Salivary endocannabinoids and N-acylethanolamines upon mastication of a semisolid food: implications in fat taste, appetite and food liking
Ilario
Mennella
 a,
Rossella
Di Monaco
a,
Rossella
Di Monaco
 ab,
Adriana
Balazy
a,
Rosalia
Ferracane
ab,
Adriana
Balazy
a,
Rosalia
Ferracane
 a,
Nicoletta A.
Miele
a,
Nicoletta A.
Miele
 b,
Silvana
Cavella
b,
Silvana
Cavella
 ab and
Paola
Vitaglione
ab and
Paola
Vitaglione
 *a
*a
aDepartment of Agricultural Sciences, University of Naples “Federico II”, 80055 Portici, Italy. E-mail: paola.vitaglione@unina.it; Fax: +39 0817762580; Tel: +39 0812539357
bCenter of Food Innovation and Development in the Food Industry, University of Naples Federico II, 80055 Portici, Italy
First published on 27th November 2017
Abstract
This study aimed to evaluate whether salivary endocannabinoid (EC) and N-acylethanolamine (NAE) concentrations upon mastication of a semisolid food were involved in the sensory perception of fat taste, food liking and appetite in humans. A fat-enriched (FEP) and a low-fat control (CP) pudding were developed and used in a randomized cross-over study with 19 healthy volunteers. The study protocol combined a Modified Sham-Feeding (MSF) with a multiple-spoon Temporal Dominance of Sensations method. Subjects masticated and expectorated 10 spoons of the pudding and selected the dominant sensations among a list of attributes. Saliva samples, appetite and food liking scores were collected at baseline, immediately after the MSF of the pudding and every 5 min until 20 min after MSF. Salivary concentrations of all monitored ECs and NAEs increased during pudding mastication compared to baseline (except for palmitoylethanolamide with FEP). The raise was lower with FEP than with CP for all compounds except for 2-arachidonoylglycerol whose increase was higher than the other compounds and independent of pudding type. Salivary N-arachidonoylethanolamine, linoleoylethanolamide and palmitoylethanolamide were significantly lower at 10 and 20 min after MSF of FEP than CP. Fatty taste at the 2nd spoon and creaminess at the 5th spoon were perceived as dominant with FEP whereas only wateriness was dominant with CP at the 2nd spoon. No difference between puddings for individual appetite or food liking over the 20 min of the protocol was recorded. During mastication of a semisolid fat-enriched food, the fatty taste and the creaminess were perceived as dominant. Salivary ECs and NAEs were not associated with the individual perception of fatty taste, pudding liking and appetite sensations.
Introduction
Dietary fats are important nutrients for humans because they supply energy, support the structural aspects of the body, and provide substances that regulate several physiological processes. A consumption of dietary fats that exceeds the individual needs is detrimental for the body because it contributes to body fat accumulation and to serious health issues.1 Among food constituents, fats and sugars are positively associated with food palatability, overeating and hedonic eating.2 This is a condition leading people to eat even in the absence of a metabolic need driven by the reward system through the gustatory system.3,4 Several studies focused on the effect of fats on the reward system and appetite.5,6 The discovery of fatty acid receptors including the G-protein coupled receptor (GPCR)120, GPR40, GPR119 and the cluster of differentiation (CD) 36, on the surface of taste cells in the oral cavity and on the entero-endocrine cells in the gastrointestinal tract explained how fatty acids could trigger both fat taste and metabolic responses induced by fats.7–9 However, also lysophosphatidylcholine, palmitoylethanolamine (PEA), oleoylethanolamide (OEA), retinoic acid, as well as 2-monoacylglycerols are well-known agonists of some of these receptors, such as GPR119. That property might explain the ability of those compounds to modulate food intake and glucose metabolism through GLP-1-mediated pathways.10–12 Modulation of food preference and intake occurs through the taste circuitry by the orosensory perception of food during the early phase of eating even before food ingestion (i.e. during the cephalic phase). The amounts of fatty acids delivered from triglycerides by salivary lipase were sufficient to activate the fat taste receptors in the mouth thus being strong contributors to fat perception.13–15 Moreover, the individual lipolytic activity of saliva was associated with both salivary free fatty acids and preference for fat foods.16,17 On the other hand, a very recent study demonstrated that OEA and linoleoylethanolamide (LEA) increased in the saliva during biscuit mastication by reaching micromolar concentrations and that post-prandial salivary OEA correlated with the actual liking of biscuits and appetite.18 Since a certain amount of NAEs was present in the biscuits, it was suggested that, during mastication, those compounds were delivered in the mouth at concentrations dependent on the food matrix and the enzymatic processes in the oral cavity.18 In that study, endocannabinoids (ECs) were not investigated. However, an animal study showed that the activation of EC receptors on the surface of taste cells could enhance the sweet taste in mice.19 In humans, circulating levels of the ECs 2-arachidonoylglycerol (2-AG) and arachidonoylethanolamide (AEA) were associated with food liking20 and we demonstrated that the plasmatic concentration of 2-AG and pancreatic polypeptide were significantly higher upon mastication of a sweet and palatable pudding compared to a bitter and unpalatable pudding.21Overall, those observations raised the hypothesis that salivary ECs and NAEs during the cephalic phase of eating might influence sensory and appetite cues in humans. To test that hypothesis and to evaluate whether the fat content of a semisolid food could influence the salivary concentrations of ECs and NAEs during mastication, two puddings were developed and a cross-over randomized study was carried out combining a physiological and sensory approach. The innovative protocol applied here was based on a combination of MSF22 and the multi-spoon Temporal Dominance of Sensations (TDS)23 procedures. The contemporary MSF and TDS multispoon methodologies allowed us to collect food boluses after ∼3 min mastication of the pudding and to individuate the dominant sensations upon food tasting. The concentrations of ECs and NAEs in saliva collected before MSF, in food boluses and in saliva collected over 20 min following MSF were assessed by LC/MS/MS analysis.
Subjects and methods
Foods
Two types of vanilla pudding having similar macronutrient composition but different fat contents were developed. The control pudding (CP) was made by mixing water (73%), milk powder (9.5%), sucrose (8.8%), whey protein (7.2%), gelatin (1.5%), and vanillin (0.1%). In fat-enriched pudding (FEP), 2.5% of high-oleic sunflower oil was added. The high-oleic sunflower oil was provided by Oleifici Mataluni (Montesarchio, Benevento, Italy). The nutritional composition of the two puddings as well as the content of ECs and NAEs is reported in Table 1.| FEP | CP | ||
|---|---|---|---|
| Proteins | G | 10.8 | 11.1 |
| % E | 32% | 38% | |
| Carbohydrates | G | 12.0 | 12.4 |
| % E | 35% | 43% | |
| Fats | G | 5.0 | 2.5 |
| % E | 33% | 19% | |
| Energy (E) | Kcal | 136.6 | 115.9 |
| ECs | |||
| 2-AG | Mg | 0.04 | 0.03 |
| AEA | Mg | 0.18 | 0.11 |
| NAEs | |||
| OEA | Mg | 11.8 | 5.97 |
| LEA | Mg | 3.65 | 1.75 |
| PEA | Mg | 4.75 | 2.71 |
Subject selection and enrolment
Nineteen subjects were selected from the students and employees of the Department of Agricultural Sciences of Federico II University of Naples (Italy). Subjects were eligible if they did not suffer from any disease (hyperlipidaemia, gastro-intestinal disease, chronic infections, dental diseases, food allergies), were non-smokers, did not regularly consume alcohol, were not taking any medications, did not undertake a restrictive diet or experienced body weight variations over three months before the study. Eating behaviour was assessed for Restraint, Disinhibition, and Hunger factors using a validated Italian translation of the Three Factor Eating Questionnaire (TFEQ) as described by Stunkard & Messick24 and for the preference and the consumption of high fat foods using the “Fat Preference questionnaire”.25 Descriptive characteristics of participants are reported in Table 2. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committee of University of Naples “Federico II”. Eligible subjects participated in the study after reading and signing an informed consent.| Men | Women | Total | |
|---|---|---|---|
| Number (n) | 8 | 11 | 19 |
| Age, mean (years) | 23.1 | 21.7 | 22.4 |
| BMI, mean ± SD (kg m−2) | 26.3 ± 2.7 | 22.2 ± 2.9 | 23.9 ± 3.4 |
| Restraint (score) | 8.9 ± 4.6 | 11.7 ± 4.1 | 10.5 ± 4.4 |
| Disinhibition (score) | 6.6 ± 3 | 6.6 ± 3.1 | 6.6 ± 3 |
| Hunger (score) | 5.4 ± 2.3 | 4.8 ± 3.7 | 5.0 ± 3.1 |
| Taste (%) | 56.3 ± 16.7 | 67.6 ± 17.5 | 62.8 ± 17.6 |
| Freq. (%) | 44.7 ± 11.1 | 49.4 ± 13.1 | 47.4 ± 12.2 |
| Diff. (%) | 16.6 ± 11.7 | 23.1 ± 17.6 | 20.4 ± 15.4 |
Study design
The study had a single blind randomized cross-over design with repeated measures. Fasting subjects participated in four sessions separated by one week of each other. The first two sessions aimed at selecting the sensory attributes for the TDS evaluation and to train subjects on the procedure. Subjects were instructed on the temporality of sensations and on the concept of “dominant sensation”, defined as the sensation that captures one's attention.26 Subsequently, the list of sensory attributes to use for TDS evaluation was generated: the two pudding types were simultaneously presented and the attributes cited by at least 50% of subjects, in at least one pudding, were included in the list.23During the training phase, subjects responded also to the Visual Analogue Scale (VAS) questionnaires27 and performed the MSF protocol inside the booths. Training made volunteers more comfortable with the procedure and permitted correcting the volunteers unable to perform the study. During the other two sessions the combined MSF and TDS protocol was performed.
Modified Sham Feeding and Temporal Dominance of Sensations protocol
Subjects were instructed to have a standardised dinner consisting of a portion of meat or fish, vegetables and white bread on the evening before each experimental session, to restrain from eating and drinking energy-containing foods and beverages from 10:00 pm until the experiments and to clean their teeth no later than 1 h before the study time.On the experimental days at 8:30 am, fasting subjects arrived at the Sensory Science laboratory of the Department of Agricultural Sciences (University of Naples). Subjects were asked about their actual health status and the study session started for those who did not report any health issue or psychological discomfort and was postponed for the others.
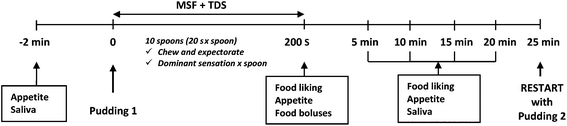
Each participant performed the tests in a sensory booth. A schematic representation of the experimental session is reported in Fig. 1. Participants rated the baseline appetite feelings (fullness, satiety, hunger and desire to eat) on 100 mm VAS questionnaires and collected an unstimulated saliva sample for 2 min (baseline saliva, T0). Then each participant was given the first pudding coded with 3 digits following a William's Latin square design and the combined MSF and TDS procedure was carried out. Subjects took a spoon of pudding (6–8 g), chewed it for 20 s and then expectorated the sample into a plastic cup. They avoided swallowing the food and repeated this procedure ten times (10 spoons) during 200 s, which was adequate for eliciting a cephalic phase response.22 During the MSF procedure, participants filled out a TDS questionnaire and during mastication of each spoon they had to choose the dominant sensory attribute from a presented list.28
The sequence of attributes on the computer screen was randomized among the participants to minimize the effect of the attribute order in the list. During the mastication of each spoon, subjects could change the dominance according to their perception, with no restrictions for the number of chosen attributes.29
After the MSF and TDS procedure, participants collected saliva samples for 2 min every 5 min (5, 10, 15 and 20 min time points). Before those collections they rated appetite feelings and actual food liking on VAS.
Five minutes after the last saliva sample collection, subjects were provided with the second pudding and the protocol was repeated. The whole protocol was conducted under artificial light and water was provided to rinse the mouth between the puddings and to ensure appropriate hydration before and after saliva collection.
During the second experimental session (one week later), the order of puddings was changed for each participant.
Saliva and sample treatment
Saliva samples were collected in 50 mL tubes and they were immediately centrifuged at 4000 rpm for 5 min. Supernatants were aliquoted in 2 mL Eppendorf tubes and stored at −40 °C until the analysis. One milliliter of samples from the chewed pudding was used for EC and NAE analysis; the remaining part of chewed, not-chewed left-over and reference puddings were freeze-dried and used for the analysis of recovery.30Endocannabinoid and N-acylethanolamine measurement by LC/MS/MS analysis
EC (2-AG, AEA, and AEAd8) and NAE (OEA, LEA and PEA) standards were purchased from Cayman (Cayman Chemical, Ann Arbor, MI). The extraction, purification and quantification of the ECs and NAEs in saliva were performed using the method described by Di Marzo,31 adapted to saliva samples. Samples were centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, for 10 min, and after addition of the internal standard (AEA-d8, 100 pg mL−1) and acetone to allow protein precipitation, the extraction of lipids by chloroform
000 rpm, for 10 min, and after addition of the internal standard (AEA-d8, 100 pg mL−1) and acetone to allow protein precipitation, the extraction of lipids by chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (2
methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) was performed. The organic phase was dried under nitrogen and re-suspended in 100 μL of acetonitrile
1 v/v) was performed. The organic phase was dried under nitrogen and re-suspended in 100 μL of acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 10 min before LC/MS/MS analysis by an API 3000 Triple Quadrupole instrument. The analysis was repeated in duplicate for each sample. The concentrations were calculated by isotope dilution using a 5-point calibration curve and expressed as ng per mL of saliva.
1 v/v) 10 min before LC/MS/MS analysis by an API 3000 Triple Quadrupole instrument. The analysis was repeated in duplicate for each sample. The concentrations were calculated by isotope dilution using a 5-point calibration curve and expressed as ng per mL of saliva.
Statistical analysis
The sample size was calculated by a power analysis based on a previous study.18 It showed that, at α = 0.05 with a power of 80%, 18 subjects were sufficient to detect 30% variations of salivary EC and NAE concentrations.Statistical analysis of biochemical data was performed using SPSS® software (IBM, version 21).
Salivary ECs and NAEs were analysed and expressed as the absolute change from the baseline to reduce possible effects of the inter-subjects fasting variability. Using analysis of variance (ANOVA) for repeated measures, the subjective appetite sensations recorded before and after MSF of the two puddings as well as the salivary EC and NAE time–concentration curves were compared and tested for the effect of treatment and time as factors. Influence of the pudding type on the overall liking and on sensory attributes was analysed and compared. The Pearson's product moment correlation test was used to analyse possible correlation among the variables. The results were considered as significant at p < 0.05.
TDS responses were recorded on a Fizz 2.4 computerized system (Biosystèmes, Couternon, France).
The dominant attribute at every time was recorded by each participant. For each spoon, the dominance rate (%) for each attribute at a given time (every 1 s) was determined.28
The dominance rate is the percentage of selections of an attribute as dominant at a particular time and it is calculated by dividing the number of citations of an attribute by the number of runs (subject × replication). Dominance rates were plotted against time for each sample to obtain TDS curves describing each spoon. Chance (P0) and significance level (Ps) were also calculated and graphically reported on the TDS curves. The chance level stands for the dominance rate that an attribute can obtain by chance. The significance level indicates the minimum value that must be reached for the dominance rate to be considered significantly higher than the chance level.23,29
Results
Salivary endocannabinoids and N-acylethanolamines upon the cephalic phase of eating
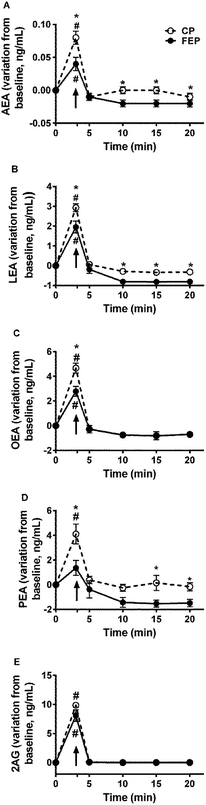
There was no difference of salivary ECs and NAEs at baseline, when the subjects tasted FEP or CP. Baseline concentrations of AEA were 0.006 ± 0.001 ng mL−1 and 0.006 ± 0.004 ng mL−1, LEA 1.45 ± 0.41 ng mL−1 and 0.95 ± 0.11 ng mL−1, OEA 1.30 ± 0.44 ng mL−1 and 1.28 ± 0.35 ng mL−1, PEA 3.08 ± 1.08 ng mL−1 and 2.87 ± 0.37 ng mL−1, respectively, at the experiments with FEP and CP (p > 0.05), whereas 2-AG was not retrieved.Fig. 2 shows the variations of salivary EC and NAE concentrations during the MSF of CP and FEP and the following 20 min. All ECs and NAEs (except for PEA with FEP) increased in the saliva samples collected during mastication of the two puddings (∼3 min) compared to baseline saliva concentrations. Lower concentrations of all monitored compounds (being p = 0.041 for AEA, p = 0.032 for LEA, p = 0.005 for OEA and p = 0.006 for PEA), except for 2-AG (p = 0.25), in saliva collected during the mastication (3 min) of FEP than CP were found. Significant differences between the two puddings for the salivary concentration of AEA (p < 0.01 at 10, 15 and 20 min), LEA (p < 0.001 at 10, 15 and 20 min), and PEA (p = 0.037 and p = 0.008 at 15 and 20 min, respectively) over the 20 min following the MSF were observed.
Appetite and food liking
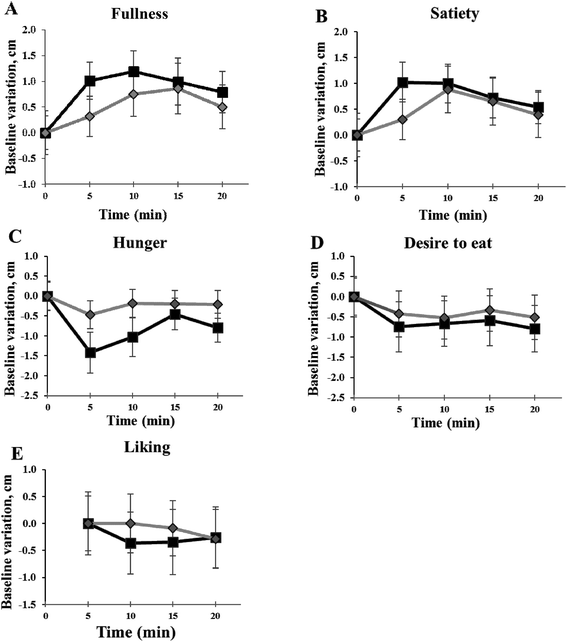
Fig. 2 shows appetite ratings recorded over the experimental sessions. No significant difference of individual fullness (panel A), satiety (panel B), hunger (panel C) and desire to eat (panel D) between the experimental sessions with the two puddings was found. Although individual liking of FEP was rated lower than CP for appearance (p = 0.036), colour (p = 0.028), texture (p = 0.047) and aftertaste (p = 0.032), no significant difference between puddings for the overall liking (panel E) was recorded.Temporal Dominance of Sensations
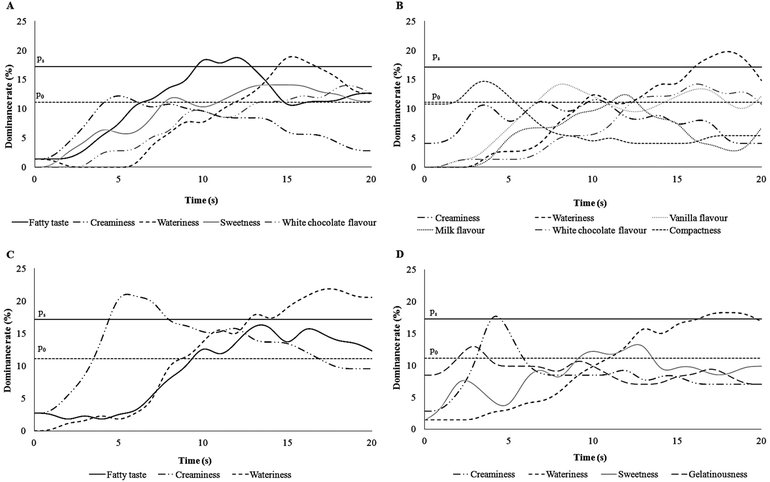
The list of sensory attributes chosen by participants included sweetness, creaminess, vanilla flavour, milk flavour, fatty taste, white chocolate flavour, compactness, wateriness, and gelatinousness. Dominance curves were obtained comparing the % of dominance of each attribute over time during the TDS evaluation of each pudding (Fig. 3). Attributes with a dominance rate higher than the chance level were illustrated in each curve. Fatty taste was significantly dominant with 18% of dominance during the 2nd spoon of FEP, followed by the wateriness sensation (Fig. 4, panel A); the 2nd spoon of CP (Fig. 4, panel B) was characterised only by a significant dominant sensation of wateriness. Additionally, during the 5th spoon a significant (21%) dominant sensation of creaminess was recorded for FEP over 5 s (Fig. 4, panel C) and for CP over 1 s (Fig. 4, panel D). In both puddings, wateriness was dominant during the late phase of the procedure. The dominance of fatty taste in FEP was recorded higher than chance level (P0) for a time longer than 10 seconds.Discussion
In the present study for the first time, the individual cephalic response to fat was evaluated using a combined sensory and physiological approach. A protocol based on MSF combined with TDS and with saliva sample collection was applied. The MSF method permitted to observe effects on appetite sensations specifically linked to the orosensory properties of FEP or CP by blunting the homeostatic negative feedbacks of food intake.22Data showed that salivary ECs and NAEs increased in saliva upon pudding mastication compared to baseline and that the salivary concentrations of AEA, OEA, LEA and PEA (but not of 2-AG) were lower with FEP than with CP. The increase of NAEs in saliva upon food mastication was in accordance with our previous study that was conducted with biscuits.18 The present data confirmed the hypothesis that upon eating the level of NAEs in saliva was influenced by NAEs and/or their phosphorylated precursors (N-acyl phosphatidylethanolamines, NAPEs) already present in the food that could be delivered and transformed by the combined mechanical and enzymatic activity in the oral cavity. Moreover, data pointed out the role of the food form, structure as well as individual mastication behaviour on salivary NAEs upon eating. Indeed, the presence of oil in FEP may have formed a less homogeneous microstructure compared to CP32 and might have slowed individual mastication to avoid FEP swallowing during MSF. On the other hand, a slower mastication could reduce the release of NAEs from FEP into saliva thus resulting in a more efficient passage in saliva from CP than FEP. It is known that coalescence occurs during mastication and the release of oil droplets during the oral processing of aggregated particle gel is difficult because a thick protein coating protects them.33 Therefore, a stronger mastication would be needed to permit the release of NAEs from FEP into saliva compared to CP. In the present study, the choice to not standardize individual mastication and to let subjects be free to chew as they preferred, avoided obtaining biased results in the contemporary TDS procedure.
Interestingly, despite 2-AG not being retrieved in saliva samples at baseline and being less abundant in puddings compared to NAEs, it enormously increased in saliva upon pudding mastication, independently of the type of pudding. This finding suggested that 2-AG could be released more efficiently from the pudding upon mastication compared to the other monitored compounds. It is in line with the physicochemical properties of ECs and NAEs. In fact, salivary flow increases in response to food and during mastication saliva acts as a solvent affecting the release of food substances. This function of saliva is regulated by its main component, water.34 Therefore, water solubility plays a major role in the extraction of food constituents into saliva. Both 2-AG and AEA are arachidonyl-derivates, but the substitution of the ethanolamide with a glycerol makes 2-AG six-fold more water soluble than the other: predicted water solubility for 2-AG is 1.4 μg mL−1 and for AEA it is 0.47 μg mL−1.35 Similarly, the other NAEs are less water soluble than 2-AG.
On the other hand, the similar concentration of salivary 2-AG between MSF with FEP and CP was in line with Di Patrizio et al.36 who showed that mastication of a lipid-based meal did not change 2-AG in tongue tissue.
Regarding the TDS multi-spoon procedure, data showed that at the 2nd spoon of FEP subjects perceived the fatty taste as the dominant sensation whereas at the 5th spoon creaminess became dominant. A recent study37 reported that fatty mouthfeel and afterfeel perception increased with repeated ingestion of high-fat emulsions (50% fat), whereas the data of the present study showed that the fat taste was dominant only during the 2nd spoon. This incongruence could be due to the different sensory methodology used, because a dominant attribute is not necessarily the most intense sensation,23 and to the different fat contents tested in the two studies, 5% (this study) and 50% (in ref. 37).
The time sequence of dominant sensations (i.e. fatty taste and creaminess) for FEP was physiologically explained considering that during the early phase of chewing, salivary lipase could hydrolyse triglycerides and derived free fatty acids could bind taste receptors in the oral cavity thus eliciting the fatty taste perception.38–40 After some spoons and the saturation of the fatty acid receptors, creaminess, that is perceived through multimodal canals and is mainly associated with food viscosity, could result as dominant. In other words, data indicated that different sensory perceptions elicited by fat can be recognised at different moments upon eating a semisolid food in accordance with their complexity. The fatty taste is related to a simple sensory experience and it is perceived first, whereas the sensation and perception of creaminess is a complicated sensory experience and it is perceived later during chewing a food.41 Indeed creaminess is not a primary sensory property detected only via either chemical or physical mechanisms, but it involves olfactory, gustatory, tactile, as well as visual mechanisms.42
The present study had also some limitations. First, it did not clarify the contribution of the food-derived NAEs and ECs on the salivary concentrations upon mastication and whether salivary glands were in some way involved in the mechanism underpinning the increased NAEs and ECs. Second, the form of the tested food (i.e. semisolid) did not allow a high addition of fats and needed a weaker chewing than a solid food thus possibly limiting the concentration of ECs and NAEs released from food during mastication (as discussed above) or from other unrecognized sources (i.e. salivary glands). Therefore, if a threshold of salivary ECs and/or NAEs does exist and physiologically modulates fat taste perception and taste-induced liking or appetite, it might be unreached in the present study. On the other hand, the amount of fats added in FEP was optimized in order to test two puddings (FEP and CP) as much as possible similar for structure and individual overall liking. Further study should evaluate if salivary EC and NAE concentration upon mastication of solid foods may give similar results to those found in the present study.
Conclusions
In this study for the first time the combination of MSF and multiple-spoon TDS was used to study the physiological and sensory mechanisms underlying appetite and food liking during the cephalic phase of eating.Data demonstrated that the mastication of a semisolid dessert with a 2.5% enrichment of fats caused dominant perceptions of the fatty taste at the 2nd spoon and of creaminess at the 5th spoon, whereas the same attributes were not dominant for the low-fat version of the food. It was unlikely that the nanomolar concentrations of ECs and NAEs present in the mouth upon pudding mastication played a main role in the mechanisms underpinning those perceptions as well as appetite and liking.
Further studies are needed to evaluate the effect of individual nutritional status and eating behaviour on the physiological and sensory responses to dietary fat tasting.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
P. V. and R. D. M. designed the research; A. B. and N. M. conducted research; R. F. and I. M. analysed food and saliva samples; I. M. analysed physiological data; N. A. M. analysed sensory data; P. V., I. M., R. D. M., and S. C. wrote the paper; P. V. had primary responsibility for the final content. All authors read and approved the final manuscript.References
- W. C. Willett, Dietary fats and coronary heart disease, J. Intern. Med., 2012, 272, 13–24 CrossRef CAS PubMed.
- J. R. Ifland, H. G. Preuss, M. T. Marcus, K. M. Rourke, W. C. Taylor, K. Burau, W. S. Jacobs, W. Kadish and G. Manso, Refined food addiction: a classic substance use disorder, Med. Hypotheses, 2009, 72, 518–526, DOI:10.1016/j.mehy.2008.11.035.
- H. R. Berthoud, Metabolic and hedonic drives in the neural control of appetite: who is the boss?, Curr. Opin. Neurobiol., 2011, 21, 888–896 CrossRef CAS PubMed.
- C. D'Addario, M. V. Micioni Di Bonaventura, M. Pucci, A. Romano, S. Gaetani, R. Ciccocioppo, C. Cifani and M. Maccarrone, Endocannabinoid signaling and food addiction, Neurosci. Biobehav. Rev., 2014, 47, 203–224, DOI:10.1016/j.neubiorev.2014.08.008.
- T. Fushiki, Why fat is so preferable: from oral fat detection to inducing reward in the brain, Biosci., Biotechnol., Biochem., 2014, 78, 363–369, DOI:10.1080/09168451.2014.905186.
- F. Grabenhorst and E. T. Rolls, The representation of oral fat texture in the human somatosensory cortex, Hum. Brain Mapp., 2014, 35, 2521–2530, DOI:10.1002/hbm.22346.
- T. J. Little and C. Feinle-Bisset, Effects of dietary fat on appetite and energy intake in health and obesity — Oral and gastrointestinal sensory contributions, Physiol. Behav., 2011, 104, 613–620 CrossRef CAS PubMed.
- P. J. Simons, J. A. Kummer, J. Luiken and L. Boon, Apical CD36 immunolocalization in human and porcine taste buds from circumvallate and foliate papillae, Acta Histochem., 2011, 113, 839–843, DOI:10.1016/j.acthis.2010.08.006.
- M. M. Galindo, N. Voigt, J. Stein, J. van Lengerich, J. D. Raguse, T. Hofmann, W. Meyerhof and M. Behrens, G Protein-Coupled Receptors in Human Fat Taste Perception, Chem. Senses, 2012, 37, 123–139, DOI:10.1093/chemse/bjr069.
- H. A. Overton, A. J. Babbs, S. M. Doel, M. C. Fyfe, L. S. Gardner, G. Griffin, H. C. Jackson, M. J. Procter, C. M. Rasamison, M. Tang-Christensen, P. S. Widdowson, G. M. Williams and C. Reynet, Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents, Cell Metab., 2006, 3, 167–175 CrossRef CAS PubMed.
- H. A. Overton, M. C. Fyfe and C. Reynet, GPR119, a novel G protein-coupled receptor target for the treatment of type 2 diabetes and obesity, Br. J. Pharmacol., 2008, 153(Suppl 1), S76–S81 CAS.
- K. B. Hansen, M. M. Rosenkilde, F. K. Knop, N. Wellner, T. A. Diep, J. F. Rehfeld, U. B. Andersen, J. J. Holst and H. S. Hansen, 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans, J. Clin. Endocrinol. Metab., 2011, 96, E1409–E1417, DOI:10.1210/jc.2011–0647.
- B. Kulkarni and R. Mattes, Evidence for Presence of Nonesterified Fatty Acids as Potential Gustatory Signaling Molecules in Humans, Chem. Senses, 2013, 38, 119–127, DOI:10.1093/chemse/bjs095.
- M. Y. Pepino, L. Love-Gregory, S. Klein and N. A. Abumrad, The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects, J. Lipid Res., 2012, 53, 561–566, DOI:10.1194/jlr.M021873.
- J. M. Heinze, A. Costanzo, I. Baselier, A. Fritsche, M. Lidolt, J. Hinrichs, S. Frank-Podlech and R. Keast, Oil Perception-Detection Thresholds for Varying Fatty Stimuli and Inter-individual Differences, Chem. Senses, 2017, 42, 585–592 CrossRef PubMed.
- E. Neyraud, S. Cabaret, H. Brignot, C. Chabanet, H. Labouré, E. Guichard and O. Berdeaux, The basal free fatty acid concentration in human saliva is related to salivary lipolytic activity, Sci. Rep., 2017, 7, 5969, DOI:10.1038/s41598-017-06418-2.
- I. Mennella, P. Vitaglione and V. Fogliano, Salivary lipase and α-amylase activities are higher in overweight than in normal weight subjects: Influences on dietary behaviour, Food Res. Int., 2014, 463–468 CrossRef CAS.
- X. Kong, R. Ferracane, L. De Luca and P. Vitaglione, Salivary concentration of N-acylethanolamines upon food mastication and after meal consumption: influence of food dietary fiber, Food Res. Int., 2016, 89, 186–193 CrossRef CAS PubMed.
- M. Jyotaki, N. Shigemura and Y. Ninomiya, Modulation of sweet taste sensitivity by orexigenic and anorexigenic factors, Endocr. J., 2010, 57, 467–475 CrossRef CAS PubMed.
- P. Monteleone, F. Piscitelli, P. Scognamiglio, A. M. Monteleone, B. Canestrelli, V. Di Marzo and M. Maj, Hedonic eating is associated with increased peripheral levels of ghrelin and the endocannabinoid 2-arachidonoyl-glycerol in healthy humans: a pilot study, J. Clin. Endocrinol., 2012, 97, E917–E924 CrossRef CAS PubMed.
- I. Mennella, R. Ferracane, F. Zucco, V. Fogliano and P. Vitaglione, Food Liking Enhances the Plasma Response of 2-Arachidonoylglycerol and of Pancreatic Polypeptide upon Modified Sham Feeding in Humans, J. Nutr., 2015, 145, 2169–2175, DOI:10.3945/jn.114.207704.
- K. L. Teff, Cephalic phase pancreatic polypeptide responses to liquid and solid stimuli in humans, Physiol. Behav., 2010, 99, 317–323 CrossRef CAS PubMed.
- R. Di Monaco, C. Su, P. Masi and S. Cavella, Temporal Dominance of Sensations: A review, Trends Food Sci. Technol., 2014, 38, 104–112 CrossRef CAS.
- A. J. Stunkard and S. Messick, The three-factor eating questionnaire to measure dietary restraint, disinhibition, and hunger, J. Psychosom. Res., 1985, 29, 71–83 CrossRef CAS PubMed.
- J. H. Ledikwe, J. Ello-Martin, C. L. Pelkman, L. L. Birch, M. L. Mannino and B. J. Rolls, A reliable, valid questionnaire indicates that preference for dietary fat declines when following a reduced-fat diet, Appetite, 2007, 49, 74–83 CrossRef PubMed.
- R. Di Monaco, N. A. Miele, S. Volpe, P. Masi and S. Cavella, Temporal Dominance of sensations and dynamic liking evaluation of polenta sticks, Br. Food J., 2016, 118, 749–760 CrossRef.
- A. Flint, A. Raben, J. E. Blundell and A. Astrup, Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies, Int. J. Obes. Relat. Metab. Disord., 2000, 24, 38–48 CrossRef CAS PubMed.
- S. Zorn, F. Alcaire, L. Vidal, A. Giménez and G. Ares, Application of multiple-sip temporal dominance of sensations method to evaluate sweeteners, Food Qual. Prefer., 2014, 36, 135–143 CrossRef.
- N. Pineau, P. Schlich, S. Cordelle, C. Mathonnière, S. Issanchou, A. Imbert, M. Rogeauxe, P. Etiévantc and E. Kösterf, Temporal dominance of sensations: Construction of the TDS curves and comparison with time–intensity, Food Qual. Prefer., 2009, 20, 450–455 CrossRef.
- A. J. Smeets, M. P. Lejeune and M. S. Westerterp-Plantenga, Effects of oral fat perception by modified sham feeding on energy expenditure, hormones and appetite profile in the postprandial state, Br. J. Nutr., 2009, 101, 1360–1368 CrossRef CAS PubMed.
- V. Di Marzo, S. K. Goparaju, L. Wang, J. Liu, S. Batkai, Z. Jarai, F. Fezza, G. I. Miura, R. D. Palmiter, T. Sugiura and G. Kunos, Leptin-regulated endocannabinoids are involved in maintaining food intake, Nature, 2001, 410, 822–825 CrossRef CAS PubMed.
- E. A. Foegeding, M. Stieger and F. van de Velde, Moving from molecules, to structure, to texture perception, Food Hydrocolloids, 2016, 68, 31–42 CrossRef.
- Q. Guo, A. Ye, M. Lad, D. Dalgleish and H. Singh, Behaviour of whey protein emulsion gel during oral and gastric digestion: effect of droplet size, Soft Matter, 2014, 10, 4173–4183 RSC.
- H. Mese and R. Matsuo, Salivary secretion, taste and hyposalivation, J. Oral Rehabil., 2007, 34, 711–723 CrossRef CAS PubMed.
- I. V. Tetko and G. I. Poda, Application of ALOGPS 2.1 to predict log D distribution coefficient for Pfizer proprietary compounds, J. Med. Chem., 2004, 47, 5601–5604 CrossRef CAS PubMed.
- N. V. Di Patrizio, G. Astarita, G. Schwartz, X. Li and D. Piomelli, Endocannabinoid signal in the gut controls dietary fat intake, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 12904–12908 CrossRef CAS PubMed.
- I. A. M. Appelqvist, A. A. M. Poelman, M. Cochet-Broch and C. M. Delahunty, Impact of model fat emulsions on sensory perception using repeated spoon to spoon ingestion, Physiol. Behav., 2016, 160, 80–86 CrossRef CAS PubMed.
- E. Dransfield, The taste of fat, Meat Sci., 2008, 80, 37–42 CrossRef CAS PubMed.
- C. A. Running, B. A. Craig and R. D. Mattes, Oleogustus: the unique taste of fat, Chem. Senses, 2015, 40, 507–516 CrossRef CAS PubMed.
- N. Voigt, J. Stein, M. M. Galindo, A. Dunkel, J. D. Raguse, W. Meyerhof and M. Behrens, The role of lipolysis in human orosensory fat perception, J. Lipid Res., 2014, 55, 870–882 CrossRef CAS PubMed.
- J. Chen and J. R. Stokes, Rheology and tribology: Two distinctive regimes of food texture sensation, Trends Food Sci. Technol., 2012, 25, 4–12 CrossRef CAS.
- J. Chen and L. Eaton, Multimodal mechanisms of food creaminess sensation, Food Funct., 2012, 3, 1265–1270 CAS.
| This journal is © The Royal Society of Chemistry 2018 |