previously described by Vallverdú-Queralt et al. (2013).10| Electric field strength (kV cm−1) | Number of pulses | Specific energy input (kJ kg−1) |
|---|---|---|
| 0 | 0 | Untreated |
| 0.4 | 5 | 0.02 |
| 0.4 | 18 | 0.06 |
| 0.4 | 30 | 0.09 |
| 1.2 | 5 | 0.14 |
| 1.2 | 18 | 0.50 |
| 1.2 | 30 | 0.83 |
| 2 | 5 | 0.38 |
| 2 | 18 | 1.38 |
| 2 | 30 | 2.31 |
2.4. Preparation of a tomato-based product
Twenty-four hours after PEF processing, tomatoes from each PEF treatment batch were cut into pieces and ground for 90 seconds using a blender (Solac Professional Mixter BV5722, Spain). Then, 5% olive oil (w/w) was added and mixed using a grinder (Moulinex DP700G-BP, France) for 10 seconds in order to obtain a homogeneous puree. Untreated tomatoes were used as reference. An aliquot of this homogenate was directly freeze-dried and stored at −40 °C until carotenoid extraction in order to determine the carotenoid profile in the non-digested samples. A second fraction was subjected to in vitro gastrointestinal digestion.2.5. In vitro gastrointestinal digestion
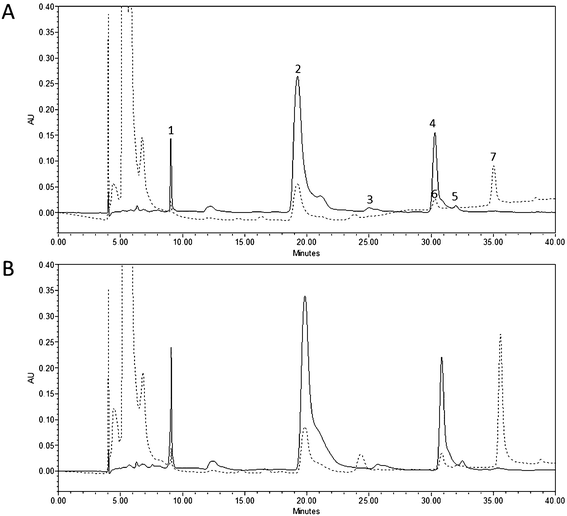
Each product obtained from either untreated or PEF-treated tomatoes was subjected to static in vitro gastrointestinal digestion consisting of oral, gastric and small intestinal phases, following the methodology previously proposed by Tagliazucchi et al. (2012)24 and Rodríguez-Roque et al. (2013)25 with slight modifications.To quantify the amount of carotenoids released from the tomato matrix and incorporated into the micellar fraction, the small intestinal digesta was centrifuged at 33.768g for 20 min at 4 °C (Beckman Coulter, Avanti J-26 XP, California, United States). The aqueous-micellar phase was collected and filtered using Whatman No. 1 filter paper and subsequently a cellulose filter (1–3 μm pore size, 70 mm diameter, Filtros Anoia S.A., Barcelona, Spain) in order to eliminate any crystalline carotenoids or undigested lipids. The micellar phase was eventually freeze-dried and stored at −40 °C until carotenoid extraction.
2.6. Quantification of carotenoids
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane (4
hexane (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v). The mixture was homogenized using an Ultraturrax (T-25 Basic, IKA®-Werke GmbH & Co., Staufen, Germany) for 2 min in an ice-bath. Then, it was filtered once through Whatman No. 1 paper under reduced pressure. The residue was re-extracted with a second volume of 10 mL of ethanol
3 v/v). The mixture was homogenized using an Ultraturrax (T-25 Basic, IKA®-Werke GmbH & Co., Staufen, Germany) for 2 min in an ice-bath. Then, it was filtered once through Whatman No. 1 paper under reduced pressure. The residue was re-extracted with a second volume of 10 mL of ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane (4
hexane (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v). The mixture was again filtered and the pellet was washed twice with 5 mL ethanol and once with 5 mL hexane. All the filtrates were placed in an amber round-bottom flask and rotoevaporated (rotovapor R-3000, BUCH, Switzerland) at 45 °C for 15 min to dryness. The residue was then saponified by adding 10 mL of methanolic KOH 0.5 M + 0.1% BHT (v/w) and 10 mL diethyl ether, under a N2 atmosphere for 30 min with continuous agitation. Afterwards, the saponified extract was placed in an amber decanting funnel and washed twice with 25 mL of 10% NaCl solution and thrice with 25 mL distilled water. The aqueous phase was discarded after each wash. The organic phase was collected and rotoevaporated at 45 °C for 20 min to dryness. The residue was dissolved with 4 mL diethyl ether and placed in an amber glass vial. Finally, the solvent was evaporated under a N2 atmosphere and stored at −40 °C until analysis. All the extractions were repeated twice. Prior to HPLC injection, extracts from non-digested and digested samples were reconstituted with 1 mL and 200 μL methylene chloride, respectively, and passed through a 0.45 μm filter.
3 v/v). The mixture was again filtered and the pellet was washed twice with 5 mL ethanol and once with 5 mL hexane. All the filtrates were placed in an amber round-bottom flask and rotoevaporated (rotovapor R-3000, BUCH, Switzerland) at 45 °C for 15 min to dryness. The residue was then saponified by adding 10 mL of methanolic KOH 0.5 M + 0.1% BHT (v/w) and 10 mL diethyl ether, under a N2 atmosphere for 30 min with continuous agitation. Afterwards, the saponified extract was placed in an amber decanting funnel and washed twice with 25 mL of 10% NaCl solution and thrice with 25 mL distilled water. The aqueous phase was discarded after each wash. The organic phase was collected and rotoevaporated at 45 °C for 20 min to dryness. The residue was dissolved with 4 mL diethyl ether and placed in an amber glass vial. Finally, the solvent was evaporated under a N2 atmosphere and stored at −40 °C until analysis. All the extractions were repeated twice. Prior to HPLC injection, extracts from non-digested and digested samples were reconstituted with 1 mL and 200 μL methylene chloride, respectively, and passed through a 0.45 μm filter.
2.7. Bioaccessibility calculation
The bioaccessibility of each individual compound was determined using eqn (1). The results were expressed as percentage.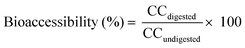 | (1) |
2.8. Statistical analysis
Statistical analyses were performed using JMP Pro v.12.0.1 software (SAS Institute, Cary, NC, USA). Results are reported as mean ± standard deviation (n = 8). Analysis of variance (ANOVA) followed by the Tukey–Kramer post hoc test was carried out in order to establish statistical differences among mean values. A correlation analysis was performed using Pearson's test. The significance level was set at 5%.3. Results and discussion
3.1. Carotenoid profile in the derived product obtained from PEF-treated tomato fruit
The application of PEFs to tomato fruit as a pre-processing treatment significantly increased (p < 0.05) the concentration of total and individual carotenoids in the subsequently obtained derived product (Table 2). Carotenoid concentrations were shown to be significantly influenced (p < 0.0001) by the specific energy input applied to intact fruit. The highest carotenoid concentrations in the tomato product (52% greater than in purees produced from untreated fruit) were obtained when applying 30 pulses at 2 kV cm−1 (specific energy of 2.31 kJ kg−1). These results are consistent with those reported in a previous paper,27 where a 50% increase in the concentration of coloured carotenoids was observed under the same treatment conditions in comparison with untreated tomato fruit.| Specific energy input (kJ kg−1) | Electric field strength (kV m−1) | Number of pulses | Carotenoid concentration (μg kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phytofluene | Phytoene | Lycopene | δ-Carotene | Lutein | γ-Carotene | β-Carotene | Total carotenoids | |||
| Values are expressed as the mean ± standard deviation (n = 8). Different letters within the same column mean significant differences (p < 0.05). | ||||||||||
| Untreated | — | — | 622 ± 30 e | 664 ± 10 e | 4000 ± 192 e | 81 ± 2 f | 240 ± 12 e | 112 ± 11 c | 3000 ± 53 bc | 8718 ± 288 f |
| 0.02 | 0.4 | 5 | 778 ± 37 d | 841 ± 18 d | 3920 ± 188 e | 93 ± 4 e | 278 ± 44 de | 122 ± 14 bc | 3129 ± 131 ab | 9162 ± 353 f |
| 0.06 | 0.4 | 18 | 815 ± 15 d | 889 ± 21 d | 4853 ± 233 de | 104 ± 4 e | 269 ± 14 e | 125 ± 10 bc | 3220 ± 71 a | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 275 ± 138 de 275 ± 138 de |
| 0.09 | 0.4 | 30 | 791 ± 14 d | 919 ± 47 d | 4400 ± 211 de | 96 ± 2 e | 273 ± 5 de | 137 ± 9 ab | 3216 ± 39 a | 9833 ± 176 e |
| 0.14 | 1.2 | 5 | 1391 ± 71 ab | 1748 ± 106 ab | 5046 ± 243 b | 153 ± 5 b | 367 ± 25 ab | 144 ± 14 a | 3221 ± 95 a | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 071 ± 102 c 071 ± 102 c |
| 0.38 | 2 | 5 | 1411 ± 90 ab | 1650 ± 112 b | 5960 ± 286 a | 155 ± 9 ab | 270 ± 31 e | 78 ± 7 d | 2930 ± 70 d | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 341 ± 430 bc 341 ± 430 bc |
| 0.5 | 1.2 | 18 | 1305 ± 68 b | 1717 ± 80 ab | 5240 ± 252 b | 117 ± 20 d | 337 ± 30 bc | 111 ± 7 c | 2818 ± 82 cd | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 097 ± 847 bc 097 ± 847 bc |
| 0.83 | 1.2 | 30 | 1143 ± 71 c | 1354 ± 124 c | 4560 ± 219 cd | 134 ± 4 c | 316 ± 22 cd | 88 ± 6 d | 2913 ± 118 cd | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 524 ± 238 d 524 ± 238 d |
| 1.38 | 2 | 18 | 1381 ± 97 ab | 1705 ± 88 b | 5888 ± 283 a | 157 ± 4 ab | 386 ± 27 a | 120 ± 10 bc | 3158 ± 69 a | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 796 ± 518 ab 796 ± 518 ab |
| 2.31 | 2 | 30 | 1438 ± 45a | 1846 ± 55 a | 6072 ± 292 a | 165 ± 5 a | 382 ± 38 ab | 134 ± 14 ab | 3233 ± 111 a | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 271 ± 265 a 271 ± 265 a |
The concentration of individual carotenoids of the product obtained from PEF-treated tomatoes was differently affected depending on the PEF treatment intensity and the carotenoid compound at stake (Table 2). The concentrations of phytoene and phytofluene were increased by 178% and 131%, respectively, in the derived products obtained from tomatoes treated with 30 pulses at 2 kV cm−1 (2.31 kJ kg−1) compared to those obtained when processing untreated fruit. An increase in lycopene concentration was also noted in purees obtained from PEF-treated tomatoes, ranging from 4400 ± 211 μg kg−1 to 6072 ± 292 μg kg−1. The maximum lycopene concentration was attained when tomatoes were subjected to the most intense PEF treatment (2.31 kJ kg−1), leading to a 1.5-fold increase in relation to the product prepared from untreated tomatoes. Although the increase in the carotenoid content in the derived product obtained from PEF-treated tomatoes may be difficult to explain due to the complexity of biological systems, a well-established explanation for this observation is the activation of the secondary metabolism in the fruit as a strategy to overcome unfavourable conditions.11,23,26 The significant correlation found between lycopene and its precursors, phytoene and phytofluene (p < 0.0001; r = 0.7612 and r = 0.7661, respectively), have led us to hypothesize that PEF treatments may activate the transcription of genes encoding enzymes such as phytoene synthase (SIPSY) and carotenoid isomerase enzyme (CRTISO), responsible for the biosynthesis of these carotenoids in the fruit, as previously suggested by Vallverdú-Queralt et al. (2013).10
After lycopene, the carotenoid biosynthetic pathway is divided into two branches. One route, ε,β-branch, produces δ-carotene, α-carotene and lutein. The alternative pathway, β,β-branch, leads to the synthesis of γ-carotene, β-carotene and β,β-xanthophylls, providing precursors for the synthesis of abscisic acid (ABA).28 In this study, the concentration of δ-carotene, lutein, γ-carotene and β-carotene significantly increased (p < 0.05) in the derived product obtained from tomatoes subjected to PEF treatments (Table 2). The concentration of δ-carotene in the product obtained from untreated tomatoes was 80.8 ± 1.6 μg kg−1, and increased by 104% when treatments delivering a specific energy input of 2.31 kJ kg−1 (30 pulses at 2 kV cm−1) were applied. Under these treatment conditions, the concentration of β-carotene rose by 8% in comparison with the same product obtained from untreated tomatoes. The concentration of γ-carotene increased from 112 μg kg−1 to 144 μg kg−1 when treatments were conducted at 1.2 kV cm−1 and 5 pulses (0.14 kJ kg−1). Lutein showed its maximum concentration (386 ± 27 μg kg−1) in the products obtained from tomatoes subjected to 18 pulses at 2 kV cm−1 (1.38 kJ kg−1), which corresponds to a 61% increase. The lesser increase found in the amount of carotenoids related to β,β-branch (γ-carotene and β-carotene) after the application of PEF treatments allows hypothesising that these carotenoids could be finally converted into ABA. This phytohormone is considered as a carotenoid-derived compound that is predominantly involved in abiotic stress adaptation.28,29 In this regard, Manzi et al. (2016)30 have previously reported a decreased pool of β,β-carotenoids together with a significant ABA accumulation when plants were subjected to stressful conditions. However, further studies focusing on quantifying ABA and/or intermediary carotenoids in PEF-treated samples should be carried out in order to confirm this hypothesis.
Furthermore, the increased concentrations of total and individual carotenoids in the derived products obtained from PEF-treated tomatoes could be related not only to the activation of the secondary metabolism but also to the improvement of the extraction of intracellular components as a result of the electropermeabilization of cell membranes.8,31,32 It is well established that PEF treatments are related to selective damage of biological cell membranes, which may produce reversible or irreversible pore formation depending on the treatment intensity. The maximum carotenoid content found in the product obtained from tomatoes subjected to the highest specific energy input suggests that carotenoid extraction could be facilitated by irreversible pore formation induced by PEFs. This is consistent with the results reported in a previous study, which reveal that treatments with electric field strengths ranging from 0.4 to 2 kV cm−1 significantly impacted the texture and colour of tomato tissues, while leading to an increase in the content of carotenoids.27 Similar results were obtained by Luengo et al. (2014),32 who found that the extraction of carotenoids from tomato peels was improved after the application of PEF treatments with an electric field strength of below 5 kV cm−1.
3.2. Bioaccessibility of individual carotenoids of the derived product obtained from PEF-treated tomato fruit
The bioaccessibility of total and individual carotenoids of the derived product obtained from untreated and PEF-treated tomatoes greatly depended on the compound at stake and the PEF treatment conditions. Carotenoid bioaccessibility widely varied depending on the individual compound analyzed, ranging from 2.4 to 43.2% (Table 3). Among all the carotenoids analysed, lycopene exhibited the lowest bioaccessibility values (2.4 ± 0.2%). In contrast, phytoene and phytofluene had the highest bioaccessibilities, exhibiting values of 43.2 ± 5.0% and 23.8 ± 3.0%, respectively. This fact was already observed by Mapelli-Brahm et al.33 who concluded that not only the hydrophobicity, but also the structure and shape of the molecule, characterized by its chain length and number of conjugated double bonds, play an important role in the bioaccessibility of carotenoids.| Specific energy input (kJ kg−1) | Electric field strength (kV m−1) | Number of pulses | Bioaccessibility (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phytofluene | Phytoene | Lycopene | δ-Carotene | Lutein | γ-Carotene | β-Carotene | Total carotenoids | |||
| Values are expressed as the mean ± standard deviation (n = 8). Different letters within the same column mean significant differences (p < 0.05). | ||||||||||
| Untreated | — | — | 23.8 ± 3.0 a | 43.2 ± 5.0 a | 4.1 ± 0.7 de | 18.0 ± 1.1 a | 9.5 ± 1.7 c | 5.5 ± 0.3 f | 14.1 ± 1.7 bcd | 12.4 ± 1.1 bc |
| 0.02 | 0.4 | 5 | 13.5 ± 3.2 cde | 27.5 ± 4.8 cd | 2.4 ± 0.2 f | 1.4 ± 1.2 bcd | 8.7 ± 0.7 cd | 6.0 ± 0.7 ef | 10.9 ± 0.9 e | 8.8 ± 1.1 de |
| 0.06 | 0.4 | 18 | 17.6 ± 0.8 bc | 32.5 ± 5.8 bcd | 2.5 ± 0.4 f | 13.5 ± 2.7 b | 6.5 ± 1.1 d | 5.8 ± 2.0 f | 11.9 ± 1.4 de | 10.3 ± 2.4 bcde |
| 0.09 | 0.4 | 30 | 21.3 ± 4.0 a | 36.1 ± 3.4 b | 7.5 ± 1.4 b | 18.5 ± 2.3 a | 14.9 ± 2.7 b | 11.3 ± 1.8 bc | 14.5 ± 1.5 bc | 13.1 ± 2.5 b |
| 0.14 | 1.2 | 5 | 10.4 ± 0.9 e | 13.4 ± 1.6 e | 9.7 ± 0.8 a | 9.2 ± 0.6 d | 13.6 ± 1.8 b | 10.1 ± 1.7 cd | 15.3 ± 0.8 b | 11.9 ± 0.4 bcd |
| 0.38 | 2 | 5 | 21.4 ± 3.5 ab | 30.4 ± 5.9 bcd | 9.5 ± 1.3 a | 18.4 ± 2.5 a | 21.4 ± 2.2 a | 34.5 ± 1.9 a | 21.6 ± 2.3 a | 17.1 ± 2.5a |
| 0.5 | 1.2 | 18 | 10.3 ± 2.4 e | 16.1 ± 1.7 e | 3.7 ± 0.5 ef | 10.2 ± 1.2 cd | 6.5 ± 1.3 d | 5.5 ± 0.7 f | 12.7 ± 0.6 cde | 8.1 ± 1.2 e |
| 0.83 | 1.2 | 30 | 15.5 ± 2.4 cd | 25.1 ± 3.1 d | 5.6 ± 1.4 cd | 12.0 ± 1.1 bcd | 8.1 ± 1.7 cd | 8.6 ± 1.5 de | 14.9 ± 1.4 bc | 12.0 ± 2.3 bcd |
| 1.38 | 2 | 18 | 13.9 ± 2.1 cde | 18.4 ± 1.8 e | 7.0 ± 1.1 bc | 12.7 ± 2.4 bc | 10.5 ± 1.9 c | 12.9 ± 2.5 b | 15.0 ± 2.1 bc | 11.4 ± 2.5 bcd |
| 2.31 | 2 | 30 | 12.4 ± 0.9 de | 14.9 ± 0.9 e | 6.3 ± 0.9 bc | 10.7 ± 1.9 bcd | 8.3 ± 0.9 cd | 10.6 ± 1.6 bcd | 14.0 ± 1.6 bcd | 9.5 ± 2.0 cde |
The effect of the application of PEF treatments to whole tomatoes on the bioaccessibility of carotenoids of the subsequently obtained tomato-based product is shown in Table 3. The concentration of carotenoids released from the food matrix into the micellar phase was significantly influenced (p < 0.0001) by their initial concentration in the tomato-based product, apart from β-carotene and γ-carotene (p > 0.05). The statistical analysis displayed that the amount of each individual carotenoid found in the micellar fraction of the digested tomato product was strongly influenced (p < 0.0001) by the electric field strength applied to whole tomatoes. Nevertheless, the number of pulses did not appear to exert a major effect on the amount of carotenoids released from the tomato matrix. The maximum increase (1.37-fold increase) in total carotenoid bioaccessibility was attained in the derived product obtained from tomatoes treated with 5 pulses at 2 kV cm−1 (0.38 kJ kg−1). These treatment conditions also led to maximal increases in the bioaccessibility of δ-carotene (2%), β-carotene (53%), lutein (125%) and γ-carotene (527%). Lycopene bioaccessibility in the derived product increased by 137% when whole tomatoes were treated at 1.2 kV cm−1 and 5 pulses (0.14 kJ kg−1). To the best of our knowledge, this is the first study evaluating the bioaccessibility of carotenoids when PEF treatments are applied to tomato fruit. It is well known that the structure of the food matrix is one of the most important factors affecting the bioaccessibility of carotenoids.21,34 In this regard, the results evidenced that PEFs would facilitate the release of carotenoid compounds from the tomato matrix. There are several studies that demonstrate that processing operation could disrupt cell walls and favour the release of carotenoids from the food matrix, thus leading to the enhancement of their bioaccessibility.19,35 Rodríguez-Roque et al. (2015)21 reported that the application of high intensity PEF treatments to fruit juice-based beverages allowed releasing the carotenoids from the food matrix, thus improving the bioaccessibility of some of these compounds. Moreover, the mechanical disruption of the food matrix induced by PEFs could enlarge the contact surface for interaction with digestive enzymes, thus favouring the release of carotenoids for incorporation into mixed micelles.
It is worth highlighting that a further increase in the amount of energy delivered to tomato fruit resulted in a reduction in the bioaccessibility of these carotenoids in the derived product compared to the reported maximum values (Table 3). Moreover, the bioaccessibilities of phytoene and phytofluene of tomato purees generally diminished (p < 0.05) when tomatoes were subjected to PEF treatments (Table 3), thus leading to less bioaccessible values (4–65% lower), in comparison with those observed in products obtained from untreated tomatoes. The decreased carotenoid bioaccessibilities as the specific energy input applied increased could be explained by the probable competitive inhibition between carotenoids at the level of micellar incorporation. It has been reported that a high-dose carotenoid intake, such as those found in the product obtained from tomatoes treated with high intense PEF treatments, could antagonize the bioaccessibility of some individual compounds.36–38 In addition, carotenoids could be entrapped within aggregates formed because of the cell wall depolymerisation triggered by PEFs. Hence, the highest intensities could lead to a higher release of intracellular and cell wall constituents, which could explain the decrease in the bioaccessibility of carotenoids in the samples subjected to the most intense conditions. This fact could decrease the amount of carotenoids available to be dissolved into micelles, thus affecting their bioaccessibility, as previously reported by Colle et al. (2010)39 and Svelander et al. (2011)40 in tomato-based products processed with high pressure homogenization (HPH). Due to the number of factors influencing the micellarization of carotenoids, further investigations are required to gain better understanding of main factors affecting the incorporation of carotenoids into mixed-micelles after applying PEF treatments to whole fresh commodities.
4. Conclusions
PEF treatments may be applied to whole tomatoes as a pre-processing treatment to obtain a derived product with increased antioxidant potential. The maximum concentration of total and individual carotenoids was found in the derived product obtained from tomatoes subjected to 30 pulses at 2 kV cm−1; nevertheless, the maximum bioaccessibility values were generally found in the products obtained from the fruit treated with 5 pulses at 2 kV cm−1. Therefore, the concentration and bioaccessible fraction of carotenoids in the tomato-based product can be improved under selected PEF conditions in order to enhance its health-related properties. Further studies focussing on the effects of PEFs on the fruit metabolism and structure, as well as on their impact on the digestive stability of carotenoids, should be carried out to gain knowledge regarding the processes associated with the changes in the concentration and bioaccessibility of carotenoids in the tomato-based products. In addition, it is necessary to evaluate the potential effect of these products on human health.Abbreviations
| PEF | Pulsed electric field |
| BHT | Butylated hydroxytoluene |
| SSF | Simulated salivary fluid |
| HPLC | High performance liquid chromatography |
| CCdigested | Carotenoid concentration in the micellar fraction |
| CCundigested | Carotenoid concentration in non-digested samples |
| ANOVA | Analysis of variance |
| SIPSY | Phytoene synthase |
| CRTISO | Carotenoid isomerase enzyme |
| ABA | Abscisic acid |
| HPH | High pressure homogenization |
Conflicts of interest
The authors declare no competing financial interest.Funding
This research was financed by the Ministerio de Economía y Competitividad (Spain) reference AGL2013-44851-R. S. G.-C. thanks the Agència de Gestió d′Ajuts Universitaris i de Recerca (AGAUR) for the predoctoral grant.References
- S. A. Tanumihardjo and Z. Yang, Encyclopedia of Human Nutrition, 2010, 339–345, DOI:10.1016/B0-12-226694-3/00048-X.
- I. Colle, L. Lemmens, S. Van Buggenhout, A. Van Loey and M. Hendrickx, J. Food Sci., 2010, 75(9), 753–759 CrossRef PubMed.
- C. Svelander, E. A. Tibäck, L. M. Ahrné, M. I. Langton, U. S. Svanberg and M. A. Alminger, J. Sci. Food Agric., 2010, 90(10), 1665–1672 CrossRef CAS PubMed.
- S. Toepfl, V. Heinz and D. Knorr, in Emerging technologies for food processing, ed. D.-W. Sun, Elsevier Ltd, 2005, pp. 69–98 Search PubMed.
- I. Álvarez, S. Condón and J. Raso, in Pulsed Electric Fields Technology for the Food Industry: Fundamentals and Applications, Springer US, Boston, MA, 2006, pp. 97–129 Search PubMed.
- O. Martín-Belloso and P. Elez-Martínez, in Emerging Technologies for Food Processing, Elsevier Ltd, 2005, pp. 155–181. DOI:10.1016/B978-012676757-5/50009-8.
- F. J. Barba, O. Parniakov, S. A. Pereira, A. Wiktor, N. Grimi, N. Boussetta, J. A. Saraiva, J. Raso, O. Martín-Belloso, D. Witrowa-Rajchert, N. Lebovka and E. Vorobiev, Food Res. Int., 2015, 77, 773–798 CrossRef.
- A. Zderic, E. Zondervan and J. Meuldijk, Chem. Eng. Trans., 2013, 32, 1795–1800 Search PubMed.
- R. Soliva-Fortuny, A. Balasa, D. Knorr and O. Martín-Belloso, Trends Food Sci. Technol., 2009, 20(11–12), 544–556 CrossRef CAS.
- A. Vallverdú-Queralt, G. Oms-Oliu, I. Odriozola-Serrano, R. M. Lamuela-Raventós, O. Martín-Belloso and P. Elez-Martínez, Food Chem., 2013, 136(1), 199–205 CrossRef PubMed.
- A. Balasa and D. Knorr, in Encyclopedia of Biotechnology in Agriculture and Food, Taylor & Francis, 2011, pp. 208–211, DOI:10.1081/E-EBAF-120045407.
- A. Vallverdú-Queralt, G. Oms-Oliu, I. Odriozola-Serrano, R. M. Lamuela-Reventós, O. Martín-Belloso and P. Elez-Martínez, J. Agric. Food Chem., 2012, 60, 3126–3134 CrossRef PubMed.
- A. Vallverdú-Queralt, I. Odriozola-Serrano, G. Oms-Oliu, R. M. Lamuela-Raventós, P. Elez-Martínez and O. Martín-Belloso, Food Chem., 2013, 141, 3131–3138, DOI:10.1016/j.foodchem.2013.05.150.
- E. Fernández-García, I. Carvajal-Lérida and A. Pérez-Gálvez, Nutr. Res., 2009, 29(11), 751–760 CrossRef PubMed.
- G. Knockaert, S. K. Pulissery, I. Colle, S. Van Buggenhout, M. Hendrickx and A. Van Loey, Food Chem., 2012, 135(3), 1290–1297 CrossRef CAS PubMed.
- M. L. Failla, T. Huo and S. K. Thakkar, Asia Pac. J. Clin. Nutr., 2008, 17(Suppl. 1), 200–203 CAS.
- K. T. Amorim-Carrilho, A. Cepeda, C. Fente and P. Regal, Trends Anal. Chem., 2014, 56, 49–73, DOI:10.1016/j.trac.2013.12.011.
- P. Palmero, L. Lemmens, M. Hendrickx and A. Van Loey, Food Chem., 2014, 157, 275–282, DOI:10.1016/j.foodchem.2014.02.055.
- J. Parada and J. M. Aguilera, J. Food Sci., 2007, 72(2), 21–32 CrossRef PubMed.
- I. J. P. Colle, L. Lemmens, S. Van Buggenhout, K. Met, A. M. Van Loey and M. E. Hendrickx, Food Res. Int., 2013, 51(1), 32–38 CrossRef CAS.
- M. J. Rodríguez-Roque, B. de Ancos, R. Sánchez-Vega, C. Sánchez-Moreno, M. P. Cano, P. Elez-Martínez and O. Martín-Belloso, Food Funct., 2015, 7, 380–389, 10.1039/c5fo01060h.
- USDA ( 1991). United States Standards for Grades of Fresh Tomatoes.
- R. Soliva-Fortuny, M. Vendrell-Pacheco, O. Martín-Belloso and P. Elez-Martínez, Postharvest Biol. Technol., 2017, 132, 195–201, DOI:10.1016/j.postharvbio.2017.03.015.
- D. Tagliazucchi, E. Verzelloni and A. Conte, J. Food Biochem., 2012, 36(5), 555–568 CrossRef.
- M. J. Rodríguez-Roque, M. A. Rojas-Graü, P. Elez-Martínez and O. Martín-Belloso, J. Agric. Food Chem., 2013, 61(8), 1859–1867, DOI:10.1021/jf3044204.
- F. Khachik, M. B. Goli, G. R. Beecher, J. Holden, W. R. Lusby, M. D. Tenorio and M. R. Barrera, J. Agric. Food Chem., 1992, 40(3), 390–398 CrossRef CAS.
- S. González-Casado, O. Martín-Belloso, P. Elez-Martínez and R. Soliva-Fortuny, Postharvest Biol. Technol., 2018, 137, 113–118 CrossRef.
- L. Liu, Z. Shao, M. Zhang and Q. Wang, Mol. Plant, 2015, 8(1), 28–39 CrossRef CAS PubMed.
- E. Sabbagh, M. Lakzayi, A. Keshtehgar and K. Rigi, Int. J. Farm., 2014, 3(9), 988–993 Search PubMed.
- M. Manzi, J. Lado, M. J. Rodrigo, V. Arbona and A. Gómez-Cadenas, Plant Sci., 2016, 252, 151–161, DOI:10.1016/j.plantsci.2016.07.017.
- M. Guderjan, S. Töpfl, A. Angersbach and D. Knorr, J. Food Eng., 2005, 67(3), 281–287 CrossRef.
- E. Luengo, I. Álvarez and J. Raso, Front. Nutr., 2014, 1, 1–10 Search PubMed.
- P. Mapelli-Brahm, J. Corte-Real, A. J. Meléndez-Martínez and T. Bohn, Food Chem., 2017, 229, 304–311, DOI:10.1016/j.foodchem.2017.02.074.
- J. Jeffery, A. Holzenburg and S. King, J. Sci. Food Agric., 2012, 92(13), 2594–2602, DOI:10.1002/jsfa.5767.
- S. Kamiloglu, D. Boyacioglu and E. Capanoglu, J. Berry Res., 2013, 3(2), 65–77 CAS.
- G. Maiani, M. J. Periago-Castón, G. Giovina, E. Toti, I. Goñi-Cambrodón, A. Bysted, F. Granado-Lorencio, B. Olmedilla-Alonso, P. Knuthsen, M. Valoti, V. Böhm, E. Mayer-Miebach, D. Behsnilian and U. Schlemmer, Mol. Nutr. Food Res., 2009, 53(Suppl. 2), 194–218 Search PubMed.
- D. Kostic, W. S. White and J. A. Olson, Am. J. Clin. Nutr., 1995, 62(3), 604–610 CrossRef CAS PubMed.
- V. Tyssandier, B. Lyan and P. Borel, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2001, 1533(3), 285–292, DOI:10.1016/S1388-1981(01)00163-9.
- I. Colle, S. Van Buggenhout, A. Van Loey and M. Hendrickx, Food Res. Int., 2010, 43(8), 2193–2200 CrossRef CAS.
- C. A. Svelander, P. Lopez-Sanchez, P. D. A. Pudney, S. Schumm and M. A. G. Alminger, J. Food Sci., 2011, 76(9), 215–225 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |