Prophylactic role of vitamin K supplementation on vascular inflammation in type 2 diabetes by regulating the NF-κB/Nrf2 pathway via activating Gla proteins
Anjum
Dihingia
ab,
Dibyajyoti
Ozah
c,
Pranab Kumar
Baruah
c,
Jatin
Kalita
ab and
Prasenjit
Manna
 *ab
*ab
aBiological Science and Technology Division, CSIR-North East Institute of Science and Technology, Jorhat, Assam, India. E-mail: pmanna2012@gmail.com; Fax: +91-376-2370011; Tel: +91-376-2370012
bAcademy of Scientific and Innovative Research (AcSIR), CSIR-NEIST Campus, Jorhat, Assam, India
cClinical Centre, CSIR-North East Institute of Science and Technology, Jorhat, Assam, India
First published on 27th November 2017
Abstract
There is no previous study that has examined the relationship between circulating vitamin K1 (VK1) and vascular inflammation in type 2 diabetes (T2D). This study aims to examine the hypothesis that circulating VK1 deficiency may be associated with higher inflammation and insulin resistance in T2D patients and that VK1 supplementation regulates the NF-κB/Nrf2 pathway via activating VK-dependent Gla proteins and reduces vascular inflammation. The results showed that plasma VK1 levels were significantly lower and MCP-1, fasting glucose, HbA1c, and insulin resistance (HOMA-IR) were significantly higher in T2D patients compared to those in the controls. The lower levels of VK1 in T2D patients were significantly and inversely correlated with MCP-1 and HOMA-IR, which suggests that VK1 supplementation may reduce the vascular inflammation and insulin resistance in T2D. Using a high fat diet-fed T2D mice model this study further demonstrated that VK1 supplementation (1, 3, 5 μg per kg BW, 8 weeks) dose-dependently decreased the body weight gain, glucose intolerance, fasting glucose, glycated hemoglobin, HOMA-IR, and cytokine secretion (MCP-1 and IL-6) in T2D mice. Further cell culture studies showed that VK1 supplementation (1, 5, or 10 nM) decreased NF-κB phosphorylation and MCP-1 secretion and increased Nrf2 protein expression in high glucose (HG, 25 mM)-treated monocytes. Signal silencing studies with GGCX siRNA again depicted the role of VK-dependent Gla proteins in mediating the effect of VK1 on vascular inflammation in HG-treated cells. In conclusion, this study suggests that circulating VK1 has a positive effect in lowering vascular inflammation in T2D by regulating NF-κB/Nrf2 transcription factors via activating VK-dependent Gla proteins.
1. Introduction
Vitamin K (VK) is blooming as an essential micronutrient on the health products market.1 VK is known to be important for blood coagulation, and unlike other fat-soluble vitamins, VK acts as a cofactor for a single microsomal enzyme, namely γ-glutamate carboxylase (GGCX), which catalyses the post-translational conversion of protein-bound glutamate residues into γ-carboxyglutamate.2 Recently, however, various studies increasingly indicate the coagulation-unrelated functions of this antihaemorrhagic vitamin, such as bone and cardiovascular mineralization, vascular hemostasis, immune response, brain metabolism, energy metabolism, and in cellular growth, survival, and signaling.3,4 In contrast to newborn babies, VK deficiency in relation to blood coagulation is rare among adults; however, long-term VK deficiency among the elderly population is an independent risk factor for the development of different degenerative diseases, such as osteoarthritis,5 atherosclerosis,4 vascular calcification,6 obesity,7 chronic kidney disease,6etc.Sub-clinical, chronic, low-grade inflammation coupled with insulin resistance plays an important role in the pathogenesis of several diseases including type 2 diabetes (T2D) and cardiovascular diseases (CVD).8 Using LPS-treated different cell culture models, various studies demonstrated the anti-inflammatory role of VK.9,10 An inverse relationship between the circulating VK status and the measurement of vascular inflammation has also been observed among normal subjects.11 Dietary VK intake or VK supplementation has been found to improve glycemic status and reduce vascular inflammation, T2D risk factors, and the prevalence of metabolic syndrome among normal subjects.3,12 The hypoglycemic effect of VK1 has also been investigated using diabetic animal models.13–15 However, there is no information about the relationship between circulating VK status, vascular inflammation, and insulin resistance in individuals with T2D. Moreover, the mechanism underlying the beneficial effect of VK supplementation on vascular inflammation in diabetes remains unclear. Using plasma samples from both T2D patients and age-matched healthy controls, this study examined the hypothesis that plasma VK deficiency may be associated with the state of higher degree of vascular inflammation and insulin resistance in T2D. Furthermore, this study also investigated whether VK supplementation can reduce the secretion of pro-inflammatory cytokine and insulin resistance and maintain the glucose homeostasis in T2D by using a high fat diet (HFD)-fed animal model of T2D.
Nuclear factor-κB (NF-κB) and nuclear factor-erythroid 2-related factor 2 (Nrf2) are the two well-known transcription factors, which have been reported to play an important role in modulating inflammatory responses.16,17 Earlier studies reported that treatment with VK reduced the activation of NF-κB and the secretion of pro-inflammatory cytokines in LPS-treated cells.9,10 Interestingly, coagulation-unrelated VK-dependent Gla proteins are gaining scientific attention in the regulation of obesity, T2D, and CVD risk factors.18,19 Using a human monocyte cell culture model and a signal silencing approach with GGCX siRNA (γ-glutamate carboxylase, an enzyme which causes the activation of VK-dependent Gla proteins), this study further dissected the molecular mechanism underlying the beneficial effect of VK against vascular inflammation associated with T2D.
2. Materials and methods
2.1 Materials
Anti-GGCX, anti-phosphorylated NF-κB (p65) (Ser 536), and anti-Nrf2 primary antibodies were purchased from Abcam, Inc. (Cambridge, MA). Anti-NF-κB (p65) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Lipopolysaccharide (LPS), VK1, and all other chemicals were purchased from Sigma Chemical Co. (St Louis, MO) unless otherwise mentioned.2.2 Study enrollment and blood collection from T2D patients and normal subjects
Informed written consent was obtained from all patients and normal subjects according to the protocol approved by the Institutional Ethics Committee for Human Experimentation. All subjects included in this study were T2D patients (n = 19) attending the clinical centre at the North East Institute of Science and Technology (NEIST). Volunteers for age-matched controls (n = 14) were enrolled from a group including siblings of patients or employees at NEIST. T2D patients and healthy controls were excluded if they had any history of cardiovascular disease, sickle cell disease, smoking habits, uncontrolled hypertension, hypothyroidism, hyperthyroidism, and treatment with an anticoagulant or insulin as evident from the records maintained at the clinical centre. Subjects were also excluded if they showed signs of coagulation complications, defined as a prothrombin time greater than 15 s. Hepatic and renal dysfunctions were defined as the liver function tests 1.5 times higher than the upper limit and the serum creatinine value greater than 1.5 mg%, respectively. Women with a positive pregnancy test or those nursing infants were also excluded. Subjects taking any supplemental vitamins or herbal products were excluded. All patients and normal subjects who gave written informed consent were invited to return to have blood drawn after fasting overnight (8 h). Serum tubes containing blood for chemistry profiles, sodium citrate (3.2%) vials for prothrombin time test, and EDTA-blood tubes for HbA1c were promptly delivered to the NEIST clinical laboratory. Additional EDTA-blood was brought to the research laboratory, plasma was separated via centrifugation at 3000 rpm for 15 min, then transferred to cryovials, and stored at −80 °C for further analysis.2.3 Estimation of plasma vitamin K1 (VK1) levels among human subjects
Circulating VK1 levels in T2D patients and normal subjects were measured using a HPLC system (Waters) equipped with a C30 column (Accucore, 3 × 100 mm, 2.6 μm) followed by a post-column on-line zinc metal reactor and a fluorometric detector (λex 248 and λex 430).20 A proprietary VK1 derivative was used as an internal standard (Immundiagnostik AG, Wiesenstrasse, Bensheim, Germany, KC2400is).2.4. Animal studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 h light–dark cycle, 22–24 °C). The study was also approved by the CPCSEA (Committee for the Purpose of Control & Supervision on Experiments on Animals), Ministry of Environment & Forests, New Delhi, India (1796/PO/Ere/S/14/CPCSEA) and the Institutional Animal Ethical Committee (IAEC), Bose Institute, Kolkata. All animal experiments were carried out following the guidelines of IAEC.
12 h light–dark cycle, 22–24 °C). The study was also approved by the CPCSEA (Committee for the Purpose of Control & Supervision on Experiments on Animals), Ministry of Environment & Forests, New Delhi, India (1796/PO/Ere/S/14/CPCSEA) and the Institutional Animal Ethical Committee (IAEC), Bose Institute, Kolkata. All animal experiments were carried out following the guidelines of IAEC.
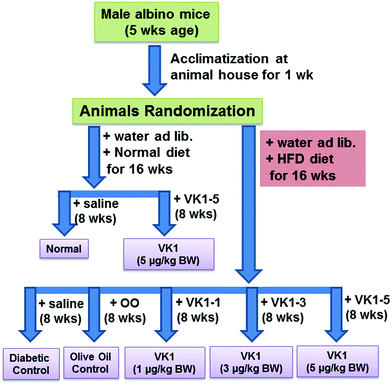
Normal – low fat diet (providing 10% calories as fat) feeding for 16 weeks.
VK1-5 – low fat diet feeding for 16 weeks and VK1 administered at a dose of 5 μg per kg BW daily by oral gavage for the last 8 weeks.
T2D – high fat diet (HFD, providing 45% calories as fat) feeding for 16 weeks.
T2D + OO – HFD feeding for 16 weeks and OO administered at a dose of 100 μL per 100 g BW daily by oral gavage for the last 8 weeks.
T2D + VK1-1, T2D + VK1-3, T2D + VK1-5 – HFD feeding for 16 weeks and VK1 administered at a dose of 1, 3, and 5 μg per kg BW, respectively, daily by oral gavage for the last 8 weeks.
The composition of the experimental diet is presented in Table 1. The high fat diet-fed animal model is a well-recognized model for T2D. The recommended adequate intake for VK1 is 1 μg per kg BW;18 however, it is not known whether this adequate intake is sufficient to meet all the physiological needs, especially coagulation-unrelated health outcomes like diabetes. At present, no known toxicity has been reported with higher doses of VK1 supplementation.2 Thus, no tolerable upper intake limit has been set yet. Body weight and blood glucose levels were measured weekly, and food intake was recorded daily. At the end of 16 weeks, the animals were fasted overnight, weighed and euthanized by exposure to isoflurane. Blood was collected via heart puncture with a 191/2 gauge needle into EDTA Vacutainer tubes. Plasma was isolated after centrifuging the blood in a 4 °C centrifuge at 3000 rpm for 15 min and stored immediately at −80 °C until further use.
| Selected nutrient information | Normal diet | High fat diet (HFD) | ||
|---|---|---|---|---|
| g (%) | kcal (%) | g (%) | kcal (%) | |
| Protein | 19 | 20 | 24 | 20 |
| Carbohydrate | 67 | 70 | 41 | 35 |
| Fat | 4 | 10 | 24 | 45 |
| Ingredient | g | kcal | g | kcal |
|---|---|---|---|---|
| Casein | 200 | 800 | 200 | 800 |
| L-Cystine | 3 | 12 | 3 | 12 |
| Corn starch | 550 | 2200 | 0 | 0 |
| Maltodextrin | 150 | 600 | 0 | 0 |
| Fructose | 0 | 0 | 345.6 | 1382 |
| Cellulose | 50 | 0 | 50 | 0 |
| Soybean oil | 25 | 225 | 25 | 225 |
| Beef tallow | 20 | 180 | 177.5 | 1598 |
| Mineral mix | 10 | 0 | 10 | 0 |
| Calcium carbonate | 5.5 | 0 | 5.5 | 0 |
| Potassium citrate | 16.5 | 0 | 16.5 | 0 |
| Dicalcium phosphate | 13 | 0 | 13 | 0 |
| Vitamin mix | 10 | 40 | 10 | 40 |
| Choline bitartrate | 2 | 0 | 2 | 0 |
| Total | 1055 | 4057 | 858.1 | 4057 |
| kcal g−1 | 3.8 | 4.7 | ||
2.5 Cell culture and treatment with high glucose (HG) and vitamin K1 (VK1)
The human THP-1 monocyte cell line was obtained from the National Centre for Cell Science (Pune, India), and the cells were maintained at 37 °C in RPMI medium following the procedure as reported earlier.22 For cell culture studies, VK1 was dissolved in DMSO and the final concentration of DMSO was below 0.5% in the cell culture media. Control cells were also treated with the same concentration of DMSO. Cells were treated with HG (25 mM) with or without VK1. Different concentrations (1, 5, or 10 nM) of VK1 were supplemented for 2 h followed by HG exposure for the next 20 h. Doses of VK1 used in this study are within the physiological range.23 Mannitol was used as an osmolarity control. LPS was used to stimulate the secretion of cytokines by HG.24 Cells were treated with LPS (2 ng mL−1) either alone or in combination with VK1 against HG exposure. Cells treated with LPS alone served as the control for this study. After treatment, cells were lysed in a radioimmunoprecipitation assay (RIPA) buffer, supplemented with protease and phosphatase inhibitors. Lysates were cleared by centrifugation, and total protein concentrations were determined by using the BCA assay (Pierce/Thermo Scientific, Rockford, IL).2.6 Signal silencing studies
GGCX-siRNA and control siRNA were purchased from Sigma (St Louis, MO). Cells were transiently transfected with 100 nM siRNA complex using LipofectamineTM2000 transfection reagent (Invitrogen, Carlsbad, CA) following the method as reported earlier.24 After transfection, cells were treated with VK1 and HG following the method as described above.2.7 Determination of cell viability, cytokine secretion, and intracellular ROS level
Cell viability was determined using the Alamar Blue reduction bioassay (Alamar Biosciences, Sacramento, CA).24 MCP-1 levels either in human plasma or the cell culture supernatant were determined by using the sandwich ELISA method with a commercially available kit from Sigma. The levels of ucMGP in the cell culture supernatant were measured by using an ELISA kit from MyBioSource (San Diego, CA). The levels of intracellular reactive oxygen species (ROS) were measured using the fluorescent dye H2DCFDA (2′,7′-dichlorofluorescein diacetate).24 The change in the intracellular ROS level was plotted as mean fluorescence intensity (MFI).2.8 Immunoblotting
Protein expression was investigated through immunoblotting studies using appropriate anti-NF-κB (#sc-109) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 dilution), anti-phosphorylated NF-κB (p65) (serine 536) (#ab86299) (1
500 dilution), anti-phosphorylated NF-κB (p65) (serine 536) (#ab86299) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), anti-Nrf2 (#ab137550) (1
1000), anti-Nrf2 (#ab137550) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), anti-GGCX (#ab197982) (1
1000), anti-GGCX (#ab197982) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilution), or anti-β-actin (#ab49900) (1
1000 dilution), or anti-β-actin (#ab49900) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) primary antibodies following the procedure as reported earlier.25 The intensity of each immunoblotting band was calculated using the histogram tool of Adobe Photoshop CS5.
000) primary antibodies following the procedure as reported earlier.25 The intensity of each immunoblotting band was calculated using the histogram tool of Adobe Photoshop CS5.
2.9 Statistical analysis
Multiple linear regression analyses were used to determine whether a dependent variable can be predicted from a linear combination of independent variables. Body weight was always used as an additional independent variable to determine the regression and P-value. The y-axis parameter was used as a dependent variable, and the x-axis and body weight were used as independent variables using the SigmaStat software (San Jose, CA, USA). The data from human, animal, and cell culture studies were analyzed statistically using one-way ANOVA with the SigmaStat statistical software (San Jose, CA, USA). When the data passed a normality test, all groups were compared using the Student–Newman–Keuls method. A p value less than 0.05 was considered significant for a statistical test.3. Results
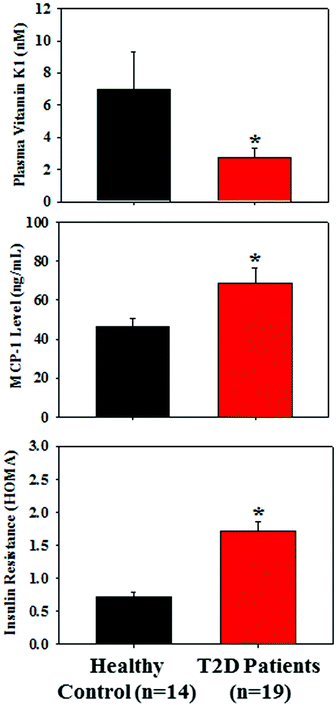
3.1 Plasma VK1, fasting glucose, insulin resistance (HOMA), and MCP-1 levels in T2D patients and healthy controls
Table 2 presents the similar distribution of age, male/female ratio, body weight, and BMI in both groups; however, the levels of fasting glucose and HbA1c were significantly higher in T2D patients compared to those of the control subjects. Fig. 1 demonstrates that the plasma level of VK1 was significantly lower, and the levels of pro-inflammatory cytokine, MCP-1 and insulin resistance (HOMA-IR) were significantly higher in T2D patients compared to those of the control subjects. All the studied subjects showed no signs of coagulation-associated complication (data not shown).| Parameter | Healthy control | T2D patients |
|---|---|---|
| n | 14 | 19 |
| Age (years) | 51.5 ± 2.1 | 52.8 ± 1.2 |
| Male/female | 9/5 | 11/8 |
| Body weight (kg) | 60.21 ± 3.8 | 57.1 ± 3.4 |
| BMI (kg m−2) | 22.41 ± 1.04 | 22.71 ± 1.3 |
| Fasting glucose (mg dL−1) | 79.72 ± 8.95 | 176.77 ± 10.71* |
| HbA1c (%) | 4.36 ± 0.11 | 7.52 ± 0.26* |
3.2 Relationship between VK1, MCP-1, and HOMA-IR among T2D patients
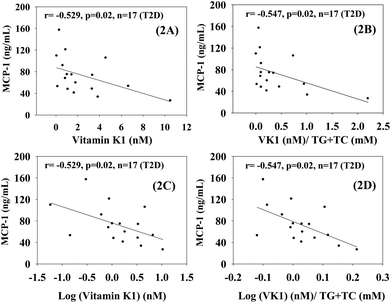
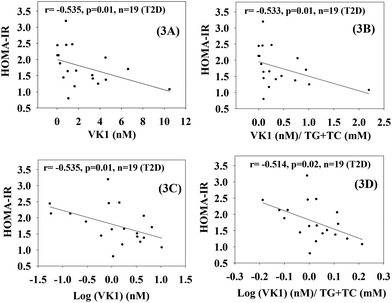
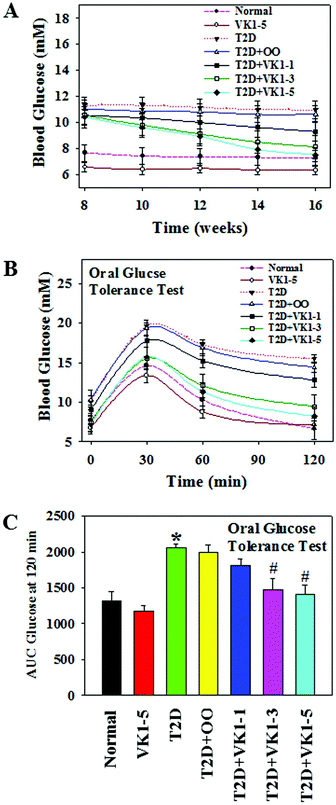
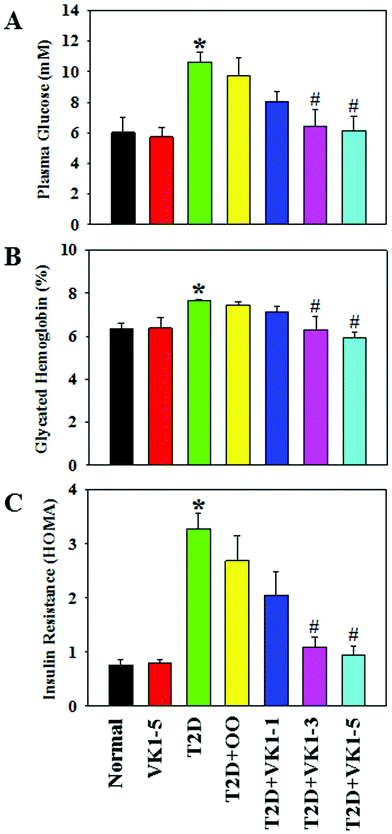
Fig. 2 shows that there was a statistically significant inverse dependence of MCP-1 with the circulating levels of VK1 among T2D patients, whether it is expressed as per volume of plasma (2A and C) or lipid level (2B and D). This difference was examined because VK is a lipid-soluble vitamin, and it may be more appropriate to express its concentration after normalization with lipid.23 Similarly, Fig. 3 illustrates that a significant negative correlation also exists between the blood levels of VK1 and HOMA-IR. The regression analyses given here were determined while controlling for body weights using multiple linear regression analyses. These results suggest that boosting plasma VK1 may have a positive effect in lowering vascular inflammation and insulin resistance among the T2D patient population.3.3. Effect of VK1 supplementation on glucose tolerance, fasting glucose, glycated haemoglobin (GHb), and insulin resistance (HOMA) in T2D mice
To investigate the role of VK1 supplementation on hyperglycemia in T2D, we first examined the weekly blood glucose levels. As shown in Fig. 5A, following an 8-week HFD feeding, the mice in the T2D group showed higher blood glucose levels compared to the normal group. The oral administration of VK1 dose-dependently reduced the blood glucose levels to close to normal levels by the end of the study. The effect of VK1 on glycemic control in T2D was further confirmed by using the OGTT assay (Fig. 5B and C). The results showed that VK1 supplementation (1, 3, and 5 μg per kg BW) dose-dependently reduced the plasma glucose and AUC in T2D mice compared to those observed in the T2D and T2D + OO groups. The plasma levels of fasting glucose, GHb, and HOMA-IR were significantly higher in T2D mice compared to those of the normal group (Fig. 6). Supplementation with VK1 dose-dependently reduced the fasting glucose, percentage of GHb, and HOMA-IR in T2D mice. Moreover, a significant increase in body weight gain and the levels of both triglyceride and total cholesterol has also been observed in T2D mice, which could be attenuated by VK1 supplementation (Table 3). OO supplementation did not cause any significant effect on body weight gain, fasting glucose, GHb, HOMA-IR, and lipid profile compared to those observed in T2D mice. The urine output was higher in T2D mice compared to that in the normal group and that was found to be decreased by VK1 supplementation (data not shown). There was no significant difference in food intake among the groups and the blood levels of liver and kidney function tests did not differ significantly. All the studied animals showed no signs of coagulation-associated complication (data not shown) suggesting that no negative side effects result from VK1 supplementation.| Parameters | Normal | VK1-5 | T2D | T2D + OO | T2D + VK1-1 | T2D + VK1-3 | T2D + VK1-5 |
|---|---|---|---|---|---|---|---|
| Body weight gain (%) | 5.22 ± 1.17 | 5.69 ± 0.89 | 11.69 ± 0.93* | 11.46 ± 1.43 | 11.16 ± 1.3 | 7.85 ± 0.86# | 7.25 ± 0.38# |
| Food intake (g per mouse per day) | 2.53 ± 0.029 | 2.53 ± 0.017 | 2.48 ± 0.008 | 2.48 ± 0.013 | 2.52 ± 0.009 | 2.52 ± 0.004 | 2.53 ± 0.01 |
| ALT (U L−1) | 8.12 ± 0.74 | 7.85 ± 0.43 | 7.39 ± 0.45 | 7.52 ± 0.45 | 7.29 ± 0.57 | 8.09 ± 1.18 | 7.68 ± 0.86 |
| AST (U L−1) | 31.31 ± 3.81 | 35.95 ± 4.92 | 36.06 ± 9.27 | 33.17 ± 5.75 | 33.39 ± 3.89 | 34.56 ± 2.11 | 30.38 ± 3.48 |
| Creatinine (mg dL−1) | 0.041 ± 0.0035 | 0.051 ± 0.0039 | 0.042 ± 0.005 | 0.044 ± 0.0025 | 0.041 ± 0.002 | 0.046 ± 0.0021 | 0.044 ± 0.0025 |
| Triglyceride (mM) | 0.59 ± 0.012 | 0.63 ± 0.03 | 1.65 ± 0.057* | 1.86 ± 0.21 | 1.48 ± 0.088 | 1.18 ± 0.062# | 0.74 ± 0.103# |
| Total cholesterol (mM) | 0.29 ± 0.015 | 0.33 ± 0.041 | 0.53 ± 0.079* | 0.47 ± 0.059 | 0.41 ± 0.014 | 0.34 ± 0.055# | 0.26 ± 0.026# |
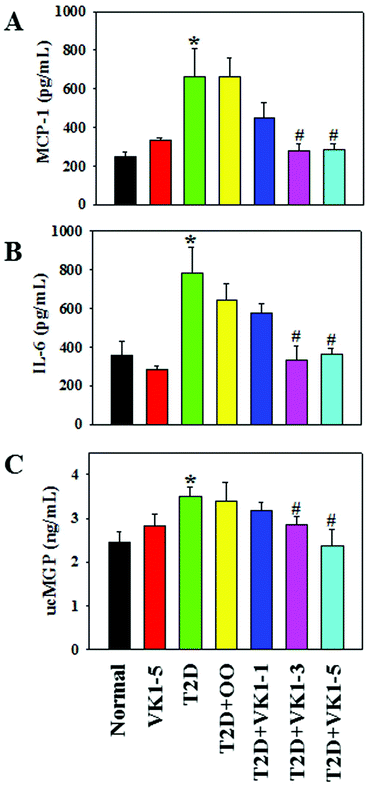
3.4. Effect of VK1 on the secretion of pro-inflammatory cytokines in normal and T2D mice
The effects of VK1 on the secretion of pro-inflammatory cytokines are shown in Fig. 7. The results show that HFD feeding caused an increase in the secretion of pro-inflammatory cytokines, MCP-1 and IL-6 in the sera of T2D mice. Supplementation with olive oil did not cause any effect on the inflammation. However, VK1 supplementation significantly decreased the secretion of both MCP-1 and IL-6 in a dose-dependent manner in T2D mice. Measurement of the blood levels of uncarboxylated vitamin K-dependent proteins, such as uncarboxylated matrix Gla protein (ucMGP), has been suggested as a functional indicator of circulating VK status.23Fig. 7C shows the plasma level of ucMGP in the experimental animals. The results show that HFD feeding caused an increase in the plasma levels of ucMGP compared to those in the normal diet-fed mice suggesting a functional VK deficiency in T2D mice. Supplementation with VK1 dose-dependently decreased the ucMGP level in T2D mice compared to that in the olive oil control group. This study suggests a beneficial effect of VK1 supplementation in maintaining circulating VK status and reducing vascular inflammation in T2D.3.5 Effect of VK1 supplementation on vascular inflammation in high glucose-treated cells
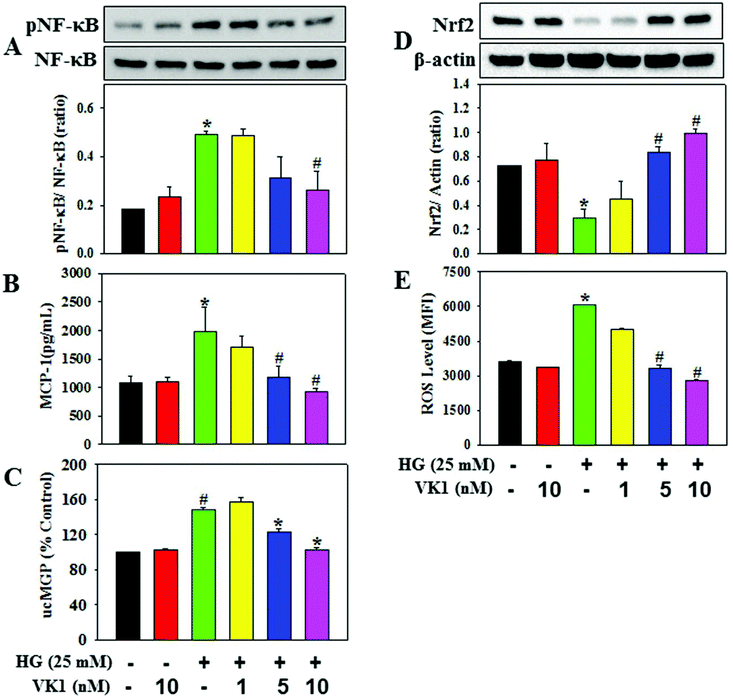
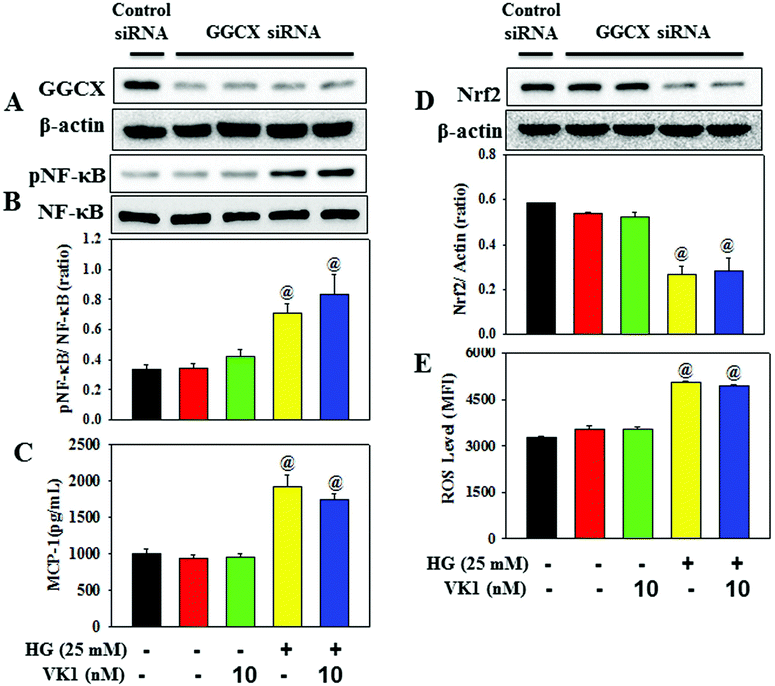
To dissect the molecular mechanism underlying the beneficial effect of VK1 against the secretion of pro-inflammatory cytokines in T2D, human monocytic cells were treated with different doses of VK1 (1, 5, or 10 nM) with or without high glucose (HG). The results showed that HG exposure up-regulated the NF-κB phosphorylation, down-regulated the Nrf2 protein expression, and increased the intracellular ROS production and MCP-1 secretion compared to those observed in the control cells (Fig. 8). Supplementation with VK1 dose-dependently down-regulated the NF-κB phosphorylation, up-regulated the Nrf2 protein expression, and decreased the ROS production and MCP-1 secretion in HG-treated cells suggesting the anti-inflammatory properties of VK1 in the pathogenesis of vascular inflammation associated with T2D. Moreover, VK1 supplementation also reduced the HG-induced increased secretion of ucMGP, which further ascertained the cellular entry of VK1.3.6 Role of VK-dependent Gla proteins in mediating the effect of VK1 on vascular inflammation in high glucose (HG)-treated cells
Coagulation-unrelated VK-dependent Gla proteins have attracted scientific attention in the regulation of obesity, T2DM, and CVD risk factors.26 The enzyme GGCX catalyses the activation of Gla proteins using VK as a co-factor. This study further investigated the role of Gla proteins in mediating the effect of VK1 on vascular inflammation in HG-treated cells (Fig. 9) by using GGCX siRNA. The results showed that in GGCX knockdown cells, VK1 supplementation could not prevent the HG-induced activation of NF-κB phosphorylation and the down-regulation of Nrf2 protein expression, ROS production, and MCP-1 secretion. This suggests that the GGCX-mediated carboxylation of VK-dependent Gla proteins facilitates the beneficial activity of VK1 in reducing hyperglycemia-induced vascular inflammation.4. Discussion
The number of studies that have evaluated the circulating VK1 status in relation to chronic diseases is relatively less compared to the studies that assessed the dietary VK1 intake and its health benefits. VK1 is the primary circulating form of VK and has been successfully measured in various population and clinical-based studies worldwide to assess the circulating VK status.23The present study showed that plasma VK1 levels were significantly lower among T2D patients compared to those observed in age-matched normal subjects. Earlier studies demonstrated that the dietary intake of VK significantly influenced its circulating status.27 In addition, an increase in dietary VK intake has also been found to reduce the risk of T2D among young and aged volunteers.3 Therefore, an inadequate dietary VK1 intake may play a significant role in reducing its circulating status in T2D patients compared to that in the healthy controls enrolled in this study. Moreover, VK1 is transported on triglyceride-rich lipoproteins in circulation and plasma VK1 has been found to be positively associated with triglyceride levels among the aged population participating in the VK1 supplementation trial.23 Interestingly, in the present study we observed that triglyceride levels were ∼15% lower in T2D patients compared to those in the control (data not shown), which may also reflect a decrease in VK1 levels in T2D patients. In addition, the levels of fasting glucose, HbA1c, HOMA-IR, and MCP-1 were significantly higher in T2D patients compared to those in the controls. Multiple linear regression analyses after adjustment of body weight showed that the circulating levels of VK1 were significantly and inversely associated with MCP-1 and HOMA-IR among T2D patients. Due to its lipophilic characteristic, it is appropriate to express the concentration of circulating VK1 after normalization with lipid, and the same has also been argued for other lipid-soluble vitamins.28,29 The results demonstrate that even after normalization with total lipid, circulating VK1 is significantly and inversely correlated with MCP-1 and HOMA-IR among T2D populations, which suggests that boosting plasma VK1 may reduce the vascular inflammation and insulin resistance in T2D patients.
Using a HFD-fed T2D animal model this study further demonstrated that VK1 supplementation dose-dependently decreased glucose intolerance, fasting glucose, glycated hemoglobin, and HOMA-IR in T2D mice. Various studies reported an increase in body weight in HFD-fed T2D mice.30–32 A recent clinical trial demonstrated the beneficial effect of VK supplementation on fat mass and body composition.33 The present study showed that supplementation with VK1 dose-dependently reduced the body weight gain in the T2D animal. Besides lowering body weight gain, insulin resistance, and hyperglycemia, VK1 supplementation also reduced the secretion of pro-inflammatory cytokines (MCP-1 and IL-6) in T2D mice. The measurement of the blood levels of uncarboxylated vitamin K dependent proteins, such as ucMGP, is considered to be an indicator of circulating VK status.23 Interestingly, plasma ucMGP level was significantly higher in T2D mice compared to that in the normal group and that was reduced by VK1 supplementation, which suggests a beneficial effect of VK1 supplementation in reducing the sub-clinical VK deficiency in T2D.
Using monocyte and endothelial cell culture models, several studies reported that treatment with HG caused an increased secretion of pro-inflammatory cytokines (MCP-1, interleukins), which suggests a direct involvement of vascular cells in HG-induced cytokine production.24,34,35 Two redox-sensitive transcription factors, namely NF-κB and Nrf2, have been reported to play an important role in modulating the inflammatory responses in several diseases including diabetes;16,17 however, the relationship between NF-κB and Nrf2 activity is controversial. Interestingly, it has been observed that hyperglycemia-induced O-GlcNAcylation of the NF-κB p65 subunit decreased the binding affinity to IkappaB alpha and increased nuclear translocation,36 which induces the transcription of target genes involved in inflammatory responses.37 Moreover, recent experimental evidence showed that the activation of NF-κB unidirectionally antagonized the transcriptional activity of Nrf2 by either promoting the localization of transcriptional repressors38 or activating the cytosolic inhibitor Keap1.39 A decline in Nrf2 activity leads to the decreased transcription of different antioxidant defence enzymes and the increased accumulation of ROS.40 The present study demonstrates that treatment with HG up-regulated the NF-κB phosphorylation, down-regulated the Nrf2 expression, and increased the ROS production and MCP-1 secretion in monocytes. Supplementation with VK1 decreased the NF-κB phosphorylation, MCP-1 secretion, and ROS production and increased the Nrf2 expression in HG-treated cells. Based upon the earlier findings, the present study suggests that the beneficial effect of VK1 in reducing hyperglycemia may reduce the extent of O-GlcNAcylation of NF-κB and its transcriptional activity in monocytes, which may lead to an increase in Nrf2 expression and a decrease in ROS production and cytokine secretion. This study suggests a beneficial effect of VK1 in preventing HG-induced vascular inflammation via the modulation of the NF-κB/Nrf2 signaling pathway. In addition, VK1 supplementation also reduces the HG-induced increased secretion of ucMGP, which further ascertained the intracellular entry of VK1.
VK functions as a cofactor for the enzyme GGCX, which catalyses the carboxylation of VK-dependent Gla proteins. Recently, coagulation-unrelated VK-dependent Gla proteins have attracted scientific attention in the regulation of obesity, T2DM, and CVD risk factors.18,19 Viegas et al. showed that coagulation-unrelated VK-dependent Gla proteins, such as Gla-rich protein (GRP) and matrix Gla protein (MGP), are synthesized and γ-carboxylated in the majority of human immune system cells.41 Treatment with γ-carboxylated GRP reduced the LPS-induced increased secretion of TNF-α in THP-1 monocytes suggesting a role of γ-carboxylated GRP in reducing inflammatory response.41 Using GGCX siRNA, this study further demonstrated that in GGCX knockdown cells, VK1 supplementation could not prevent the HG-induced NF-κB phosphorylation, Nrf2 down-regulation, and MCP-1 secretion, which suggests that the GGCX-mediated carboxylation of VK-dependent Gla proteins facilitated the anti-inflammatory activity of VK1 in diabetes.
In conclusion, this is the first report to demonstrate that circulating VK1 levels were significantly lower among T2D patients, and it is significantly and inversely associated with the levels of MCP-1 and HOMA-IR among T2D patients. Supplementation with VK1 also reduced the secretion of pro-inflammatory cytokines and insulin resistance and maintained the glucose homeostasis in T2D mice. Using a monocyte cell culture model, this study also showed that VK1 supplementation reduced MCP-1 secretion and ROS production via the regulation of the NF-κB/Nrf2 signaling pathways in HG-treated cells. Moreover, signal silencing studies with GGCX siRNA also depicted a role of coagulation-unrelated VK-dependent Gla proteins in mediating the beneficial effect of VK1 on vascular inflammation in T2D (Fig. 10).
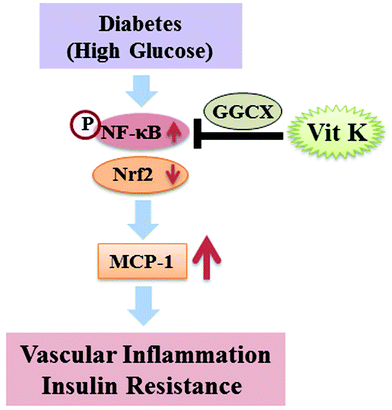 | ||
| Fig. 10 Schematic diagram of the proposed mechanism underlying the beneficial effect of vitamin K1 on vascular inflammation in T2D. | ||
The findings of this study will allow for a better understanding of and care for the excess diabetes and cardiovascular disease associated with the VK deficient population. Although the recommended adequate intake for VK1 is 1 μg kg−1,1 it is not known whether this adequate intake is sufficient to meet all the physiological needs, especially coagulation-unrelated health outcomes like diabetes. Moreover, no known toxicity has been reported so far with higher doses of VK1 supplementation.3 Thus, the tolerable upper intake limit has not been set yet. Further pre-clinical and clinical trials are needed to examine the role of VK supplementation on vascular inflammation in T2D and investigate its link with VK-dependent Gla proteins.
Author contributions
D. O. and P. K. B. performed the clinical studies. A. D. and P. M. performed the animal experiment, and compiled and analyzed the data. A. D. and P. M. designed and performed the cell culture experiments, VK analysis, biochemical analyses, and immunoblotting studies. P. M., A. D., and J. K. wrote the manuscript. All authors reviewed the results and approved the final version of the manuscript.Abbreviations
| BMI | Body mass index |
| GGCX | γ-Glutamyl carboxylase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| NF-κB | Nuclear factor kappa B |
| Nrf2 | Nuclear factor-erythroid 2-related factor 2 |
| ROS | Reactive oxygen species |
| VK1 | Vitamin K1 |
Conflicts of interest
The authors have declared that no conflict of interest exists.Acknowledgements
The authors are thankful to the Director, CSIR-NEIST, Jorhat, for his support and the DBT, Government of India, for providing the Ramalingaswami Re-Entry Fellowship to Dr Manna (BT/RLF/Re-Entry/34/2013). The authors thank Ms. Cassandra Warden (Vanderbilt University Medical Center, Department of Ophthalmology) for the excellent editing of this manuscript.References
- U. Grober, J. Reichrath, M. F. Holick and K. Kisters, Vitamin K: an old vitamin in a new perspective, Dermatoendocrinol, 2014, 6, e968490 CrossRef CAS PubMed.
- D. W. Stafford, The vitamin K cycle, J. Thromb. Haemostasis, 2005, 3, 1873–1878 CrossRef CAS PubMed.
- P. Manna and J. Kalita, Beneficial role of vitamin K supplementation on insulin sensitivity, glucose metabolism, and the reduced risk of type 2 diabetes: A review, Nutrition, 2016, 32, 732–739 CrossRef CAS PubMed.
- S. L. Booth, Roles for vitamin K beyond coagulation, Annu. Rev. Nutr., 2009, 29, 89–110 CrossRef CAS PubMed.
- D. Misra, S. L. Booth, I. Tolstykh, D. T. Felson, M. C. Nevitt, C. E. Lewis, J. Torner and T. Neogi, Vitamin K deficiency is associated with incident knee osteoarthritis, Am. J. Med., 2013, 126, 243–248 CrossRef CAS PubMed.
- M. K. Shea and R. M. Holden, Vitamin K status and vascular calcification: evidence from observational and clinical studies, Adv. Nutr., 2012, 3, 158–165 CrossRef CAS PubMed.
- M. K. Shea, S. L. Booth, C. M. Gundberg, J. W. Peterson, C. Waddell, B. Dawson-Hughes and E. Saltzman, Adulthood obesity is positively associated with adipose tissue concentrations of vitamin K and inversely associated with circulating indicators of vitamin K status in men and women, J. Nutr., 2010, 140, 1029–1034 CrossRef CAS PubMed.
- P. Manna and S. K. Jain, Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies, Metab. Syndr. Relat. Disord., 2015, 13, 423–444 CrossRef CAS PubMed.
- Y. Ohsaki, H. Shirakawa, A. Miura, P. E. Giriwono, S. Sato, A. Ohashi, M. Iribe, T. Goto and M. Komai, Vitamin K suppresses the lipopolysaccharide-induced expression of inflammatory cytokines in cultured macrophage-like cells via the inhibition of the activation of nuclear factor kappaB through the repression of IKKalpha/beta phosphorylation, J. Nutr. Biochem., 2010, 21, 1120–1126 CrossRef CAS PubMed.
- K. Reddi, B. Henderson, S. Meghji, M. Wilson, S. Poole, C. Hopper, M. Harris and S. J. Hodges, Interleukin 6 production by lipopolysaccharide-stimulated human fibroblasts is potently inhibited by naphthoquinone (vitamin K) compounds, Cytokine, 1995, 7, 287–290 CrossRef CAS PubMed.
- M. K. Shea, G. E. Dallal, B. Dawson-Hughes, J. M. Ordovas, C. J. O'Donnell, C. M. Gundberg, J. W. Peterson and S. L. Booth, Vitamin K, circulating cytokines, and bone mineral density in older men and women, Am. J. Clin. Nutr., 2008, 88, 356–363 CAS.
- J. W. Beulens, A. D. van der, D. E. Grobbee, I. Sluijs, A. M. Spijkerman and Y. T. van der Schouw, Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes, Diabetes Care, 2010, 33, 1699–1705 CrossRef CAS PubMed.
- M. K. Varsha, R. Thiagarajan, R. Manikandan and G. Dhanasekaran, Vitamin K1 alleviates streptozotocin-induced type 1 diabetes by mitigating free radical stress, as well as inhibiting NF-kappaB activation and iNOS expression in rat pancreas, Nutrition, 2015, 31, 214–222 CrossRef CAS PubMed.
- M. K. Sai Varsha, T. Raman and R. Manikandan, Inhibition of diabetic-cataract by vitamin K1 involves modulation of hyperglycemia-induced alterations to lens calcium homeostasis, Exp. Eye Res., 2014, 128, 73–82 CrossRef CAS PubMed.
- J. Iwamoto, A. Seki, Y. Sato, H. Matsumoto, T. Takeda and J. K. Yeh, Vitamin K(2) prevents hyperglycemia and cancellous osteopenia in rats with streptozotocin-induced type 1 diabetes, Calcif. Tissue Int., 2011, 88, 162–168 CrossRef CAS PubMed.
- V. Ganesh Yerra, G. Negi, S. S. Sharma and A. Kumar, Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-kappaB pathways in diabetic neuropathy, Redox Biol., 2013, 1, 394–397 CrossRef CAS PubMed.
- M. Buelna-Chontal and C. Zazueta, Redox activation of Nrf2 & NF-kappaB: a double end sword?, Cell. Signalling, 2013, 25, 2548–2557 CrossRef CAS PubMed.
- S. L. Booth, A. Centi, S. R. Smith and C. Gundberg, The role of osteocalcin in human glucose metabolism: marker or mediator?, Nat. Rev. Endocrinol., 2013, 9, 43–55 CrossRef CAS PubMed.
- A. Dihingia, J. Kalita and P. Manna, Implication of a novel Gla-containing protein, Gas6 in the pathogenesis of insulin resistance, impaired glucose homeostasis, and inflammation: A review, Diabetes Res. Clin. Pract., 2017, 128, 74–82 CrossRef CAS PubMed.
- R. Paroni, E. M. Faioni, C. Razzari, G. Fontana and M. Cattaneo, Determination of vitamin K1 in plasma by solid phase extraction and HPLC with fluorescence detection, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2009, 877, 351–354 CrossRef CAS PubMed.
- J. Cacho, J. Sevillano, J. de Castro, E. Herrera and M. P. Ramos, Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats, Am. J. Physiol. Endocrinol. Metab., 2008, 295, E1269–E1276 CrossRef CAS PubMed.
- P. Manna and S. K. Jain, Effect of PIP3 on adhesion molecules and adhesion of THP-1 monocytes to HUVEC treated with high glucose, Cell. Physiol. Biochem., 2014, 33, 1197–1204 CrossRef CAS PubMed.
- M. K. Shea and S. L. Booth, Concepts and Controversies in Evaluating Vitamin K Status in Population-Based Studies, Nutrients, 2016, 8(1) DOI:10.3390/nu8010008[pii:E8].
- P. Manna and S. K. Jain, L-cysteine and hydrogen sulfide increase PIP3 and AMPK/PPARgamma expression and decrease ROS and vascular inflammation markers in high glucose treated human U937 monocytes, J. Cell. Biochem., 2013, 114, 2334–2345 CrossRef CAS PubMed.
- P. Manna and S. K. Jain, Vitamin D up-regulates glucose transporter 4 (GLUT4) translocation and glucose utilization mediated by cystathionine-gamma-lyase (CSE) activation and H2S formation in 3 T3L1 adipocytes, J. Biol. Chem., 2012, 287, 42324–42332 CrossRef CAS PubMed.
- R. L. Kennedy, V. Vangaveti, U. H. Malabu and D. McCulloch, The vitamin K-dependent Gla proteins and risk of type 2 diabetes, Diabetologia, 2013, 56, 2100–2101 CrossRef CAS PubMed.
- B. Walther, J. P. Karl, S. L. Booth and P. Boyaval, Menaquinones, bacteria, and the food supply: the relevance of dairy and fermented food products to vitamin K requirements, Adv. Nutr., 2013, 4, 463–473 CrossRef CAS PubMed.
- S. K. Jain, D. Micinski, L. Huning, G. Kahlon, P. F. Bass and S. N. Levine, Vitamin D and L-cysteine levels correlate positively with GSH and negatively with insulin resistance levels in the blood of type 2 diabetic patients, Eur. J. Clin. Nutr., 2014, 68, 1148–1153 CrossRef CAS PubMed.
- R. J. Sokol, J. E. Heubi, S. T. Iannaccone, K. E. Bove and W. F. Balistreri, Vitamin E deficiency with normal serum vitamin E concentrations in children with chronic cholestasis, N. Engl. J. Med., 1984, 310, 1209–1212 CrossRef CAS PubMed.
- E. Arunkumar, S. Bhuvaneswari and C. V. Anuradha, An intervention study in obese mice with astaxanthin, a marine carotenoid–effects on insulin signaling and pro-inflammatory cytokines, Food Funct., 2012, 3, 120–126 CAS.
- S. Bhuvaneswari and C. V. Anuradha, Astaxanthin prevents loss of insulin signaling and improves glucose metabolism in liver of insulin resistant mice, Can. J. Physiol. Pharmacol., 2012, 90, 1544–1552 CrossRef CAS PubMed.
- M. S. Winzell and B. Ahren, The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes, Diabetes, 2004, 53(Suppl 3), S215–S219 CrossRef PubMed.
- M. H. J. Knapen, K. M. Jardon and C. Vermeer, Vitamin K-induced effects on body fat and weight: results from a 3-year vitamin K2 intervention study, Eur. J. Clin. Nutr., 2017 DOI:10.1038/ejcn.2017.146.
- S. K. Ku, S. Kwak and J. S. Bae, Orientin inhibits high glucose-induced vascular inflammation in vitro and in vivo, Inflammation, 2014, 37, 2164–2173 CrossRef CAS PubMed.
- Q. Zhao, C. Gao and Z. Cui, Ginkgolide A reduces inflammatory response in high-glucose-stimulated human umbilical vein endothelial cells through STAT3-mediated pathway, Int. Immunopharmacol., 2015, 25, 242–248 CrossRef CAS PubMed.
- W. H. Yang, S. Y. Park, H. W. Nam, D. H. Kim, J. G. Kang, E. S. Kang, Y. S. Kim, H. C. Lee, K. S. Kim and J. W. Cho, NFkappaB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 17345–17350 CrossRef CAS PubMed.
- K. E. Wellen and G. S. Hotamisligil, Inflammation, stress, and diabetes, J. Clin. Invest., 2005, 115, 1111–1119 CAS.
- G. H. Liu, J. Qu and X. Shen, NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK, Biochim. Biophys. Acta, 2008, 1783, 713–727 CrossRef CAS PubMed.
- M. Yu, H. Li, Q. Liu, F. Liu, L. Tang, C. Li, Y. Yuan, Y. Zhan, W. Xu, W. Li, H. Chen, C. Ge, J. Wang and X. Yang, Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway, Cell. Signalling, 2011, 23, 883–892 CrossRef CAS PubMed.
- A. K. Jaiswal, Nrf2 signaling in coordinated activation of antioxidant gene expression, Free Radical Biol. Med., 2004, 36, 1199–1207 CrossRef CAS PubMed.
- C. S. B. Viegas, R. M. Costa, L. Santos, P. A. Videira, Z. Silva, N. Araujo, A. L. Macedo, A. P. Matos, C. Vermeer and D. C. Simes, Gla-rich protein function as an anti-inflammatory agent in monocytes/macrophages: Implications for calcification-related chronic inflammatory diseases, PLoS One, 2017, 12, e0177829 Search PubMed.
| This journal is © The Royal Society of Chemistry 2018 |