 Open Access Article
Open Access ArticleCircular reuse of bio-resources: the role of Pleurotus spp. in the development of functional foods
Vera
Lavelli
 *,
Cristina
Proserpio
*,
Cristina
Proserpio
 ,
Francesca
Gallotti
,
Francesca
Gallotti
 ,
Monica
Laureati
,
Monica
Laureati
 and
Ella
Pagliarini
and
Ella
Pagliarini

DeFENS, Department of Food, Environmental and Nutritional Sciences, Università degli Studi di Milano, via Celoria 2, 20133 Milano, Italy. E-mail: vera.lavelli@unimi.it; Fax: +39 2 50316632; Tel: +39 2 50319172
First published on 26th February 2018
Abstract
The basidiomycetes fungi belonging to the genus Pleurotus could make an important contribution to sustainable functional food design because they possess an elevated protein content with a valuable essential amino acid scoring pattern, a unique dietary fibre profile, mainly comprised of branched β-glucan, high levels of some vitamins of the B group, vitamin D, Fe, Zn, Cu, Se and some bioactive mycochemicals, while the Na and fat contents are low. Moreover, Pleurotus spp. can grow efficiently on various clean by-products of food processing, such as wheat straw, wheat stalk and spent beer grain, thus representing a sustainable food source. This review illustrates the compositional variability of Pleurotus spp. grown on various by-products, in order to clarify its potential ability to address the needs of populations with endemic nutritional deficiencies as well as those populations at risk or affected by some chronic diseases. The perspectives for Pleurotus applications in functional foods decisively depend on consumers’ acceptability. Hence, the sensory properties of Pleurotus spp. are also clarified herein. Lastly, the three main strategies of functional food development using Pleurotus spp. are summarized, namely its use as a fortifying agent, high-cost protein replacer and prebiotic ingredient.
Introduction
Global food production is facing an uphill task to address many relevant challenges nowadays, including growing populations, the effects of climatic change on agricultural production, the noticeable impact of the agro-food system on the environment and an imbalanced economic situation caused by the worldwide financial crisis.1,2 Additionally, with the surge in the incidence of cardiovascular disease, type-2 diabetes and cancer, there is a need to develop new dietary strategies, and to develop foods that could potentially support disease prevention.3In this context, the basidiomycetes fungi belonging to the genus Pleurotus can make a valuable contribution because they combine the ability to grow with a negligible use of bio-resources and can support the production of value-added foods. Indeed, Pleurotus spp. are a fast growing fungi that can be obtained with limited capital investment and technical skill, both in temperate and in tropical regions. Moreover, Pleurotus spp. can use various by-products from the food industry as growth substrates, since they efficiently decompose lignocellulose-rich substrates due to their enzymatic complexes, including phenol oxidases and peroxidases.4 Through this conversion, Pleurotus spp. yield a fungal biomass that represents a source of protein with good levels of essential amino acids, dietary fibre with unique structural features (branched β-glucans), vitamins, minerals and low-molecular weight bioactive compounds, also known as mycochemicals.5 The nutritional value of Pleurotus spp. has long been recognized.6–8 Moreover, Pleurotus spp. are becoming increasing attractive as sources for the development of new drugs and functional foods due to their potential antioxidant, antimicrobial, anti-proliferative, immunomodulatory, anti-inflammatory and anti-hypertensive properties.5
Around 200 species of Pleurotus have been identified, but only a few have been used for food applications to date, namely P. ostreatus, P. eryngii, “P. sajor-caju” and P. pulmonarius. The name “P. sajor-caju” is considered improper because either it has been used for a tropical ecotype of P. pulmonarius or it has been incorrectly used for a species belonging to the genus Lentinus, which was later named as Lentinus sajor-caju (Fr.) Fries.9 A number of studies have been performed to characterize Pleurotus spp. compositions. However, these produced some contradictory results regarding the identification and quantification of some of its components, which raises attention to the methodology applied.10,11 There are also a growing number of studies on the use of this mushroom in new functional foods. The aim of this review is to summarize the existing literature information on the composition, nutritional value, health studies performed on humans, perception of sensory attributes and acceptability, and food applications of the most common species of Pleurotus, in order to evaluate the potential ability of this mushroom to address the needs of populations with endemic nutritional deficiencies and/or to act as a dietary supplement in the prevention of some diseases.
Composition and nutritional value
Dietary fibres and purified β-glucan fractions
The health effects of Pleurotus spp. are mainly due to its fibre fraction, which is comprised of glucans, chitin, mannoproteins, galactomannans, cellulose and polyglucuronic acids.12 The total dietary fibre content found in various studies is in the range 10.58–56.99 g per 100 g of fruit body dry weight (d.w.) (Table 1).7,8,13–21 The AOAC enzymatic gravimetric method is the most frequently used to determine dietary fibre contents in mushrooms. However, the presence in the residue of non-protein nitrogen (N) originating from chitin (which is generally not mentioned) impairs the calculation and could be one of the reasons for the different values found by various authors. Nevertheless, the effect of the growth substrate on the dietary fibre content seems to be important, whereby the use of wheat stalk and straw has resulted in high dietary fibre contents,13–15 while olive by-products have yielded a lower dietary fibre content in the literature studies.15| Pleurotus species and growth substratea | Total dietary fibreb | Proteinb,c,d | Fatb | Ashb | Ref. |
|---|---|---|---|---|---|
| a Unspecified substrate means that data refer to wild or cultivated mushrooms collected from the market. b Data obtained by the AOAC procedure. c Data were expressed as N × 4.38. d Data were recalculated as N × 4.38. e N.R.: not reported. | |||||
| P. ostreatus | |||||
| Unspecified | N.R.e | 19.93 ± 0.20c | N.R.e | 7.80 ± 0.74 | 6 |
| Unspecified | N.R.e | 34.73 ± 0.35c | N.R.e | 8.49 ± 1.40 | 6 |
| Unspecified | 47.3 ± 0.7 | 18.59 ± 0.23c | 4.16 ± 0.23 | 10.3 ± 0.1 | 7 |
| Unspecified | 30.0 ± 0.1 | 21.8 ± 0.1d | 4.4 ± 0.1 | 8.0 ± 0.1 | 8 |
| Unspecified | N.R.e | 14.7 ± 0.04c | 1.53 ± 0.25 | 5.69 ± 0.64 | 32 |
| Blank paper | N.R.e | 9.71 ± 0.02c | 1.18 ± 0.01 | 15.9 ± 1.2 | 32 |
| Printed paper | N.R.e | 9.29 ± 0.08c | 1.68 ± 0.49 | 10.5 ± 0.8 | 32 |
| Spent beer grain + wheat bran | N.R.e | 32.4 ± 0.1d | 4.4 ± 0.1 | 7.3 ± 0.1 | 33 |
| Spent beer grain + wheat bran | N.R.e | 37.4 ± 0.1d | 4.3 ± 0.1 | 6.7 ± 0.1 | 33 |
| Maize straw | N.R.e | 22.25 ± 0.51d | N.R.e | N.R.e | 34 |
| Pumpkin straw | N.R.e | 21.24 ± 0.51d | N.R.e | N.R.e | 34 |
| Wheat stalk | 30.25 ± 0.12 | 17.99 ± 0.65d | 2.60 ± 0.22 | 4.78 ± 0.04 | 13 |
| Wheat stalk | 30.25 ± 0.06 | 17.10 ± 0.56d | 2.59 ± 0.12 | 4.79 ± 0.03 | 14 |
| Cotton stalk | 29.80 ± 0.04 | 14.97 ± 0.76d | 2.90 ± 0.10 | 4.60 ± 0.01 | 14 |
| Soybean stalk | 27.0 ± 0.06 | 22.15 ± 0.15d | 2.45 ± 0.05 | 4.85 ± 0.03 | 14 |
| Millet stalk | 31.32 ± 0.12 | 14.77 ± 0.19d | 3.15 ± 0.21 | 4.71 ± 0.04 | 14 |
| Olive mill by-products | 12.50 ± 5.44 | 19.74 ± 1.19c | 2.72 ± 0.23 | 9.48 ± 1.93 | 15 |
| Almond + walnut shells | 13.00 ± 0.53 | 31.36 ± 0.57c | 2.49 ± 0.25 | 9.86 ± 0.27 | 15 |
| Beech sawdust | 15.78 ± 0.61 | 16.06 ± 1.76c | 3.46 ± 1.14 | 6.21 ± 0.12 | 15 |
| Corn cobs | 13.76 ± 4.13 | 15.41 ± 0.78c | 3.37 ± 0.65 | 8.02 ± 0.49 | 15 |
| Wheat straw | 19.07 ± 2.33 | 14.64 ± 1.38c | 2.56 ± 0.17 | 8.56 ± 0.89 | 15 |
| Olive-press cake | 13.68 ± 2.54 | 21.41 ± 2.34c | 1.64 ± 0.35 | 6.98 ± 1.26 | 15 |
| Pine needles | 13.68 ± 0.16 | 22.74 ± 0.04c | 2.44 ± 0.25 | 7.50 ± 0.67 | 15 |
| P. eryngii | |||||
| Unspecified | N.R.e | 22.89 ± 0.17c | N.R.e | 10.55 ± 0.31 | 6 |
| Unspecified | N.R.e | 22.74 ± 0.11c | N.R.e | 9.16 ± 0.26 | 6 |
| Unspecified | 25.9 ± 3.2 | 16.42 ± 0.75c | 5.97 ± 0.01 | 9.0 ± 0.74 | 16 |
| Unspecified | 43.34 ± 1.04 | N.R.e | N.R.e | N.R.e | 17 |
| Wheat stalk | 28.45 ± 0.09 | 12.55 ± 0.98d | 7.50 ± 0.08 | 4.89 ± 0.06 | 13 |
| P. sajor-caju | |||||
| Unspecified | 56.99 ± 0.01 | 22.41 ± 0.01c | 2.30 ± 0.01 | 7.79 ± 0.01 | 18 |
| Straw | 13.3 ± 0.1 | 18.6 ± 0.1d | 2.00 | 6.5 ± 0.1 | 19 |
| Cotton waste | 14.1 ± 0.1 | 21.2 ± 0.1d | 1.70 | 6.7 ± 0.1 | 19 |
| Cotton waste + straw | 11.4 ± 0.1 | 21.3 ± 0.1d | 2.00 | 6.6 ± 0.1 | 19 |
| Cotton waste + tea leaves | 14.5 ± 0.1 | 25.0 ± 0.1d | 1.70 | 6.4 ± 0.1 | 19 |
| Paddy straw | 12.3 ± 0.26 | 29.03 ± 1.03c | 0.9 ± 0.06 | 6.8 ± 0.48 | 20 |
| Wheat stalk | 30.67 ± 0.12 | 17.59 ± 1.07d | 1.15 ± 0.18 | 5.84 ± 0.09 | 13 |
| Bean straw | 16.55 ± 0.01 | 16.30 ± 0.01c | 3.26 ± 0.01 | 6.26 ± 0.01 | 21 |
| Apple pomace | 10.58 ± 0.01 | 24.44 ± 0.01c | 3.84 ± 0.01 | 6.12 ± 0.01 | 21 |
| Grape bagasse | 19.60 ± 0.01 | 27.83 ± 0.01c | 3.12 ± 0.01 | 7.05 ± 0.01 | 21 |
| Wheat straw | N.R.e | 29.36 ± 0.44c | 2.07 ± 0.06 | 8.05 ± 0.13 | 35 |
| P. pulmonarius | |||||
| Unspecified | N.R.e | 30.48 ± 0.22c | N.R.e | 8.35 ± 0.77 | 6 |
| Straw + wheat bran | N.R.e | 15.9 ± 2.5d | N.R.e | N.R.e | 36 |
Among the constituents of the dietary fibre of Pleurotus spp., β-glucans are the major components. These polysaccharides have a backbone of D-glucose-linked β-(1→3) with no branches or variable amounts of β-(1→6) branches.12 The glucose chains of β-glucans are twisted and create a single or a triple helix stabilized by inter-chain hydrogen bonds.22,23 The array of relative molecular weights of β-glucans is quite wide ranging, from tens to thousands of kilodaltons.23 Regarding the amount of β-glucans, assays based on enzymatic hydrolysis with β-glucanase have yielded low values. Hence, it has been recommended to calculate the β-glucan content as the difference between the total glucans (by measuring glucose obtained through a controlled acid hydrolysis) and the α-glucans (by measuring glucose released from α-glucans through enzymatic hydrolysis with α-amylase and amyloglucosidase).10 Using this latter approach, a study on the intraspecific variability among 16 strains of P. ostreatus mushrooms revealed that the total glucan content varied in the range 14–25 g per 100 g d.w., with β-glucans in the range of 10.9–22.9 g per 100 g d.w.24 A higher β-glucan content, i.e. 32.3 g per 100 g d.w., was also observed in one strain of P. ostreatus.10 Similarly, considering two strains of P. eryngii, the β-glucan content was found to vary between 23.85 and 37.1 g per 100 g d.w.10,17 Beside the genetic factors, the growing conditions affect the β-glucan content: substrates with a high content of polyphenolic compounds induce an increased synthesis of β-(1→3) D-glucan synthetase in their fruiting bodies. Factors such as the C/N ratio, pH of the substrate and the incubation temperature are also important and species specific.23 However, knowledge of the effects of the growing conditions on the β-glucan content is still scarce.
Parameters such as the main chain structure, degree of branching and molecular weight affect the solubility of the β-glucans. Procedures to recover concentrated hot-water-soluble, alkali-soluble and insoluble β-glucan fractions from Pleurotus spp. have been proposed (Table 2, Fig. 1). Nevertheless, the structure–bioactivity relationship of Pleurotus β-glucans has not been clarified yet.25 Karacsonyi et al.26 purified an alkali-insoluble fraction obtained from one strain of P. ostreatus and found that it was composed of branched β-(1→3),β-(1→6)-D-glucans with trace branched β-(1→3),β-(1→4)-D-glucans. This fraction was referred to as pleuran and accounted for 4.6% of the fruit body d.w.26 Carbonero et al.27 obtained a highly purified β-glucan fraction from both one strain of P. ostreatus and one strain of P. eryngii, through freezing of the hot water soluble fraction followed by mild thawing at 4 °C. However, the recovery yields for this purified fraction were low, i.e. 2.7 and 2.5 g per 100 g of the fruit body d.w. for P. ostreatus and P. eryngii, respectively.27 By another approach, Synytsya et al.28 isolated and characterized both hot-water-soluble, alkali-soluble and insoluble-glucan rich fractions from four strains of P. ostreatus and one strain of P. eryngii. The hot-water-soluble fraction mainly contained branched β-(1→3),β-(1→6)-D-glucans (44.2–72.0 g per 100 g d.w. in P. ostreatus and 33.6 g per 100 g d.w. in P. eryngii) with proteins and traces of both heteropolysaccharides and starch; while the alkali-soluble fraction mainly contained linear α-(1→3)-D-glucans (45.9–71.2 g per 100 g d.w. in P. ostreatus and 55.4 g per 100 g d.w. in P. eryngii) with proteins and traces of both heteropolysaccharides and starch. The residue mainly contained branched β-(1→3),β-(1→6)-D-glucans (65.8–86.9 g per 100 g d.w. in P. ostreatus and 66.4 g per 100 g d.w. in P. eryngii) with starch, heteropolysaccharides and chitin. Considering a moisture content of 10% for the fruit body, the yields of the water-soluble and alkali-soluble fractions were ∼5% d.w., while that of the residue was ∼30% d.w. In P. ostreatus, removal of proteins from the hot-water-soluble and alkali-soluble fractions increased the glucan contents to 78.9–85.0 g per 100 g d.w. (deproteinized hot-water-soluble fraction) and 84.3–89.2 g per 100 g d.w. (deproteinized alkali-soluble fraction).28
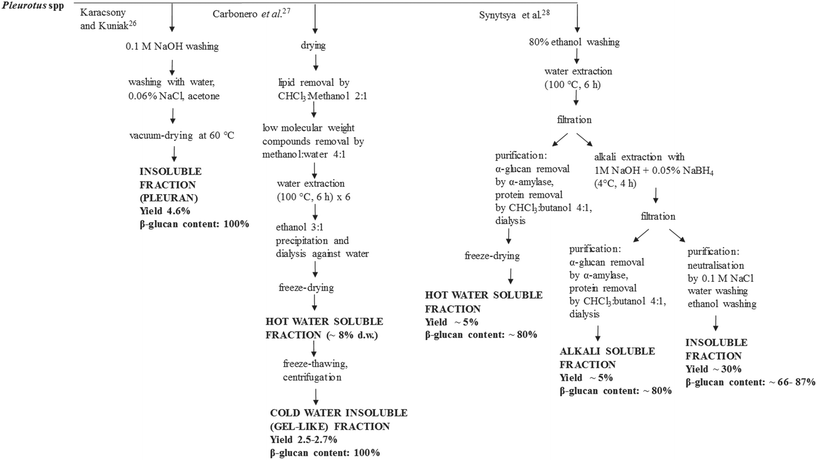 | ||
| Fig. 1 Proposed processes to obtain concentrated and purified β-glucan rich fractions from Pleurotus spp. The yield, β-glucan content and composition of the fractions are shown in Table 2. | ||
| Pleurotus species and fruit body/fraction | β-Glucan content | Yielda | Composition | Ref. |
|---|---|---|---|---|
| a The yield was transformed from a fresh basis to a dry basis considering a dry matter content of the fruit body of 10 g per 100 g f.w. b N.R.: not reported. | ||||
| P. ostreatus | ||||
| Fruit body | 10.9 ± 0.01–32.3 ± 0.1 | 10 and 24 | ||
| Alkali-insoluble fraction (pleuran) | 100 | 4.6 | Branched β-(1→3),β-(1→6)-D-glucans; branched β-(1→3),β-(1→4)-D-glucans (traces) | 26 |
| Hot-water-soluble fraction | 100 | 2.7 | Branched β-(1→3),β-(1→6)-D-glucans | 27 |
| Hot-water-soluble fraction | 44.2 ± 0.1–72.0 ± 0.1 | 5a | Branched β-(1→3),β-(1→6)-D-glucans; heteropolysaccharides and starch (traces); proteins | 28 |
| Alkali-soluble fraction | 45.9 ± 0.1–71.2 ± 0.1 | 5a | Linear α-(1→3)-D-glucans glucan; heteropolysaccharides and starch (traces); proteins | 28 |
| Insoluble fraction | 65.8 ± 0.1–86.9 ± 0.1 | 30a | Branched β-(1→3),β-(1→6)-D-glucans; heteropolysaccharides and starch; chitin | 28 |
| Deproteinized hot-water-soluble fraction | 78.9 ± 0.1–85.0 ± 0.1 | N.R.b | Branched β-(1→3),β-(1→6)-D-glucans; heteropolysaccharides and starch (traces); proteins (traces) | 28 |
| Deproteinized alkali-soluble fraction | 84.3 ± 0.1–89.2 ± 0.1 | N.R.b | Branched 1,3-1,6-β-D-glucan; heteropolysaccharides and starch (traces); proteins (traces) | 28 |
| P. eryngii | ||||
| Fruit body | 23.85 ± 1.60–37.1 ± 0.1 | 10 and 17 | ||
| Hot-water-soluble fraction | 100 | 2.5 | Branched β-(1→3),β-(1→6)-D-glucans | 27 |
| Hot-water-soluble fraction | 33.6 ± 0.1 | 5a | Branched β-(1→3),β-(1→6)-D-glucans; heteropolysaccharides and starch (traces); proteins | 28 |
| Alkali-soluble fraction | 55.4 ± 0.1 | 5a | Linear α-(1→3)-D-glucans glucan; heteropolysaccharides and starch (traces); proteins | 28 |
| Insoluble fraction | 66.4 ± 0.1 | 30a | Branched β-(1→3),β-(1→6)-D-glucans; heteropolysaccharides and starch; chitin | 28 |
Proteins
As the world's population increases rapidly and against the constraints of limited land, water and food resources, it is important to find efficient protein sources to meet human nutritional needs.29 The protein content of foods is generally determined on the basis of total N content as evaluated by the Kjeldahl method, which is then multiplied by the conversion factor 6.25.30 However, regarding edible mushrooms, many studies have indicated a probable digestibility of 60% to 70% for protein calculated as N × 6.25, due to their noteworthy amount of non-protein N in the form of glucosamine in their chitinous cell walls.31 Hence, a conversion factor for N content equal to 4.38 (i.e. 0.7 × 6.25) was proposed. This latter conversion factor was used to fill out Table 1, to obtain a close approximation of protein content of four Pleurotus spp., which resulted in values varying from 9.29 to 37.4 g per 100 g of fruit bodies d.w. (Table 1).6–8,13–16,18–21,32–36 Indeed, these mushrooms are considered as a good source of protein, especially for vegetarians. Overall on a dry basis, the protein content of Pleurotus spp. is higher than that of rice, i.e. 7.1–8.3 g per 100 g d.w.,37 which is one of the major crops contributing to the human food supply.38 Moreover, Pleurotus spp. has a good essential amino acid scoring pattern for human needs.39 The species and strain, stage of maturation, part of the mushroom body, harvest location and most of all the composition of the substrate all have a significant effect on the protein content of Pleurotus fruit bodies.31 Interestingly, the biomass of Pleurotus spp. rich in high quality protein can be obtained through the conversion of agro-wastes (Table 1). Regarding P. ostreatus, the lowest amount of crude protein (9.29 g per 100 g d.w.) was found in the fruit body grown on printed paper,32 while the highest amount (37.4 g per 100 g d.w.) was achieved when spent beer grain added with wheat bran was used as the substrate.33 For P. eryngii and P. pulmonarius, data on the effect of the substrate on their protein content are lacking, but it is noticeable that the protein content of these species grown on wheat stalk was found to be lower than that observed in wild and commercial mushrooms of the same species. The lowest protein content of P. sajor-caju (16.30 g per 100 g d.w.) was found on a bean straw medium,21 while the highest protein content (29.36 g per 100 g d.w.) was obtained when growing P. sajor-caju on a wheat straw substrate.35It is noteworthy that the protein of Pleurotus spp. generally meets the essential amino acid scoring patterns recommended for children, adolescents and adults29 (Table 3). Regarding wild Pleurotus mushrooms, some strains of P. eryngii, P. ostreautus and P. sajor-caju were found to meet the reference pattern for children and adults, while for P. pulmonarius, leucine and lysine contents were limited.6 In general, the levels of histidine and threonine in Pleurotus spp. protein are also good with respect to those recommended for infants, but the other essential amino acids are limited for infants’ requirements. The use of wheat stalk as a growth substrate for P. ostreatus, P. eryngii and P. sajor-caju has led to very good essential amino acid scoring patterns, with isoleucine, threonine, valine13,35 and aromatic amino acids13 also meeting the infants’ requirements.
| Groups | Recommended essential amino acid scoring pattern | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a SAA (Sulphur Amino Acids): CYS + MET. b AAA (Aromatic Amino Acids): PHE + TYR. c Unspecified substrate means that data refer to wild or cultivated mushrooms collected from the market. d N.D.: not determined. e N.R.: not reported. | ||||||||||
| HIS | ILE | LEU | LYS | SAAa | AAAb | THR | TRP | VAL | ||
| Infant (birth to 6 months) | 21 | 55 | 96 | 69 | 33 | 94 | 44 | 17 | 55 | 29 |
| Child (6 months to 3 years) | 20 | 32 | 66 | 57 | 27 | 52 | 31 | 8.5 | 43 | 29 |
| Older child, adolescent, adult | 16 | 30 | 61 | 48 | 23 | 41 | 25 | 6.6 | 40 | 29 |
| Pleurotus species and growth substratec | Amino acid content | |||||||||
| P. ostreatus | ||||||||||
| Unspecified | 43.3 | 53.2 | 76.5 | 69.9 | 41.9 | 99.6 | 61.6 | 14.8 | 55.3 | 6 |
| Unspecified | 44.1 | 24.2 | 38.9 | 37.5 | 16.5 | 45.9 | 29.7 | 6.6 | 26.1 | 6 |
| Spent beer grain + wheat bran | 32.0 | 41.8 | 66.3 | 59.1 | 19.6 | 73.5 | 44.1 | 12.4 | 54.2 | 33 |
| Maize straw | 60.6 | 52.5 | 68.2 | 87.2 | N.R.e | N.R.e | N.R.e | N.R.e | 49.8 | 34 |
| Pumpkin straw | 89.6 | 44.8 | 71.2 | 86.9 | N.R.e | N.R.e | N.R.e | N.R.e | 51.1 | 34 |
| Wheat stalk | N.D.d | 54.9 | 90.9 | 62.7 | 15.0 | 100.2 | 52.4 | N.R.e | 58.4 | 13 |
| P. eryngii | ||||||||||
| Unspecified | 29.9 | 35.4 | 66.9 | 63.5 | 31.1 | 73.5 | 49.0 | 12.4 | 37.0 | 6 |
| Wheat stalk | 19.9 | 57.9 | 85.8 | 58.2 | N.R.e | 96.0 | 54.0 | N.R.e | 59.0 | 13 |
| P. sajor-caju | ||||||||||
| Unspecified | 22 | 44 | 70 | 57 | 30 | 113 | 55 | 12 | 53 | 31 |
| Wheat stalk | N.D.d | 63.7 | 83.1 | 32.7 | N.R.e | 86.4 | 50.9 | N.R.e | 57.1 | 13 |
| Wheat straw | 34.7 | 43.6 | 69.5 | 53.5 | 41.2 | N.R.e | 55.9 | N.R.e | 85.5 | 35 |
| P. pulmonarius | ||||||||||
| Unspecified | 29.5 | 43.4 | 31.6 | 28.5 | 26.2 | 51.7 | 64.2 | 11.3 | 53.1 | 6 |
Besides proteins, mushrooms contain free amino acids, among which glutamic acid (Glu) is prevalent. The typical presence of this amino acid is one of the factors that allow these mushrooms to be used as a functional food or as a raw material for functional foods.40 In fact, free Glu plays an important physiological role in the process of digestion, nutrient absorption and energy homeostasis via the gut–brain axis. These activities are mediated via several receptors in the oral cavity, where Glu is responsible for the “umami taste” (as described under the sensory attributes and perception paragraph). Moreover, Glu stimulates luminal gut glutamic acid sensors that are linked to the afferent branches of the vagus nerve, which in turn modulates a number of target areas in the brain, thus enhancing the secretion of digestive juices and insulin.41 Only a limited number of Pleurotus species have been analysed for free Glu content. In some strains of P. ostreatus and P. eryngii, contents of the free form of this amino acid were found to be in the range 0.071–4.109 g per 100 g d.w.40,42–46 The effects of the growth substrates on free Glu content have also been poorly investigated. In P. eryngii, sawdust was found to be beneficial for the free Glu content with respect to corncob.45
Fats
Pleurotus spp. are low in fat content. Previous reports have found that the fat content ranges between 1.18 and 4.4 g per 100 g d.w. in P. ostreatus, between 5.97 and 7.5 g per 100 g d.w. in P. eryngii and between 0.9 and 3.84 g per 100 g d.w. in P. sajor-caju (Table 1).7,8,13–16,18–21,32–36 The fat fraction of mushrooms includes, in general, representative compounds of all classes of lipids, namely, free fatty acids, mono-, di- and triglycerides, sterols, sterol esters and phospholipids. Triglycerides are prevalent, while squalene, ergosterol (free and esterified) and ubiquinone have also been reported as minor components.31 In an intraspecific study on 16 strains of wild P. ostreauts, polyunsaturated fatty acids were found to be the most prevalent, ranging between 58.84% and 80.63% of total fatty acids, while monounsaturated fatty acids were between 6.76% and 20.29% of total fatty acids and saturated fatty acids were between 8.77 and 17.07% of total fatty acids. Linoleic acid dominated in all samples (56.8–80.5%) followed by oleic and palmitic acids.24 A similar fatty acids profile was observed for the samples obtained with paper scraps as the substrate.32 The fatty acid profile of one strain of P. eryngii was found to be different, with a profile of 25.79%, 49.05% and 25.17% saturated fatty acids, monounsaturated and polyunsaturated fatty acids with respect to total fatty acids, respectively.47Organic acids and soluble sugars/polyols
The amounts of organic acids and sugars/polyol of Pleurotus spp. were found to vary in the ranges 3.0–9.8 g per 100 g d.w and 15.6–32.9 g per 100 g d.w., respectively.32,45,47 The patterns of organic acids reported by various studies showed some differences: in one strain of P. ostreatus, citric acid, oxalic acid, fumaric acid and malic acid were observed.48 The same pattern was found in a P. erjngii strain,47 while in another P. ostreatus strain, quinic acid was detected instead of malic acid.32 Among the sugars and polyols, trehalose, mannitol and glucose were found to be the most prevalent in the Pleurotus genus.32,35,45,47 The amount of sugars and polyols in Pleurotus spp. greatly depends on the growth substrate. Using printed paper and blank paper as the substrate for P. ostreatus, the amounts of sugars/polyols were 9.45 and 17.6 g per 100 g d.w., respectively, while the amount in the control was 26.2 g per 100 g d.w.32 In P. eryngii, a corncob substrate was beneficial to high contents of trehalose, soluble carbohydrates and polysaccharides, while sawdust produced the lowest content, being beneficial for protein.45 In P. sajor-caju, the use of increasing amounts of detoxified mahua cake up to 20% in the growth substrate, led to a decrease in total sugars/polyol from 7.54 to 5.39 g per 100 g d.w.35Minerals
Mushrooms are potential dietary sources of the minerals that are necessary for metabolic reactions, the transmission of nerve impulses, bone formation, regulation of water and for salt balance. In fact, all edible mushrooms can accumulate minerals in their fruit bodies.49 The ash content of the fruit bodies of Pleurotus spp. ranges between 4.60 and 10.55 g per 100 g d.w.6–8,13–21,32–36 (Table 1). The mineral levels are largely affected by the growth substrates since substrates high in a particular mineral produce mushrooms relatively high in the content of that mineral.49 From the mineral analysis reports of different studies, it was revealed that the major minerals in Pleurotus spp. are P (496–1647.6 mg per 100 g d.w.), K (271–4054.3 mg per 100 g d.w.), Na (13–310 mg per 100 g d.w.), Ca (1–330 mg per 100 g d.w.), Mg (137–203.4 mg per 100 g d.w.), Mn (1.1–1.6 mg per 100 g d.w.), Fe (5.4–15.62 mg per 100 g d.w.), Zn (8.3–13.7 mg per 100 g d.w.) and Cu (0.84–2.5 mg per 100 g d.w.).6,15,19,31,33,50,51 The cultivated species of Pleurotus were found to contain only low levels of the undesirable elements, such as Cd, Pb and Hg.49The distinctive presence in Pleurotus spp. of a low Na content and high K content (second major mineral after P) is beneficial from a nutritional point of view. In fact, to reduce blood pressure, the risk of cardiovascular disease, stroke and coronary heart disease in adults (≥16 years of age), the recommended upper limit for Na dietary intake is <2000 mg per day, while a dietary intake of 3510 mg per day for K is suggested. These latter amounts adjusted based on the different energy requirements have also been recommended for children (2–15 years of age) to control blood pressure.52,53 Interestingly, in both wild Pleurotus spp. and Pleurotus spp. grown on different waste substrates, the concentration range of Na in 100 g of dried fruits is notably lower than the recommended upper limit for Na daily dietary intake, while the concentration range of K in general meets the recommended K daily dietary intake (Table 4).
| Groups | Dietary reference intakes for minerals | Ref. | ||||
|---|---|---|---|---|---|---|
| a The recommended level of intake should be adjusted downward based on the energy requirements of children relative to those of adults. b Values refer to a moderate dietary Fe bioavailability (10%). c Values refer to a moderate dietary Zn bioavailability (30%). d N.R.: not reported. e Unspecified substrate means that data refer to a wild or cultivated mushrooms collected from the market. | ||||||
| Ka | Naa | Se | Feb | Znc | ||
| Infant (birth to 12 months) | N.R.d | N.R.d | 0.006–0.010 | 9.3 | 2.8–4.1 | 52–54 |
| Child (1 to 9 years) | >3510 | <2000 | 0.017–0.021 | 5.8–8.9 | 4.1–5.6 | 52–54 |
| Adolescent, adult, elderly | >3510 | <2000 | 0.025–0.033 | 13.7–32.7 | 7.0–8.6 | 52–54 |
| Pregnancy and lactation | >3510 | <2000 | 0.028–0.042 | 15 | 5.5–10 | 52–54 |
| Pleurotus species and growth substratee | Mineral content | |||||
| P. ostreatus | ||||||
| Unspecified | 2682.3 ± 53.0 | 136 ± 0.4.6 | N.R.d | N.R.d | N.R.d | 6 |
| Unspecified | 3443.8 ± 109.0 | 25.2 ± 5.7 | N.R.d | N.R.d | N.R.d | 6 |
| Unspecified | 3730 ± 1 | 13 ± 1 | 0.015 ± 0.01 | 5.4 ± 0.1 | 8.3 ± 0.1 | 50 |
| Spent beer grain and wheat bran | 2171.4 ± 0.1 | 21.9 ± 0.1 | N.R.d | 7.1 ± 0.1 | 13.7 ± 0.1 | 33 |
| Soybean straw | 2320 ± 9 | 310 ± 4 | N.R.d | 14.35 ± 0.16 | N.R.d | 51 |
| Paddy straw | 2260 ± 9 | 290 ± 4 | N.R.d | 14.94 ± 0.16 | N.R.d | 51 |
| Soybean straw and paddy straw | 2100 ± 9 | 295 ± 4 | N.R.d | 15.62 ± 0.16 | N.R.d | 51 |
| Soybean straw and wheat straw | 2000 ± 9 | 260 ± 4 | N.R.d | 14.20 ± 0.16 | N.R.d | 51 |
| Wheat straw and paddy straw | 1900 ± 9 | 275 ± 4 | N.R.d | 13.13 ± 0.16 | N.R.d | 51 |
| Wheat straw | 2100 ± 9 | 305 ± 4 | N.R.d | 13.88 ± 0.16 | N.R.d | 51 |
| Wheat straw | 352 ± 4 | 104 ± 6 | N.R.d | N.R.d | N.R.d | 15 |
| Almond and walnut shells | 371 ± 9 | 89 ± 2 | N.R.d | N.R.d | N.R.d | 15 |
| Corn cobs | 325 ± 18 | 80 ± 2 | N.R.d | N.R.d | N.R.d | 15 |
| Grape marc plus cotton gin trash | 374 ± 18 | 91 ± 3 | N.R.d | N.R.d | N.R.d | 15 |
| Olive mill by-products | 510 ± 27 | 100 ± 10 | N.R.d | N.R.d | N.R.d | 15 |
| Extracted olive-press cake | 277 ± 7 | 76 ± 3 | N.R.d | N.R.d | N.R.d | 15 |
| Date palm tree leaves | 342 ± 5 | 96 ± 2 | N.R.d | N.R.d | N.R.d | 15 |
| Pine needles | 271 ± 1 | 81 ± 3 | N.R.d | N.R.d | N.R.d | 15 |
| Coffee husk + 102 mg kg−1 Se | 85.8 ± 0.1 | 58 | ||||
| P. eryngii | ||||||
| Unspecified | 3095.0 ± 40 | 50.4 ± 1.1 | N.R.d | N.R.d | N.R.d | 6 |
| Unspecified | 4054.3 ± 244.2 | 76.6 ± 2.8 | N.R.d | N.R.d | N.R.d | 6 |
| P. sajor-caju | ||||||
| Chopped rice straw | 3260 ± 1 | N.R.a | N.R.d | 12.4 ± 0.1 | 12.9 ± 0.1 | 31 |
| Straw | 2400 ± 1 | 238 ± 1 | N.R.d | 11.5 ± 0.1 | N.R.d | 19 |
| Cotton waste | 2207 ± 1 | 158 ± 1 | N.R.d | 5.9 ± 0.1 | N.R.d | 19 |
| Cotton waste and straw | 2322 ± 1 | 172 ± 1 | N.R.d | 5.0 ± 0.1 | N.R.d | 19 |
| Cotton waste and tea leaves | 2130 ± 1 | 256 ± 1 | N.R.d | 5.6 ± 0.1 | N.R.d | 19 |
| P. pulmonarius | ||||||
| Unspecified | 2818.9 ± 36.0 | 103.4 ± 2.1 | N.R.d | N.R.d | N.R.d | 6 |
Comparing the Fe and Zn contents of Pleurotus spp. with their recommended daily dietary intake,54 it is outstanding to note that the Pleurotus species are able to provide more than adequate quantities of these minerals (Table 4). However, the amount provided by foods is not always enough to meet nutritional requirements if the bioavailability is low. This latter depends on dietary sources due to the presence of inhibitors and promoters of absorption.55,56 A diet containing at least small amounts of meat and fish is associated with good levels and bioavailability of Fe and Zn, while these minerals are found in low amounts and have low bioavailability in cereal- and tuber-based diets. Hence, a deficiency in these minerals is common in developing countries, where the diet is limited with respect to their content and bioavailability. Additionally, bioavailability is diminished in phytate-containing foods. Other reasons for Fe anaemia in many tropical countries are infestations with hookworms, which lead to intestinal blood losses. Patients who have gastric diseases and celiac subjects may also develop Fe deficiency because of impaired Fe absorption. The populations most at risk for Fe and Zn deficiency are infants, children, adolescents and women of childbearing age, especially pregnant women.54 Interestingly, a previous study carried out with a mice animal model indicated that the bioavailability of Fe present in the fruit bodies of P. sajor-caju was high.57 However, human studies are necessary to define the possible role of mushrooms in the prevention of Fe and Zn deficiencies.
P. ostreatus has also shown a great potential to improve the dietary intake of Se when grown on Se-enriched substrates. Se deficiency is endemic in regions where this mineral is poorly available from soil for staple crops, covering especially localities from northeast to southwest China and Siberia, where it the primary factor for the occurrence of Keshan and Kaschin-Beck diseases. Fluctuations in the Se status of many communities in northern Europe has also been observed, which reflect the intrinsically low Se content of glacial soil in this region. Non-endemic Se depletion is also common in subjects maintained on parenteral or enteral feeding for long periods. Additionally, the possibility that increased intakes of Se might protect against the development of cancer in humans has generated great interest, although the question of “whether Se protects against cancer” remains wide open.54P. ostreatus was able to absorb and accumulate Se from selenite added to coffee husks used as a growth substrate in the range of 3.2–100 mg of Se per kg. The lowest concentration of Se in the substrate (3.2 mg of Se per kg) resulted in mushrooms with 5.76 mg of Se per 100 g d.w., while the highest concentration used (100 mg of Se mg per kg) resulted in mushrooms with 85.8 mg of Se per 100 g d.w. Interestingly, for the enriched mushrooms, the Se bioavailability in rats was higher than that of sodium selenite.58 However, human studies on the bioavailability of mineral microelements in mushrooms are lacking and hence preclude drawing general conclusions.
Vitamins and pro-vitamins
Wild and cultivated mushrooms from Pleurotus genus are good sources of some B group vitamins (Table 5).14,19,31,50,59,60 In a few studies, the level of the B group vitamins was found to be affected by the ingredients used in the growth substrate.14,19 However, information on the effect of the growth substrate on the vitamin content in Pleurotus spp. is lacking and hence general rules cannot be drawn. The levels of B group vitamins in Pleurotus spp. were found to vary between 0.02 and 1.96 mg per 100 g for B1, 0.15 and 6.66 mg per 100 g for B2 and 0.59 and 65 mg per 100 g for B3.14,19,31 Thiamine deficiency has been observed in developing country populations as well as in Japanese elderly and people with chronic alcoholism.54 African and Asian children commonly demonstrate clinical signs of riboflavin deficiency during periods of the year when gastrointestinal infections are prevalent. However, the major cause of hyporiboflavinosis is an inadequate dietary intake as a result of a limited food supply. Niacin deficiency, which can causes pellagra disease, is also endemic in poorer areas of Africa, China and India. Interestingly, the content of niacin in mushrooms is higher than those generally found in vegetables. Information on vitamins B5 and B6 in Pleurotus spp. is limited. For vitamin B5, a level of 21.1 mg per 100 g d.w. was found in one strain of P. sajor-caju.31 For vitamin B6, a range of values between 0.0701 and 0.23 mg per 100 g was found in P. ostreatus.14,59 A nutritional deficiency of vitamins B5 or B6 alone is uncommon because it usually occurs in association with a deficit in other B complex vitamins and other nutrients.54| Groups | Recommended nutrient intakes | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|
| a Expressed as mg niacin equivalents (NE) per d. b Expressed as mg dietary folate equivalents (DFE) per d. c The vitamin D content reported for the different species of Pleurotus only refers to vitamin D2. d Unspecified substrate means that data refer to wild or cultivated mushrooms collected from the market. e N.R.: not reported. | ||||||||
| B1 | B2 | B3a | B5 | B6 | B9b | D2c | ||
| Infant (birth to 12 months) | 0.2–0.3 | 0.3–0.4 | 2–4 | 1.7–1.8 | 0.1–0.3 | 0.08 | 0.005 | 54 |
| Child (1 year to 9 years) | 0.5–0.9 | 0.5–0.9 | 6–12 | 2–4 | 0.5–1.0 | 0.15–0.3 | 0.005 | 54 |
| Adolescent, adult, elderly | 1.1–1.2 | 1.0–1.3 | 14–16 | 5 | 1.2–1.7 | 0.4 | 0.005–0.015 | 54 |
| Pregnancy and lactation | 1.4–1.5 | 1.4–1.6 | 17–18 | 6.0–7.0 | 1.9–2.0 | 0.5–0.6 | 0.005 | 54 |
| Pleurotus species and growth substrated | Vitamin content | |||||||
| P. ostreatus | ||||||||
| Unspecified | 0.9 ± 0.1 | 2.5 ± 0.1 | 65 ± 1 | N.R.e | N.R.e | 0.64 ± 0.01 | 0.0003 ± 0.0001 | 50 |
| Unspecified | 0.30 ± 0.01 | 1.62 ± 0.08 | 9.98 ± 0.57 | N.R.e | 0.0701 ± 0.0012 | N.R.e | N.R.e | 59 |
| Unspecified | N.R.e | N.R.e | N.R.e | N.R.e | N.R.e | N.R.e | 0.083 ± 0.06 | 63 |
| Millet stalk | 0.14 ± 0.00 | 0.15 ± 0.00 | 0.93 ± 0.02 | N.R.e | 0.23 ± 0.01 | N.R.e | N.R.e | 14 |
| Wheat stalk | 0.12 ± 0.01 | 0.19 ± 0.02 | 0.67 ± 0.00 | N.R.e | 0.23 ± 0.01 | N.R.e | N.R.e | 14 |
| Cotton stalk | 0.25 ± 0.00 | 0.21 ± 0.03 | 1.43 ± 0.00 | N.R.e | 0.21 ± 0.02 | N.R.e | N.R.e | 14 |
| Soybean stalk | 0.07 ± 0.02 | 0.20 ± 0.00 | 0.59 ± 0.00 | N.R.e | 0.21 ± 0.00 | N.R.e | N.R.e | 14 |
| Wheat straw | 1.92 ± 0.01 | 3.3 ± 0.1 | 35.98 ± 0.01 | N.R.e | N.R.e | N.R.e | N.R.e | 60 |
| Wheat straw | 1.96 ± 0.01 | 3.7 ± 0.1 | 36.56 ± 0.01 | N.R.e | N.R.e | N.R.e | N.R.e | 60 |
| P. sajor-caju | ||||||||
| Unspecified | 1.75 ± 0.23 | 6.66 ± 1.22 | 60.0 ± 4.7 | 21.1 ± 3.1 | N.R.e | 1.278 ± 0.130 | N.R.e | 31 |
| Straw | 0.02 ± 0.01 | 1.36 ± 0.01 | 18.2 ± 0.1 | N.R.e | N.R.e | N.R.d | N.R.e | 19 |
| Cotton waste | 0.02 ± 0.01 | 1.32 ± 0.01 | 20.7 ± 0.1 | N.R.e | N.R.e | N.R.d | N.R.e | 19 |
| Cotton waste and straw | 0.03 ± 0.01 | 1.33 ± 0.01 | 21.3 ± 0.1 | N.R.e | N.R.e | N.R.d | N.R.e | 19 |
| Cotton waste and tea leaves | 0.06 ± 0.01 | 1.21 ± 0.01 | 20.6 ± 0.1 | N.R.e | N.R.e | N.R.d | N.R.e | 19 |
Regarding the vitamin B9, mushrooms contain moderately high amounts of this, and their contents are of the same magnitude as those generally found in vegetables like spinach. In addition, the bioavailability of mushroom folates appears to be as good as that for folic acid, unlike the bioavailability of folates from some vegetables, such as peas and spinach.61 A high content of folates was found in one strain of P. ostreatus (0.64 mg per 100 g d.w.)50 and in one strain of P. sajor-caju (1.278 mg per 100 g d.w.).31 A deficiency of folate is common in people consuming a limited diet and in pregnant women, because pregnancy significantly increases the folate requirement, especially during periods of rapid foetal growth. During lactation, losses of folate in milk also increase the folate requirement.54
The content of vitamin B12 was only reported for P. ostreatus and found to be 0.6 μg per 100 g d.w.50 However, among 38 common edible fungi analysed for vitamin B12 content, only 9 were found to contain this vitamin, where one of the best producers was P. ostreatus.62 Hence, this mushroom could be a good B12 source for vegans, because otherwise it would normally enter the human food chain through incorporation in food of an animal origin. In mushrooms, the vitamin probably derives from surface microorganisms that can synthesize it.50
In addition to vitamins from the B group, the genus Pleurotus contains elevated amounts of the vitamin D2 (ergocalciferol) precursor, i.e. ergosterol – a component of the fungal cell membrane. The ergosterol content in P. ostreatus varies from 290 to 754 mg per 100 g d.w.63–65 The natural level of vitamin D2 in Pleurotus spp. is generally low and highly variable: both undetectable levels,64 and low levels, such as 0.3 μg per 100 g d.w.50 and values in the range 0.083–0.156 mg per 100 g d.w., have been reported.63 However, vitamin D2 in mushrooms is converted from ergosterol through UV irradiation during growth, after harvest and after drying too. After the exposure of P. ostreatus powder with no detectable amount of vitamin D2 to 2800–2900 mJ cm−2 UVB at 60–66 °C for 10 min, 11 mg per 100 g d.w. of vitamin D2 was obtained.64 Accordingly, treatment with 411 mJ cm−2 UVB at 20 °C for 10 min led to a vitamin D2 formation of 4.07 mg per 100 g d.w.65 The fact that the enrichment in vitamin D2 may be performed after drying, greatly facilitates the potential use of UV technology in the processing of mushrooms in order to improve their nutritional value. It is estimated that about one billion people in the world have a vitamin D deficiency: infants, adolescents, elderly, pregnant and lactating women constitute the populations most at risk.54 Moreover, mushrooms are a natural source of vitamin D for some consumer groups, including vegetarians and vegans, and people intolerant to lactose, since most of the products fortified with vitamin D include dairy products.
Another vitamin is vitamin C, where its content in Pleurotus spp. was reported to vary from 9.10 (P. ostreatus) to 111 mg per 100 g d.w. (P. sajor-caju).59,60 The vitamin C recommended dietary intake varies from 25 (for infants) to 70 (for lactating women) mg per day and a deficiency in vitamin C is associated with malnutrition.54 With regard to vitamin E precursors, the α-, β-, γ- and δ-tocopherols were found in the Pleurotus genus. P. ostreatus showed significant amounts of total tocopherols (while β-tocopherol was lacking) in the range 0.279–2.87 mg per 100 g d.w.32,59 A total tocopherol content of 0.086 mg per 100 g d.w. (while δ-tocopherol was lacking) was found in one strain of P. eryngii.47 In general, these amounts are low with respect to the vitamin E recommended intake, which is in the range 2.7–10 mg d−1.54P. ostreatus was reported to contain 1.075 mg per 100 g d.w. of the vitamin A precursor, i.e. β-carotene, and 0.638 mg of lycopene per 100 g d.w.59 However, in another study, the presence of carotenoids in Pleurotus spp. was denied.66
Ergothioneine, lovastatin and γ-aminobutiryc acid
Ergothioneine (EGT), lovastatin (also known as monacolin K, mevinolin or mevacor) and γ-aminobutyric acid (GABA) are secondary metabolites from fungal growth, occurring both in the mycelium and in the fruiting body, that are thought to be beneficial for human health.67EGT is not synthesized by higher organisms. However, in humans, EGT has been shown to accumulate in various cells and tissues at high concentrations (100 μM to 2 mM), most abundantly in erythrocytes, bone marrow, liver, kidney, seminal fluid and the lens and cornea of the eyes. EGT is not currently considered an essential dietary component and there are no reports of symptoms due to its deficiency. A wide body of evidence suggests that EGT may function as a physiological antioxidant. The biological role of EGT is under investigation for its positive impact on the inflammatory process.68 Among various fungi, the Pleurotus genus contains a considerably high amount of EGT, which is higher in the fruiting body than in the mycelium. The level of EGT in the fruiting body was found to be in the ranges of 94–259 mg per 100 g d.w. for P. ostreatus and 62.4–84.0 mg per 100 g d.w. for P. eryngii.67,69,70
Lovastatin is one of the natural statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors), which inhibit the rate-limiting enzyme in the production of cholesterol and have been proven to reduce the risk of coronary heart disease. Contrary to EGT, the lovastatin content in the fruiting body of fungi is lower than that of the mycelium. In the fruiting body, its level was found to be in the ranges of 16.5–60.6 mg per 100 g d.w. for P. ostreatus and 12.0–15.2 mg per 100 g d.w. for P. eryngii.67
Several in vivo experiments have demonstrated the hypotensive effect of GABA. In a screening study with various Pleurotus strains, the level of GABA was found to be in the ranges of 14.3–280.8 mg per 100 g d.w. in P. ostreatus, 53.3–54.6 mg per 100 g d.w in P. eryngii and 165.4 mg per 100 g d.w. in P. pulmonarius.6
Phenolic compounds
The high total phenolic content of Pleurotus spp. is likely responsible for its ability to scavenge free radicals and other reactive oxygen species that are continuously being produced in vivo, as well as its ability to chelate Fe++ ions, which catalyze oxidative processes. These properties result in the prevention of cell death and tissue damage. Indeed, phenolic extracts from Pleurotus spp. possess antioxidant, anti-inflammatory and antimicrobial activities.5,71 However, knowledge of the phenolic pattern of Pleurotus spp. is still lacking. HPLC-MS analysis of the methanolic extract for 16 strains of P. ostreatus revealed the presence of p-hydroxy-benzoic acid (n.d.–424.7 μg per 100 g d.w.), p-hydroxy-phenylacetic acid (10.3–120.9 μg per 100 g d.w.), 3-4-dihydroxy-phenylacetic acid (n.d.–35.4 μg per 100 g d.w.), protocatechuic acid (n.d.–32.3 μg per 100 g d.w.), syringic acid (n.d.–14.4 μg per 100 g d.w.), vanillic acid (n.d.–12.9 μg per 100 g d.w.), caffeic acid (0.5–5.4 μg per 100 g d.w.), cinnamic acid (n.d.–110 μg per 100 g d.w.), ferulic acid (n.d.–2.2 μg per 100 g d.w), vanillin (n.d.–30.2 μg per 100 g d.w.), tyrosol (n.d.–8.6 μg per 100 g d.w.) and resveratrol (5.4–95.8 μg per 100 g d.w.).24 Phenolic acids can also be released from the polysaccharides of the cell wall after alkaline hydrolysis. Particularly in a strain of P. ostreatus, bound coumaric and ferulic acids were found at the levels of 556 and 90 μg per 100 g d.w., respectively.72 The presence of phenolic acids in the Pleurotus genus was confirmed in other studies with P. ostreatus, P. eryngii and P. sajor-caju.73–75Some authors have identified the presence of flavonoids in Pleurotus spp. However, this identification was not confirmed by MS studies and it has been considered misleading because edible mushrooms do not have the main enzymes involved in the flavonoids metabolic pathway. Additionally, mushrooms have been found unable to accumulate flavonoids present in the growth substrates.11
Human studies on Pleurotus health properties
Immunomodulatory properties and anti-allergic effects
The immunomodulatory activity of insoluble β-glucan of Pleurotus spp. (pleuran) is well documented and recognized (Table 6). The mechanism of its action in the organism is mediated through several receptors, especially the dectin-1 receptors, toll-like receptors, complement receptor 3, scavenger receptor and lactosylceramid. After the binding of β-glucan to its receptors, it stimulates the production of many cytokines or other mechanisms of immune and non-immune reactions.76| Supplementa | Daily dose and trial period | Subjects | Main conclusionb | Ref. |
|---|---|---|---|---|
| a I-β-glucan: purified insoluble β-glucan fraction (pleuran). b TG: triglycerides; TC: total cholesterol; ox-LDL: oxidised low density lipoproteins. | ||||
| P. ostreatus I-β-glucan | 100 mg for 2 months | Athletes (n = 20) | Modulation of exercise-induced changes in natural killer cell activity | 77 |
| P. ostreatus I-β-glucan | 100 mg in combination with 100 mg of vitamin C for 3 months | Athletes (n = 50) | Decrease in the incidence of upper respiratory tract infections symptoms and increase in the activity and number of natural killer cells | 78 |
| P. ostreatus I-β-glucan | 10 mg in combination with 10 mg of vitamin C per 5 kg body weight for 6 months | Children with recurrent respiratory tract infections (n = 175) | Improvement of the humoral and cellular immunity and prevention of infectious respiratory diseases | 76 |
| P. eryngii powder | 5–10 g for 2 days | Healthy human subjects (n = 12) | Enhancement of the innate and acquired immune responses | 80 |
| P. ostreatus powder | 10 g for 6 weeks | Patients with dyslipidemia (n = 57) | Decrease in blood TG and TC levels | 81 |
| P. ostreatus powder | 30 g for 21 days | Healthy human subjects (n = 20) | Decrease in TG, ox-LDL levels and TC levels | 82 |
| P. sajor-caju | Not reported dose for 3 months | Type 2 diabetic patients (n = 120) | Reduced fasting blood glucose, glycosylated haemoglobin as well as blood cholesterol levels | 85 |
| P. ostreatus powder | 3 g for 3 months | Type 2 diabetic patients (n = 27) | Decrease in fasting plasma glucose level and reduction in the level of glycosylated haemoglobin | 86 |
| P. ostreatus powder | 50 mg per kg of body weight for 1 month | Healthy human subjects (n = 22) type 2 diabetic patients (n = 28) | Decrease in fasting plasma glucose level and increased the serum insulin levels in diabetic patients | 84 |
Since excessive and exhausting physical loads depress the immune system, the immunomodulatory activity of Pleurotus β-glucan has been studied in athletes. A P. ostreatus insoluble β-glucan supplement (Imunoglukan 1) was orally administered to athletes to investigate the effects on cellular immune response and respiratory tract infections. In a double-blind pilot study, 20 elite athletes were randomized into insoluble β-glucan (n = 9) or placebo (n = 11) groups. These groups consumed 100 mg of β-glucan (Imunoglukan®) or placebo supplements, respectively, once a day for 2 months. The study showed that insoluble β-glucan supplementation from P. ostreatus may play a role in modulating exercise-induced changes in natural killer cell activity in intensively training athletes.77 In a second study, 50 healthy male (n = 26) and female (n = 24) top-level athletes were enrolled and randomized into a pleuran or placebo group. The experimental pleuran group consisted of athletes (n = 25) who were required to take 100 mg of β-glucan (Imunoglukan®) and 100 mg of vitamin C or a placebo (100 mg of vitamin C only) in the morning on an empty stomach for 3 months. The study confirmed that pleuran reduced the incidence of upper respiratory tract infections symptoms and increased the activity and number of natural killer cells.78
Additionally, the immunomodulatory properties of pleuran were studied in children with respiratory diseases. In this study, 175 children from 2 to 5 years of age with recurrent respiratory tract infections were enrolled and randomized into an active group, treated with 1 mL per 5 kg of Imunoglukan P4H® syrup (10 mg of pleuran and 10 mg of vitamin C in 1 mL of syrup) and a placebo group treated with vitamin C only, for 6 months. The results showed that in the active group, the humoral and cellular immunity improved and prevented infectious respiratory diseases.76 Patients were also monitored for parameters for allergy against a standardized panel of inhalant and food allergens, and it was revealed that pleuran showed a potential suppressive effect on the markers of allergic inflammation in peripheral blood, especially in atopic subjects. This effect led the study researchers to conclude that pleuran could also be applied as a complementary adjuvant therapy in allergic patients.79
Besides pleuran, P. eryngii superfine powder administered at a daily dosage of 5 to 15 g to 12 healthy volunteers for 2 days enhanced their innate and acquired immune responses.80
Hypolipidemic effects
The hypolipidemic effects of Pleurotus spp. have been investigated but a precise identification of the molecules involved is still lacking. Statins, such as lovastatin, which has been found in Pleurotus spp.67 and acts as an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase, are likely to be involved.P. ostreatus showed a significant hypocholesterolemic effect in a clinical study with 57 patients with dyslipidemia (32 women and 25 men with an average age of 43 years old). Subjects were fed lyophilized powder of P. ostreatus in an average daily dose of 10 g. After 6 weeks of mushroom feeding, the blood triglycerides (TG) and total cholesterol (TC) levels of the individuals decreased significantly.81 Likewise, in a study with 20 healthy human subjects (9 male and 11 female aged 20–34 years old), treatment with 30 g of dried P. ostreatus or a tomato soup as a placebo on a daily basis for 21 days decreased the TG concentrations and oxidized low density lipoprotein (ox-LDL) levels significantly, and showed a significant tendency towards lowering the TC values in comparison with the control group.82
Hypoglycaemic effects
Pleurotus spp. intake has been proven to have hypoglycaemic effects and to be able to decrease the levels of the marker of hyperglycaemia damage (glycosylated haemoglobin). In previous studies, the hypoglycemic effect from either the fruiting body or mycelia of some edible/medicinal fungi have been investigated in vitro and in animal models. The water extract and especially, the water-soluble polysaccharide fraction have been found to have hypoglycaemic properties. However, the hypoglycaemic effects can be observed in the whole dehydrated powder without any purification step but the molecules involved have not been precisely identified.83 The mechanism of hypoglycaemic activity of Pleurotus spp. is possibly through increasing the glucokinase activity and promoting insulin secretion, thereby increasing the utilization of glucose by peripheral tissues, inhibiting glycogen synthase kinase and promoting glycogen synthesis.84A study was conducted with 120 type 2 diabetic patients (randomly divided into three groups, with 40 patients in a mushroom-fed group, and the remaining groups serving as controls). It was found that patients in the group fed the P. sajor-caju mushroom for 3 months showed significantly reduced fasting blood glucose levels and glycosylated haemoglobin as well as blood cholesterol. However, the exact amount of P. sajor-caju supplemented daily was not specified.85
P. ostreatus powder was supplemented to 27 hypertensive males with type 2 diabetes mellitus (age range: 32 to 68 years old) at a daily dose of 3 g for 3 months. Both systolic and diastolic blood pressure decreased significantly. It was also observed that P. ostreatus decreased fasting plasma glucose levels and reduced the level of glycosylated haemoglobin.86 The hypoglycaemic effect of freeze-dried and powdered P. ostreatus was also investigated with 22 healthy human volunteers and 28 type 2 diabetic patients on diet control at a dose of 50 mg per kg per body weight, followed by a glucose load. The P. ostreatus powder showed a significant reduction in fasting and the postprandial serum glucose levels of healthy volunteers and reduced the postprandial serum glucose levels and increased the serum insulin levels of type 2 diabetic patients.84 Additionally, the inclusion of 8% of P. ostreatus powder in biscuits was found to decrease postprandial glycaemic response in 11 healthy participants (four males and seven females with no histories of carbohydrate malabsorption).18
Anticancer effects
Clear clinical evidence of the anticancer activities of Pleurotus mushrooms is still not available, even though different types of extracts from Pleurotus mushrooms have been reported as potential anticancer agents in several tumour cell lines, most likely acting through distinct mechanisms.5Sensory attributes and perception
A detailed knowledge on Pleurotus spp.'s aromatic profile is lacking, because only a few strains have been characterized. The aroma components of different Pleurotus spp. were studied by combining gas chromatography and electronic nose and sensory analysis involving a trained panel of assessors.95 This study confirmed that the main aroma constituents of Pleurotus spp. were C8 compounds, mainly 1-octen-3-ol, 3-octanol and 3-ocatonone. The highest amount of 1-octen-3-ol was measured in P. ostreatus, with an optical purity of (R)-(−)-1-octen-3-ol that accounted for 97.3%.96 Previous research indicated that (R)-(−)-1-octen-3-ol has a mushroom-like odour, whereas (S)-(+)-1-octen-3-ol has a mouldy, grassy note.97 In one strain of P. eryngii, the major volatile compound was found to be benzaldehyde, which confers a highly appreciated almond flavour.94 In another strain of P. eryngii, methional (potato-like odour), 1-octen 3-ol (mushroom odour) and nonanal (described as sweet, citrus and green) were found to be the main aroma components.92 Methional (potato-like odour) and 1-octen 3-ol (mushroom odour) were also found to be the main aroma component in P. sajor-caju.98
Food applications
Application of Pleurotus spp. as a fortifying agent
It is well known that cereal-based products are consumed throughout continents and civilizations, representing one of the most consumed foodstuffs; hence, studies have been carried out to improve their nutritive value and functional effect by the substitution of some ingredients with Pleurotus spp. powder or β-glucan-rich fractions (Table 7).| Food | Ingredienta | Main results | Ref. |
|---|---|---|---|
| a I-β-glucan: insoluble β-glucan; HW-β-glucan: hot-water-soluble β-glucan. | |||
| Bread | P. pulmonarius powder 5–25% of flour | Increase in protein and dietary fibre contents from 7.96 to 14.21 and from 0.51 to 2.48 g per 100 g f.w., respectively | 102 |
| Biscuits | P. sajor-caju powder 4–12% of flour | Increase in dietary fibre contents from 3.37 to 8.62 g per 100 g f.w. Decrease in the glycaemic index; upon 8% addition: glycaemic index from 57.2 to 49 | 18 |
| Pasta | P. eryngii I-β-glucan fraction 2–6% of flour | Fortification with I-β-glucans at final levels of 0.79–2.4 g per 100 g of flour | 17 |
| Tapioca cracker | P. sajor-caju powder 5–20% | Increase in protein content from 0.47 to 3.88 g per 100 g f.w. | 107 |
| Instant drink | P. eryngii broth | Fortification with ergothioneine at final levels of 6.22–11.57 mg per g d.w. and γ-aminobutyric acid at final levels of 4.19–8.30 mg per g d.w. | 108 |
| Chicken patty | P. sajor-caju powder 25–50% | Decrease in fat content, use of a cost-effective protein source: upon 25% addition fat from 11.91 to 9.86 g per 100 g f.w. and protein from 14.79 to 13.52 g per 100 g f.w. | 109 |
| Beef patty | P. sajor-caju powder 25–50% | Use of a cost-effective protein source; upon 25% addition: fat from 13.38 to 12.07 g per 100 g f.w. and protein from 22.73 to 19.37 g per 100 g f.w. | 110 |
| Ready-to-eat paste | P. sajor-caju powder 4–20% | Decrease in fat content, use of a cost-effective protein source; upon 20% addition fat from 13.82 to 8.16 g per 100 g f.w. and protein from 7.12 to 11.67 g per 100 g f.w. | 111 |
| Milk | P. ostreatus HW-β-glucan 0.25–1% | Increase in the counts of S. thermophilus and L. bulgaricus | 113 |
| P. eryngii HW-β-glucan 0.125–0.5% | Increase in the counts of S. thermophilus | 114 | |
| Soymilk | P. eryngii HW-β-glucan 0.5% | Increase in the counts of B. longum | 115 |
In bread, Pleurotus powder was added to replace 5–25% of the flour, with an aim to increase the protein and dietary fibre contents.102 In a study by Ng et al.,18 it was demonstrated that the addition of 8% of Pleurotus powder to biscuits increased dietary fibre content from 3.37% to 8.62% and decreased the in vivo glycaemic index. This effect was attributed to the mushroom fibre, which interfered with the starch granules by reducing the sizes and inducing uneven spherical shapes, resulting in reduced starch susceptibility to digestive enzymes.
The effects of the addition of Pleurotus spp. powder on the sensory properties of bread and biscuits were also investigated (Table 8). Okafor et al.102 found that bread samples supplemented with over 15% (flour basis) of P. pulmonarius powder negatively affected the liking scores, maybe due to a poor loaf size, dark colour and a pronounced mushroom taste and flavour. Accordingly, Ndung'u et al.103 found that wheat flour could be replaced with a low concentration (5%) of P. ostreatus powder to make fortified bread without adversely affecting the sensory acceptability. Indeed, the liking scores of the colour attribute decreased with increasing the mushroom content, due to the presence of dark coloured mushroom flour. Moreover, all the composite breads had a characteristic odour that could be responsible for the poor rating in aroma. Similarly, Prodhan et al.104 found that biscuits without incorporation of the mushroom powder obtained the highest score for overall acceptability compared to the fortified samples. However, considering the three mentioned studies, it must be taken into account that the number of semi-trained panellists involved was not appropriate.105 Concerning the supplementation of biscuits with different concentrations of P. sajor-caju powder, two studies have been conducted involving an adequate number of consumers.18,106 Wan Rosli et al.106 added lower concentrations of mushroom powder and observed no significant differences in overall acceptance among samples. Ng et al.18 found that supplementation with P. sajor-caju powder up to 8% to biscuits could lead to a more desirable aroma, colour and flavour when compared with the biscuit without supplementation. Nevertheless, with higher amounts of P. sajor-caju powder, undesirable results were obtained, with decreasing liking scores due to the higher degree of firmness and the stronger aroma and flavour as well as the darker surface colour of the biscuits.
| Food | Ingredienta | Sensory attribute | Judges | Hedonic scale | Ref. |
|---|---|---|---|---|---|
| a I-β-glucan: insoluble β-glucan. | |||||
| Bread | P. pulmonarius powder 5–25% of flour | Appearance, crust and crumb colour, texture, taste, chew ability, flavour and overall acceptability | 20 | 9-Points (1 = extremely unacceptable; 9 = extremely acceptable) | 102 |
| P. ostreatus powder 5–10% of flour | Crumb colour, crumb texture, aroma, taste and overall acceptability | 20 | 9-Points (1 = dislike extremely; 9 = like extremely) | 103 | |
| Biscuits | P. sajor-caju powder 2–15% of flour | Colour, texture, taste, odour and overall acceptability | 10 | 9-Points (1 = excellent; 9 = very poor) | 104 |
| P. sajor-caju powder 4–12% of flour | Aroma, colour, appearance, crispiness, flavour, overall acceptability | 60 | 7-Points (1 = dislike the most; 7 = like the most) | 18 | |
| P. sajor-caju powder 2–6% of flour | Aroma, colour, appearance, crispiness, flavour and overall acceptability | 60 | 7-Points (1 = dislike extremely; 7 = like extremely) | 106 | |
| Pasta | P. eryngii I-β-glucan fraction 2–6% of flour | Colour, flavour, hardness, and overall acceptability | 30 | 9-Points (1 = dislike extremely; 9 = like extremely) | 17 |
| Tapioca cracker | P. sajor-caju powder 5–20% | Colour, odour, crispness, taste, and overall acceptability | 30 | 7-Points (1 = dislike extremely; 7 = like extremely) | 107 |
| Instant drink | P. eryngii broth | Colour, flavour and overall acceptability | 50 | 7-Points (1 = dislike extremely; 7 = like extremely) | 108 |
| Chicken patty | P. sajor-caju powder 25–50% | Aroma, colour, springiness, juiciness, flavour and overall acceptability | 60 | 7-Points (1 = dislike extremely; 7 = like extremely) | 109 |
| Beef patty | P. sajor-caju powder 25–50% | Colour, juiciness, elasticity, flavour and overall acceptability | 60 | 7-Points (1 = dislike extremely; 7 = like extremely) | 110 |
| Ready-to-eat paste | P. sajor-caju powder 4–20% | Aroma, colour, viscosity, hotness, sourness, aftertaste and overall acceptability | 50 | 7-Points (1 = dislike extremely; 7 = like extremely) | 111 |
In pasta, insoluble dietary fibre separated from mushroom powder was added at levels of 2–6% of semolina to fortify the product with mushroom β-glucans (Table 7) (Kim et al., 2016).17 The results of sensory evaluation showed that common wheat pasta obtained the lowest liking scores, while the acceptability increased with the addition of the insoluble β-glucan fraction. In particular, the sample with 2% of the β-glucan-rich fractions added to replace wheat flour was significantly preferred compared to the sample without supplementation. However, an unsuitable number of judges was involved (Table 8).105
Yahya et al.107 incorporated powdered P. sajor-caju in a popular snack food in Malaysia and other Asian countries. Usually, these snacks (fried crackers) are produced with tapioca flour and fresh seafood, whereas the authors used mushroom powder as an alternative protein source, which was also suitable for vegetarians (Table 7). The fortified snacks showed higher mean scores for all the sensory attributes and for overall acceptability compared to the sample without the addition, maybe due to odour and taste enhancement by P. sajor-caju powder. However, it was difficult to draw firm conclusions due to the small group of consumers and the scale used in the sensory evaluation (Table 8).105
Lin et al.108 utilized the centrifuged broth from blanched P. eryngii, rich in taste-effective and bioactive components (which is a by-product of Pleurotus spp. processing), to develop a novel functional product as an instant drink. The centrifuged broth recovered consisted of 54.2–62.8% of the total weight of blanched mushrooms. The solids of the centrifuged broth contained free amino acids (15.20–34.23%), 5′-nucleotides (7.44–9.71%), sugars and polyols (33.55–34.97%) and substantial amounts of ergothioneine (5.49–9.90%) and γ-aminobutyric acid (1.23–6.90%). The indigestible dextrin Fibersol-2 was used as the carrier for the Pleurotus broth components (Table 7). Instant drinks (centrifuged broth mixed with Fibersol-2 at ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) dissolved in hot water were rated the highest in colour, flavour and overall acceptability, suggesting that the centrifuged broth could be developed as a functional food in the form of drink (Table 8).
5) dissolved in hot water were rated the highest in colour, flavour and overall acceptability, suggesting that the centrifuged broth could be developed as a functional food in the form of drink (Table 8).
Application of Pleurotus spp. as a high-cost protein replacer
Efforts have been also made to try to replace high-cost proteins in processed meat and poultry products. In this context, some authors investigated the ability of P. sajor-caju, which also permits maintaining the same protein content while decreasing production costs. To this aim, P. sajor-caju powder was added to chicken or beef patties at levels of 25–50% (Table 7).109,110 Results of the sensory evaluation showed that the patties made with different levels of mushroom powder (25% and 50%) were accepted by the consumers, since the liking scores for all the sensory attributes (e.g. aroma, colour, elasticity, juiciness, flavour) and overall acceptability were not significantly different compared to the unfortified samples (Table 8). Saiful Bahri and Wan Rosli111 investigated the effect of P. sajor-caju addition to replace coconut milk powder at a level of 4–20% on nutritional composition and the sensory acceptability of a Malaysia ready-to-eat paste (Table 7). The formulations had increased protein content and decreased fat content. Formulations with more than 40% of mushroom powder were accepted by the consumers (Table 8).Application of Pleurotus spp. as a prebiotic ingredient
Like other dietary fibre components, β-glucans isolated from Pleurotus spp. can stimulate the growth of colon microorganisms (probiotics), i.e. act as prebiotics. The water- and alkali-soluble fractions of P. ostreatus and P. eryngii (separated as described in Fig. 1) showed potential prebiotic activity in vitro towards Lactobacillus spp., Bifidobacterium spp. and Enterococcus faecium. Indeed, these fractions supported the probiotic bacteria growth rate, biomass and short chain fatty acid production.28 The insoluble fraction of P. eryngii was applied in a mice model system and resulted in an increased abundance of Porphyromonadaceae, Rikenellaceae, Bacteroidaceae and Lactobacillaceae.112β-Glucan-rich fractions were also applied in model foods, including fermented milk and soymilk (Table 7). Pelaes Vital et al.113 formulated a hot water extract obtained from P. ostreatus powder with milk (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), corresponding to a final mushroom powder concentration in the range of 0.25–1% before fermentation with Streptocuccus thermophilus and Lactobacillus bulgaricus. Li et al.114 applied the hot-water-soluble fraction obtained from P. eryngii to milk at a level of 0.125–0.5% before fermentation with S. thermophilus. In both these studies, β-glucan-rich fractions increased the counts of probiotic bacteria at the time of production and during storage for 1 month at 4 °C. In one study the effect of β-glucan addition to fermented milk on the content of angiotensin-I-converting enzyme (ACE)–inhibitory peptides was also investigated. These latter are defined as bioactive peptides having demonstrated anti-hypertensive properties and are produced as metabolites of bacterial proteinase, which have been widely found in dairy products. The addition of 0.125% of the hot-water-soluble fraction of P. eryngii led to increased levels of ACE-inhibitory peptides. However, higher additions led to lower ACE-inhibitory activity, probably due to the increase in proteolytic activity.114
1), corresponding to a final mushroom powder concentration in the range of 0.25–1% before fermentation with Streptocuccus thermophilus and Lactobacillus bulgaricus. Li et al.114 applied the hot-water-soluble fraction obtained from P. eryngii to milk at a level of 0.125–0.5% before fermentation with S. thermophilus. In both these studies, β-glucan-rich fractions increased the counts of probiotic bacteria at the time of production and during storage for 1 month at 4 °C. In one study the effect of β-glucan addition to fermented milk on the content of angiotensin-I-converting enzyme (ACE)–inhibitory peptides was also investigated. These latter are defined as bioactive peptides having demonstrated anti-hypertensive properties and are produced as metabolites of bacterial proteinase, which have been widely found in dairy products. The addition of 0.125% of the hot-water-soluble fraction of P. eryngii led to increased levels of ACE-inhibitory peptides. However, higher additions led to lower ACE-inhibitory activity, probably due to the increase in proteolytic activity.114
The hot-water-soluble β-glucan fraction obtained from P. eryngii and the whole P. eryngii powder were also added to soymilk at a level of 0.5% before fermentation with Bifidobacterium longum. This study revealed that the β-glucan-rich fraction had a higher bifidogenic effect compared with the whole P. eryngii powder.115
Moreover, the functional fermented milk and soymilk also showed different physical properties than the control product due to having a less dense microstructure, as revealed by texture analysis and scanning electron microscopy and/or confocal laser scanning microscopy. However, liking tests with consumers were not performed.113–115
Conclusive remarks and future work
The concepts proposed in this review are to explore the use of Pleurotus spp. as a sustainable food ingredient to address the needs of populations with endemic nutritional deficiencies as well as the needs of populations at risk or affected by some chronic diseases.Even though there has been some progress in the reduction of large-scale nutritional deficiencies in the world, there are periodic reports of outbreaks of protein, vitamin and mineral deficiencies related to populations under various distress conditions. It is also worth considering that nutritional deficiencies could even be underestimated, given that many cases are not reported in the medical literature.54 From the studies above summarized, it can be concluded that Pleurotus spp. grown on various food processing by-products can meet, to a considerable extent, the daily requirements of some essential amino acids, vitamins of the B group, vitamin D, Fe, Zn and Se. However, a better knowledge on the effects of the growth substrate and species on Pleurotus composition would lead to a more efficient design of its dietary applications.
There should also be a shift towards the use of sustainable sources to be used in the dietary prevention and management of the major chronic diseases. The human studies reported above have demonstrated potential immunomodulatory, hypolipidemic and hypoglycaemic effects of Pleurotus consumption. While the role of β-glucans as an anti-inflammatory agent has been well documented, the identification of possible healthy roles of other molecules that are bioactive in vitro is still lacking and deserves further investigation.
The so-far described food applications of Pleurotus powder or β-glucan-rich fractions isolated from Pleurotus spp. have mainly considered this mushroom as a source of proteins and β-glucans. However, to take advantage of the great potential of Pleurotus spp., a major focus on its micronutrients and bioactive compounds is needed. Moreover, the sensory properties of functional foods enriched with Pleurotus spp. play a pivotal role in food acceptance by consumers. In this context, sensory evaluation with a proper number of assessors could make a fundamental contribution to product optimization.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Research funded by Cariplo Foundation. Project ReMarcForFood n. 2016-0740.References
- H. C. J. Godfray, J. R. Beddington, I. R. Crute, L. Haddad, D. Lawrence, J. F. Muir, J. Pretty, S. Robinson, S. M. Thomas and C. Toulmin, Food Security: The Challenge of Feeding 9 Billion People, Science, 2010, 327, 812–818 CrossRef CAS PubMed.
- J. A. Foley, N. Ramankutty, K. A. Brauman, E. S. Cassidy, J. S. Gerber, M. Johnston, N. D. Mueller, C. O'Connell, D. K. Ray, P. C. West, C. Balzer, E. M. Bennett, S. R. Carpenter, J. Hill, C. Monfreda, S. Polasky, J. Rockstrom, J. Sheehan, S. Siebert, D. Tilman and D. P. M. Zaks, Solutions for a cultivated planet, Nature, 2011, 478, 337–342 CrossRef CAS PubMed.
- D. M. Martirosyan and J. Singh, A new definition of functional food by FFC: what makes a new definition unique?, Funct. Foods Health Dis., 2015, 5, 209–223 Search PubMed.
- C. Sánchez, Cultivation of Pleurotus ostreatus and other edible mushrooms, Appl. Microbiol. Biotechnol., 2010, 85, 1321–1337 CrossRef PubMed.
- R. C. G. Corrêa, T. Brugnari, A. Bracht, R. M. Peralta and I. C. F. R. Ferreira, Biotechnological, nutritional and therapeutic uses of Pleurotus spp. (Oyster mushroom) related with its chemical composition: A review on the past decade findings, Trends Food Sci. Technol., 2016, 50, 103–117 CrossRef.
- P. Manzi, L. Gambelli, S. Marconi, V. Vivanti and L. Pizzoferrato, Nutrients in edible mushrooms: an inter-species comparative study, Food Chem., 1999, 65, 477–482 CrossRef CAS.
- P. Manzi, A. Aguzzi and L. Pizzoferrato, Nutritional value of mushrooms widely consumed in Italy, Food Chem., 2001, 73, 321–325 CrossRef CAS.
- P. Mattila, P. Salo-Väänänen, K. Könkö, H. Aro and T. Jalava, Basic composition and amino acid contents of mushrooms cultivated in Finland, J. Agric. Food Chem., 2002, 50, 6419–6422 CrossRef CAS PubMed.
- I. V. Zmitrovich and S. P. Wasser, Is widely cultivated “Pleurotus sajor-caju”, especially in Asia, indeed an independent species?, Int. J. Med. Mushrooms, 2016, 18, 583–588 CrossRef PubMed.
- B. V. McCleary and A. Draga, Measurement of β-glucan in mushrooms and mycelial products, J. AOAC Int., 2016, 99, 364–373 CrossRef CAS PubMed.
- A. Gil-Ramírez, C. Pavo-Caballero, E. Baeza, N. Baenas, C. Garcia-Viguera, F. R. Marín and C. Soler-Rivas, Mushrooms do not contain flavonoids, J. Funct. Foods, 2016, 25, 1–13 CrossRef.
- N. Dalonso, G. H. Goldman and R. M. M. Gern, β-(1→3), (1→6)-Glucans: medicinal activities, characterization, biosynthesis and new horizons, Appl. Microbiol. Biotechnol., 2015, 99, 7893–7906 CrossRef CAS PubMed.
- A. Dundar, H. Acay and A. Yildiz, Yield performances and nutritional contents of three oyster mushroom species cultivated on wheat stalk, Afr. J. Biotechnol., 2008, 7, 3497–3501 CAS.
- A. Dundar, H. Acay and A. Yildiz, Effect of using different lignocellulosic wastes for cultivation of Pleurotus ostreatus (Jacq.) P. Kumm. on mushroom yield, chemical composition and nutritional value, Afr. J. Biotechnol., 2009, 8, 662–666 CAS.
- G. Koutrotsios, K. C. Mountzouris, I. Chatzipavlidis and G. I. Zervakis, Bioconversion of lignocellulosic residues by Agrocybe cylindracea and Pleurotus ostreatus mushroom fungi – Assessment of their effect on the final product and spent substrate properties, Food Chem., 2014, 161, 127–135 CrossRef CAS PubMed.
- P. Manzi, S. Marconi, A. Aguzzi and L. Pizzoferrato, Commercial mushrooms: nutritional quality and effect of cooking, Food Chem., 2004, 84, 201–206 CrossRef CAS.
- S. Kim, J.-W. Lee, Y. Heo and B. Moon, Effect of Pleurotus eryngii mushroom β-glucan on quality characteristics of common wheat pasta, J. Food Sci., 2016, 81, C835–C840 CrossRef CAS PubMed.
- S. H. Ng, S. D. Robert, W. A. N. Wan Ahmad and W. R. Wan Ishak, Incorporation of dietary fibre-rich oyster mushroom (Pleurotus sajor-caju) powder improves postprandial glycaemic response by interfering with starch granule structure and starch digestibility of biscuit, Food Chem., 2017, 227, 358–368 CrossRef CAS PubMed.
- S. T. Chang, O. W. Lau and K. Y. Cho, The cultivation and nutritional value of Pleurotus sajor-caju, Eur. J. Appl. Microbiol. Biotechnol., 1981, 12, 58–62 CrossRef CAS.
- S. S. Gogavekar, S. A. Rokade, R. C. Ranveer, J. S. Ghosh, D. C. Kalyani and A. K. Sahoo, Important nutritional constituents, flavour components, antioxidant and antibacterial properties of Pleurotus sajor-caju, J. Food Sci. Technol., 2014, 51, 1483–1491 CrossRef CAS PubMed.
- M. F. Da Paz, C. A. Breyer, R. F. Longhi and M. S. V. P. Oviedo, Determining the basic composition and total phenolic compounds of Pleurotus sajor-caju cultivated in three different substrates by solid state bioprocess, J. Biotechnol. Biodiversity, 2012, 3, 11–14 Search PubMed.
- G. D. Brown and S. Gordon, Immune recognition of fungal β-glucans, Cell. Microbiol., 2005, 7, 471–479 CrossRef CAS PubMed.
- O. Rop, J. Mlcek and T. Jurikova, Beta-glucans in higher fungi and their health effects, Nutr. Rev., 2009, 67, 624–631 CrossRef PubMed.
- G. Koutrotsios, N. Kalogeropoulos, P. Stathopoulos, A. C. Kaliora and G. I. Zervakis, Bioactive compounds and antioxidant activity exhibit high intraspecific variability in Pleurotus ostreatus mushrooms and correlate well with cultivation performance parameters, World J. Microbiol. Biotechnol., 2017, 33, 1–14 CrossRef CAS PubMed.
- X. Huang and S. Nie, The structure of mushroom polysaccharides and their beneficial role in health, Food Funct., 2015, 6, 3205–3217 CAS.
- S. Karacsonyi and L. Kuniak, Polysaccharides of Pleurotus ostreatus: isolation and structure of pleuran, an alkali-insoluble β-D-glucan, Carbohydr. Polym., 1994, 24, 107–111 CrossRef CAS.
- E. R. Carbonero, A. H. P. Gracher, F. R. Smiderle, F. R. Rosado, G. L. Sassaki, P. A. J. Gorin and M. Iacomini, A β-glucan from the fruit bodies of edible mushrooms Pleurotus eryngii and Pleurotus ostreatoroseus, Carbohydr. Polym., 2006, 66, 252–257 CrossRef CAS.
- A. Synytsya, K. Míčková, I. Jablonský, J. Spěváček, V. Erban, E. Kováříková and J. Čopíková, Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: structure and potential prebiotic activity, Carbohydr. Polym., 2009, 76, 548–556 CrossRef CAS.
- Report of a FAO Expert Consultation, FAO Food and Nutrition Paper, Dietary protein quality evaluation inhuman nutrition, Auckland, New Zealand, 31 March–2 April, 2011, http://www.fao.org/documents/card/en/c/ab5c9fca-dd15–58e0–93a8-d71e028c8282/ (accessed July 2017).
- AOAC, Association of Official Analytical Chemists, Arlington VA, USA, 17th edn, 2000 Search PubMed.
- Z. Bano, S. Rajarathnam and K. H. Steinkraus, Pleurotus mushrooms. Part II. Chemical composition, nutritional value, post-harvest physiology, preservation, and role as human food, Crit. Rev. Food Sci. Nutr., 1988, 27, 87–158 CrossRef CAS PubMed.
- A. Fernandes, L. Barros, A. Martins, P. Herbert and I. C. F. R. Ferreira, Nutritional characterisation of Pleurotus ostreatus (Jacq. ex Fr.) P. Kumm. produced using paper scraps as substrate, Food Chem., 2015, 169, 396–400 CrossRef CAS PubMed.
- D. Wang, A. Sakoda and M. Suzuki, Biological efficiency and nutritional value of Pleurotus ostreatus cultivated on spent beer grain, Bioresour. Technol., 2001, 78, 293–300 CrossRef CAS PubMed.
- L. Ancona Mendez, C. A. Sandoval Castro, R. Belmar Casso and C. M. Capetillo Leal, Effect of substrate and harvest on the amino acid profile of oyster mushroom (Pleurotus ostreatus), J. Food Compos. Anal., 2005, 18, 447–450 CrossRef.
- A. Gupta, S. Sharma, S. Saha and S. Walia, Yield and nutritional content of Pleurotus sajor caju on wheat straw supplemented with raw and detoxified mahua cake, Food Chem., 2013, 141, 4231–4239 CrossRef CAS PubMed.
- S. W. Chiu, Y. H. Chan, S. C. Law, K. T. Cheung and D. Moore, Cadmium and manganese in contrast to calcium reduce yield and nutritional values of the edible mushroom Pleurotus pulmonarius, Mycol. Res., 1998, 102, 449–457 CrossRef CAS.
- B. P. Juliano, in Rice Chemistry and Technology, ed. AACC, St. Paul, MN, 2nd edn, 1985 Search PubMed.
- FAOSTAT. (2017). Retrieved July 21st, 2017 from: http://faostat.fao.org/site/567/ default.aspx#ancor.
- P. Maftoun, H. Johari, M. Soltani, R. Malik, N. Z. Othman and H. A. El Enshasy, The edible mushroom Pleurotus spp.: I. Biodiversity and Nutritional Values, Int. J. Biotechnol. Wellness Ind., 2015, 4, 67–83 CrossRef.
- Y. Zhang, C. Venkitasamy, Z. Pan and W. Wang, Recent developments on umami ingredients of edible mushrooms - A review, Trends Food Sci. Technol., 2013, 33, 78–92 CrossRef CAS.
- K. Torii, H. Uneyama and E. Nakamura, Physiological roles of dietary glutamate signaling via gut–brain axis due to efficient digestion and absorption, J. Gastroenterol., 2013, 48, 442–451 CrossRef CAS PubMed.
- S. Y. Tsai, S. J. Huang, S. H. Lo, T. P. Wu, P. Y. Lian and J. L. Mau, Flavour components and antioxidant properties of several cultivated mushrooms, Food Chem., 2009, 113, 578–584 CrossRef CAS.
- S. Beluhan and A. Ranogajec, Chemical composition and non-volatile components of Croatian wild edible mushrooms, Food Chem., 2011, 124, 1076–1082 CrossRef CAS.
- W. Li, Z. Gu, Y. Yang, S. Zhou, Y. Liu and J. Zhang, Non-volatile taste components of several cultivated mushrooms, Food Chem., 2014, 143, 427–431 CrossRef CAS PubMed.
- W. Li, X. Li, Y. Yang, F. Zhou, Y. Liu, S. Zhou and H. Yu, Effects of different carbon sources and C/N values on nonvolatile taste components of Pleurotus eryngii, Int. J. Food Sci. Technol., 2015, 50, 2360–2366 CrossRef CAS.
- C. Phat, B. Moon and C. Lee, Evaluation of umami taste in mushroom extracts by chemical analysis, sensory evaluation, and an electronic tongue system, Food Chem., 2016, 192, 1068–1077 CrossRef CAS PubMed.
- F. S. Reis, L. Barros, M. J. Sousa, A. Martins and I. C. F. R. Ferreira, Analytical methods applied to the chemical characterization and antioxidant properties of three wild edible mushroom species from northeastern Portugal, Food Anal. Methods, 2014, 7, 645–652 CrossRef.
- L. Barros, C. Pereira and I. C. F. R. Ferreira, Optimized analysis of organic acids in edible mushrooms from Portugal by ultra-fast liquid chromatography and photodiode array detection, Food Anal. Methods, 2013, 6, 309–316 CrossRef.
- P. Kalač and L. Svoboda, A review of trace element concentrations in edible mushrooms, Food Chem., 2000, 69, 273–281 CrossRef.
- P. Mattila, K. Könkö, M. Eurola, J.-M. Pihlava, J. Astola, L. Vahteristo, V. Hietaniemi, J. Kumpulainen, M. Valtonen and V. Piironen, Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms, J. Agric. Food Chem., 2001, 49, 2343–2348 CrossRef CAS PubMed.
- S. S. Patil, S. A. Ahmed, S. M. Telang and M. M. V. Baig, The nutritional value of Pleurotus ostreatus (jacq.:fr.) kumm cultivated on different lignocellulosic agrowastes, Innovative Rom. Food Biotechnol., 2010, 7, 66–76 CAS.
- WHO, Guideline: Potassium intake for adults and children, Geneva, World Health Organization (WHO), 2012, http://www.who.int/nutrition/publications/guidelines/potassium_intake/en/ (accessed September 2017) Search PubMed.
- WHO, Guideline: Sodium intake for adults and children, Geneva, World Health Organization (WHO), 2012, http://www.who.int/nutrition/publications/guidelines/sodium_intake/en/ (accessed September 2017) Search PubMed.
- WHO/FAO, Vitamin and mineral requirements in human nutrition, Geneva, Second edn, 2004, http://www.who.int/nutrition/publications/micronutrients/9241546123/en/ (accessed September 2017) Search PubMed.
- M. A. Khan and M. Tania, Nutritional and medicinal importance of Pleurotus mushrooms: an overview, Food Rev. Int., 2012, 28, 313–329 CrossRef CAS.
- S. R. Drago, in Nutraceutical and Functional Food Components - Effects of Innovative Processing Techniques, ed. C. M. Galanakis, Academic Press, 2017, pp. 129–157 Search PubMed.
- H. Memuna and C. H. Chakrabarti, A study on availability of iron in mushrooms, Indian J. Nutr. Diet., 1982, 19, 203–207 Search PubMed.
- M. C. S. Da Silva, J. Naozuka, J. M. R. Da Luz, L. S. De Assunção, P. V. Oliveira, M. C. D. Vanetti, D. M. S. Bazzolli and M. C. M. Kasuya, Enrichment of Pleurotus ostreatus mushrooms with selenium in coffee husks, Food Chem., 2012, 131, 558–563 CrossRef CAS.
- G. Jaworska, K. Pogon, E. Bernas and A. Duda-Chodak, Nutraceuticals and antioxidant activity of prepared for consumption commercial mushrooms Agaricus bis porus and Pleurotus ostreatus, J. Food Qual., 2015, 38, 111–122 CrossRef CAS.
- Z. Bano and S. Rajarathnam, Vitamin values of Pleurotus mushrooms, Qual. Plant. – Plant Foods Hum. Nutr., 1986, 36, 11–15 CrossRef CAS.
- A. J. Clifford, M. K. Heid, J. M. Peerson and N. D. Bills, Bioavailability of food folates and evaluation of food matrix effects with a rat bioassay, J. Nutr., 1991, 121, 445–453 CrossRef CAS PubMed.
- A. N. Shivrina, L. N. Koryokina and P. A. Yakimov, Vitamin B12 content of polyporaceae and Agaricus strains, in Kormovya Belk, i. Fiziol. Aktiyn. Veshchestva Dlya Zhivotnovadstva, Akad. Nauk., SSSR, Botan. Inst., 1965, pp. 88–91 Search PubMed.
- S.-J. Huang, C.-P. Lin and S.-Y. Tsai, Vitamin D2 content and antioxidant properties of fruit body and mycelia of edible mushrooms by UV-B, J. Food Compos. Anal., 2015, 42, 38–45 CrossRef CAS.
- Y. Sapozhnikova, W. C. Byrdwell, A. Lobato and B. Romig, Effects of UV-B radiation levels on concentrations of phytosterols, ergothioneine, and polyphenolic compounds in mushroom powders used as dietary supplements, J. Agric. Food Chem., 2014, 62, 3034–3042 CrossRef CAS PubMed.
- A. Sławińska, E. Fornal, W. Radzki, K. Skrzypczak, M. Zalewska-Korona, M. Michalak-Majewska, E. Parfieniuk and A. Stachniuk, Study on vitamin D2 stability in dried mushrooms during drying and storage, Food Chem., 2016, 199, 203–209 CrossRef PubMed.
- B. Ribeiro, P. G. de Pinho, P. B. Andrade, C. Oliveira, A. C. S. Ferreira, P. Baptista and P. Valentão, Do bioactive carotenoids contribute to the color of edible mushrooms?, Open Chem. Biomed. Methods J., 2011, 4, 14–18 CrossRef CAS.
- S.-Yu. Chen, K.-J. Ho, Y.-J. Hsieh, L.-T. Wang and J.-L. Mau, Contents of lovastatin, γ-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia, LWT – Food Sci. Technol., 2012, 47, 274–278 CrossRef CAS.
- I. K. Cheah and B. Halliwell, Ergothioneine; antioxidant potential, physiological function and role in disease, Biochim. Biophys. Acta, 2012, 1822, 784–793 CrossRef CAS PubMed.
- N. J. Dubost, B. Ou and R. B. Beelman, Quantification of polyphenols and ergothioneine in cultivated mushrooms and correlation to total antioxidant capacity, Food Chem., 2007, 105, 727–735 CrossRef CAS.
- J. A. Carrasco-González, S. O. Serna-Saldívar and J. A. Gutiérrez-Uribe, Nutritional composition and nutraceutical properties of the Pleurotus fruiting bodies: Potential use as food ingredient, J. Food Compos. Anal., 2017, 58, 69–81 CrossRef.
- S. Khatun, A. Islam, U. Cakilcioglu, P. Gulerd and N. C. Chatterjee, Nutritional qualities and antioxidant activity of three edible oyster mushrooms (Pleurotus spp.), NJAS, Wageningen J. Life Sci., 2015, 72–73, 1–5 Search PubMed.
- J. A. Carrasco-Gonzalez, S. O. Serna-Saldívar and J. A. Gutierrez-Uribe, Mycochemical changes induced by Selenium enrichment in P. ostreatus fruiting bodies, J. Agric. Food Chem., 2017, 65, 4074–4082 CrossRef PubMed.
- R. V. C. Cardoso, A. Fernandes, M. Beatriz, P. P. Oliveira, R. C. Calhelha, L. Barros, A. Martinsa and I. C. F. R. Ferreira, Development of nutraceutical formulations based on the mycelium of Pleurotus ostreatus and Agaricus bisporus, Food Funct., 2017, 8, 2155–2164 CAS.
- F. S. Reis, A. Martins, L. Barros and I. C. F. R. Ferreira, Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples, Food Chem. Toxicol., 2012, 50, 1201–1207 CrossRef CAS PubMed.
- N. G. Puttaraju, S. U. Venkateshaiah, S. M. Dharmesh, S. M. N. Urs and R. Somasundaram, Antioxidant activity of indigenous edible mushrooms, J. Agric. Food Chem., 2006, 54, 9764–9772 CrossRef CAS PubMed.
- M. Jesenak, J. Majtan, Z. Rennerova, J. Kyselovic, P. Banovcin and M. Hrubisko, Immunomodulatory effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections, Int. Immunopharmacol., 2013, 15, 395–399 CrossRef CAS PubMed.
- M. Bobovcák, R. Kuniaková, J. Gabriž and J. Majtán, Effect of Pleuran (β-glucan from Pleurotus ostreatus) supplementation on cellular immune response after intensive exercise in elite athletes, Appl. Physiol., Nutr., Metab., 2010, 35, 755–762 CrossRef PubMed.
- K. Bergendiova, E. Tibenska and J. Majtan, Pleuran (β-glucan from Pleurotus ostreatus) supplementation, cellular immune response and respiratory tract infections in athletes, Eur. J. Appl. Physiol., 2011, 111, 2033–2040 CrossRef CAS PubMed.
- M. Jesenak, M. Hrubisko, J. Majtan, Z. Rennerova and P. Banovcin, Anti-allergic effect of Pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections, Phytother. Res., 2014, 28, 471–474 CrossRef PubMed.
- Y. Sun, Y. Ma, Z. Xu, W. Yang, A. M. Mariga, G. Pang, G. Geng and Q. Hu, Immunoregulatory role of Pleurotus eryngii superfine powder through intercellular communication of cytokines, Food Agric. Immunol., 2014, 25, 586–599 CrossRef CAS.
- I. Kajaba, R. Simoncic, K. Frecerova and G. Belay, Clinical studies on the hypolipidemic and antioxidant effects of selected natural substances, Bratisl. Lek. Listy, 2008, 109, 267–272 CAS.
- I. Schneider, G. Kressel, A. Meyer, U. Krings, R. G. Berger and A. Hahn, Lipid lowering effects of oyster mushroom (Pleurotus ostreatus) in humans, J. Funct. Foods, 2011, 3, 17–24 CrossRef CAS.
- S. Khatun, A. Islam, P. Guler, U. Cakilcioglu and N. C. Chatterjee, Hypoglycemic activity of a dietary mushroom Pleurotus florida, on alloxan induced diabetic rats, Biol. Divers. Conserv., 2013, 6/2, 91–96 Search PubMed.
- W. J. A. B. N. Jayasuriya, C. A. Wanigatunge, G. H. Fernando, D. T. U. Abeytunga and T. S. Suresh, Hypoglycaemic activity of culinary Pleurotus ostreatus and P. cystidiosus mushrooms in healthy volunteers and type 2 diabetic patients on diet control and the possible mechanisms of action, Phytother. Res., 2015, 29, 303–309 CrossRef PubMed.
- R. P. Agrawal, A. Chopra, G. S. Lavekar, M. M. Padhi, N. Srikanth, S. Ota and S. Jain, Effect of oyster mushroom on glycemia, lipid profile and quality of life in type 2 diabetic patients, Aust. J. Med. Herb., 2010, 22, 50–54 Search PubMed.
- M. B. K. Choudhury, T. Rahman, A. J. Kakon, N. Hoque, M. Akhtaruzzaman, M. M. Begum, M. S. K. Choudhuri and M. S. Hossain, Effects of Pleurotus ostreatus on blood pressure and glycemic status of hypertensive diabetic male volunteers, Bangladesh J. Med. Biochem., 2013, 6, 5–10 Search PubMed.
- G. Jaworska and E. Bernaś, Qualitative changes in Pleurotus ostreatus (Jacq.: Fr.) Kumm. mushrooms resulting from different methods of preliminary processing and periods of frozen storage, J. Sci. Food Agric., 2009, 89, 1066–1075 CrossRef CAS.
- A. Omarini, V. Nepote, N. R. Grosso, J. A. Zygadlo and E. Albertó, Sensory analysis and fruiting bodies characterisation of the edible mushrooms Pleurotus ostreatus and Polyporus tenuiculus obtained on leaf waste from the essential oil production industry, Int. J. Food Sci. Technol., 2010, 45, 466–474 CrossRef CAS.
- S. L. Cuppett, A. M. Parkhurst, W. Chung, M. Weyer and L. B. Bullerman, Factors affecting sensory attributes of oyster mushrooms, J. Food Quality, 1998, 21, 383–395 CrossRef.
- K. Kurihara, Glutamate: from discovery as a food flavor to role as a basic taste (umami), Am. J. Clin. Nutr., 2009, 90, 719S–722S CrossRef CAS PubMed.
- T. Feng, F. Bing, Y. Yang, H. Zhuang, R. Ye, X. Li, Z. Xu and K. Wang, Discrimination of edible fungi varieties and evaluation of their umami intensities by using an electronic tongue method, Int. J. Food Sci. Technol., 2016, 51, 1393–1400 CrossRef CAS.
- A. Usami, R. Motooka, H. Nakahashi, Y. Okuno and M. Miyazawa, Characteristic odorants from bailingu oyster mushrooms (Pleurotus eryngii var. tuoliensis) and summer oyster mushrooms (Pleurotus cystidiosus), J. Oleo Sci., 2014, 63, 731–739 CrossRef CAS PubMed.
- W. Kabbaj, S. Breheret, J. Guimberteau, T. Talou, J.-M. Olivier, M. Bensoussan, M. Sobal and S. Roussos, Comparison of volatile compound production in fruit body and in mycelium of Pleurotus ostreatus identified by submerged and solid-state cultures, Appl. Biochem. Biotechnol., 2002, 102, 463–469 CrossRef PubMed.
- J.-L. Mau, Y.-P. Lin, P. T. Chen, Y.-H. Wu and J.-T. Peng, Flavor compounds in king oyster mushrooms Pleurotus eryngii, J. Agric. Food Chem., 1998, 46, 4587–4591 CrossRef CAS.
- R. Zawirska-Wojtasiak, M. Siwulski, S. Mildner-Szkudlarz and E. W
![[a with combining cedilla]](https://www.rsc.org/images/entities/char_0061_0327.gif) sowicz, Studies on the aroma of different species and strains of Pleurotus measured by GC/MS, sensory analysis and electronic nose, Acta Sci. Pol. Technol. Aliment., 2009, 8, 47–61 CAS.
sowicz, Studies on the aroma of different species and strains of Pleurotus measured by GC/MS, sensory analysis and electronic nose, Acta Sci. Pol. Technol. Aliment., 2009, 8, 47–61 CAS. - R. Zawirska-Wojtasiak, Optical purity of (R)-(-)-1-octen-3-ol in the aroma of various species of edible mushrooms, Food Chem., 2004, 86, 113–118 CrossRef CAS.
- A. Mosandl, G. Heusinger and M. Gessner, Analytical and sensory differentiation of 1-octen-3-ol enanatiomers, J. Agric. Food Chem., 1986, 34, 119–122 CrossRef CAS.
- A. Usami, S. Nakaya, H. Nakahashi and M. Miyazawa, Chemical composition and aroma evaluation of volatile oils from edible mushrooms (Pleurotus salmoneostramineus and Pleurotus sajor-caju), J. Oleo Sci., 2014, 63, 1323–1332 CrossRef CAS PubMed.
- M. Jafri, A. Jha, D. S. Bunkar and R. C. Ram, Quality retention of oyster mushrooms (Pleurotus florida) by a combination of chemical treatments and modified atmosphere packaging, Postharvest Biol. Technol., 2013, 76, 112–118 CrossRef CAS.
- S. Zivanovic, R. W. Busher and K. S. Kim, Textural changes in mushrooms (Agaricus bis porus) associated with tissue ultrastructure and composition, J. Food Sci., 2000, 65, 1404–1408 CrossRef CAS.
- J. Liu, C. Vijayakumar, C. A. Hall III, M. Hadley and C. E. Wolf-Hall, Sensory and chemical analyses of oyster mushrooms (Pleurotus sajor-caju) harvested from different substrates, J. Food Sci., 2005, 70, S586–S592 CrossRef CAS.
- J. N. C. Okafor, G. I. Okafor, A. U. Ozumba and G. N. Elemo, Quality characteristics of bread made from wheat and Nigerian oyster mushroom (Pleurotus plumonarius) powder, Pak. J. Nutr., 2012, 11, 5–10 CrossRef CAS.
- S. W. Ndung'u, C. A. Otieno, C. Onyango and F. Musieba, Nutritional composition, physical qualities and sensory evaluation of wheat bread supplemented with oyster mushroom, Am. J. Food Technol., 2015, 10, 241–253 CrossRef.
- U. K. Prodhan, K. M. M. R. Linkon, M. F. Al-Amin and M. J. Alam, Development and quality evaluation of mushroom (Pleurotus sajor-caju) enriched biscuits, Emirates J. Food Agric., 2015, 27, 542–547 CrossRef.
- H. T. Lawless and H. Heymann, in Sensory Evaluation of Food, Springer, New York, 2nd edn, 2010, pp. 19–56 Search PubMed.
- W. I. Wan Rosli, A. R. Nurhanan and M. S. Aishah, Effect of partial replacement of wheat flour with oyster mushroom (Pleurotus sajor-caju) powder on nutritional composition and sensory properties of butter biscuit, Sains Malays., 2012, 41, 1565–1570 CAS.
- F. Yahya, N. N. M. Yusof and C. K. Chen, Effect of varying ratios of oyster mushroom powder to tapioca flour on the physicochemical properties and sensory acceptability of fried mushroom crackers, Malays. Appl. Biol., 2017, 46, 57–62 Search PubMed.
- S.-D. Lin, Y.-T. Wu, Y.-C. Lo and J.-L. Mau, Quality characteristics of centrifuged broth from blanched Pleurotus eryngii and its application as instant drink, J. Food Process Preserv., 2017, 00:e13356, 1–8 Search PubMed.
- W. I. Wan Rosli, M. A. Solihah and S. S. J. Mohsin, On the ability of oyster mushroom (Pleurotus sajor-caju) conferring changes in proximate composition and sensory evaluation of chicken patty, Int. Food Res. J., 2011, 18, 1463–1469 CAS.
- W. I. Wan Rosli and M. A. Solihah, Effect on the addition of Pleurotus sajor-caju (PSC) on physical and sensorial properties of beef patty, Int. Food Res. J., 2012, 19, 993–999 CAS.
- S. Saiful Bahri and W. I. Wan Rosli, Effect of oyster mushroom (Pleurotus sajor-caju) addition on the nutritional composition and sensory evaluation of herbal seasoning, Int. Food Res. J., 2016, 23, 262–268 CAS.
- G. Ma, B. M. Kimatu, L. Zhao, W. Yang, F. Pei and Q. Hu, In vivo fermentation of a Pleurotus eryngii polysaccharide and its effects on fecal microbiota composition and immune response, Food Funct., 2017, 8, 1810–1821 CAS.
- A. C. Pelaes Vital, P. A. Goto, L. N. Hanai, S. M. Gomes-da-Costa, B. Alves de Abreu Filho, C. V. Nakamura and P. T. Matumoto-Pintro, Microbiological, functional and rheological properties of low fat yogurt supplemented with Pleurotus ostreatus aqueous extract, LWT – Food Sci. Technol., 2015, 64, 1028–1035 CrossRef.
- S. Li and N. P. Shah, Effects of Pleurotus eryngii polysaccharides on bacterial growth, texture properties, proteolytic capacity, and angiotensin-I-converting enzyme–inhibitory activities of fermented milk, J. Dairy Sci., 2015, 98, 2949–2961 CrossRef CAS PubMed.
- S. Li and N. P. Shah, Characterization, antioxidative and bifidogenic effects of polysaccharides from Pleurotus eryngii after heat treatments, Food Chem., 2016, 197, 240–249 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |





