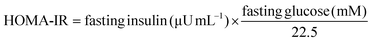Pepsin egg white hydrolysate ameliorates metabolic syndrome in high-fat/high-dextrose fed rats
S.
Moreno-Fernández
ab,
M.
Garcés-Rimón
ab,
C.
González
bc,
J. A.
Uranga
bcd,
V.
López-Miranda
bcde,
G.
Vera
bcde and
M.
Miguel
 *ac
*ac
aInstituto de Investigación en Ciencias de la Alimentación (CIAL, CSIC-UAM), Madrid, Spain. E-mail: marta.miguel@csic.es; Tel: +34 910 017 931
bUnidad Asociada I+D+i del Instituto de Investigación en Ciencias de la Alimentación (CIAL), Consejo Superior de Investigaciones Científicas (CSIC), Madrid, Spain
cCiencias Básicas de la Salud. Facultad de Ciencias de la Salud, Universidad Rey Juan Carlos, Alcorcón, Madrid, Spain
dGrupo de Excelencia Investigadora URJC-Banco de Santander- Grupo multidisciplinar de investigación y tratamiento del dolor (i+Dol), Alcorcón, Spain
eUnidad Asociada I+D+i del Instituto de Química Médica (IQM), Consejo Superior de Investigaciones Científicas (CSIC), Madrid, Spain
First published on 16th October 2017
Abstract
The aim of this study was to examine the effect of a pepsin egg white hydrolysate (EWH) on metabolic complications using a high-fat/high-dextrose diet-induced Metabolic Syndrome (MetS) experimental model. Male Wistar rats were divided into 4 groups which received: standard diet and water (C), standard diet and a solution with 1 g kg−1 day−1 of EWH (CH), high-fat/high-dextrose diet and water (MS), and high-fat/high-dextrose diet and a solution with 1 g kg−1 day−1 of EWH (MSH). EWH consumption normalized body weight gain; abdominal obesity and peripheral neuropathy developed in MetS animals, and adipose tissue and liver weight, as well as plasma glucose were reduced. Oxidative stress and inflammation biomarkers were normalized in MSH animals. In conclusion, the oral administration of EWH could be used as a functional food ingredient to improve some complications associated with MetS induced by unhealthy diets.
1. Introduction
Metabolic syndrome (MetS) is a complex disorder which refers to the clustering of central obesity, insulin resistance, impaired glucose tolerance, hypertension and dyslipidemia.1,2 This pathology increases the risk of developing diabetes, cardiovascular diseases, non-alcoholic fatty liver disease and microvascular complications including painful peripheral neuropathy and/or autonomic neuropathy.3 The prevalence of MetS is increasing fast, especially in developing areas undergoing rapid socio-environmental changes.4 One of the major causes of obesity is a diet rich in both, sugar and saturated fat.4,5 This diet, known as the “Western diet”, leads to disturbances in carbohydrate and lipid metabolism that promotes metabolic complications.6The current treatment used in MetS complications are lifestyle change interventions, pharmacotherapy and, in some cases, surgery, a dietary intervention being probably the safest and most cost-effective option. Along this line, various studies have emphasized the possibility of using food-derived compounds as natural ingredients to control metabolic complications related to MetS.7,8 Bioactive peptides are released during food processing or after the digestion of food proteins from different sources (milk, egg, rice, fish etc.) and they can exert different biological activities. Some of them may help metabolic syndrome conditions.9 In this context, egg derived peptides have demonstrated angiotensin converting enzyme (ACE) inhibitory activity,10,11 antioxidant activity,12,13 antihypertensive effects after short14 and long term administration,15 and beneficial properties regarding the lipid profile of spontaneously hypertensive rats (SHR).16 Moreover, our research group has obtained an hydrolysate from egg white which simultaneously possess antioxidant, hypocholesterolemic and DPP-IV inhibitory activities, both in vitro and in vivo in Zucker fatty rats.17,18
Currently, high-fat/high-carbohydrate diet-induced MetS is one of the most relevant animal models to mimic the diet responsible for human MetS as a basis to investigate its potential interventions.19,20 High-fat/high-carbohydrate diets induced in rats most of the symptoms of MetS such as hypertension, dyslipidemia, impaired glucose tolerance, excess fat deposition, increased proinflammatory markers and oxidative stress and also peripheral polyneuropathy.1,21
The aim of this study was to examine the effect of a pepsin egg white hydrolysate (EWH), previously characterized in our research group,17 on metabolic complications related to MetS developed in high-fat/high-dextrose diet-induced MetS rats.
2. Materials and methods
2.1. Preparation of egg white hydrolysate
The EWH was prepared according to the method of Garcés-Rimón et al.17 Briefly, pasteurized egg white was hydrolysed with food grade pepsin from pork stomach (E.C. 3.4.23.1. BC PEPSIN 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000, Biocatalysts, United Kingdom). The egg white was acidified with concentrated food grade HCl 37% (Panreac Quimica S.L.U., Spain) to pH 2. The samples were incubated at 37 °C under constant stirring in a thermostatic water bath for 8 hours. Inactivation of pepsin was achieved by increasing the pH to 7.0 with food grade NaOH 10 M (Panreac Quimica S.L.U.). The hydrolysate was centrifuged for 15 min at 4500g, and the supernatant was stored at −20 °C until analysis.
3000, Biocatalysts, United Kingdom). The egg white was acidified with concentrated food grade HCl 37% (Panreac Quimica S.L.U., Spain) to pH 2. The samples were incubated at 37 °C under constant stirring in a thermostatic water bath for 8 hours. Inactivation of pepsin was achieved by increasing the pH to 7.0 with food grade NaOH 10 M (Panreac Quimica S.L.U.). The hydrolysate was centrifuged for 15 min at 4500g, and the supernatant was stored at −20 °C until analysis.
2.2. General protocol in animals
The experiments were designed to minimize the number of animals used and were performed in accordance with the European and Spanish legislation on the care and use of experimental animals (210/63/UE; Real Decreto 53/2013), and were approved by the Ethics Committee at University Rey Juan Carlos (URJC).Thirty-four 8-week old Wistar male rats weighting 280–310 g purchased from Harlan Laboratories (Harlan Ibérica, Barcelona, Spain) were used in this study. During the experimental period the animals were maintained in a temperature-controlled room (23 °C), with 12 h light/dark cycles and ad libitum access to water and feed.
The rats were randomly divided into 4 groups which were fed, for 20 weeks, with a standard chow diet (A04, SAFE, France) and tap water (C, n = 7), a standard chow diet and an EWH solution 1 g kg−1 day−1 (CH, n = 7), a high-fat diet (Purified Diet 235 HF, SAFE, France) with a 25% dextrose solution (MS, n = 10) and a high-fat diet with a 25% dextrose and an EWH solution 1 g kg−1 day−1 (MSH, n = 10). The EWH was provided from the 10th week until the 20th week of the study. The daily doses of 1 mg kg−1 were selected according to the results obtained after in vitro studies17 and from previous in vivo studies using EWH in SHR.14–16
During the experimental period, the body weights of the animals were recorded weekly up to the 20th week of the study. Drinking fluids and food intake were estimated weekly from the different groups. The occurrence of a neuropathic sign (tactile allodynia) was assessed once every 6 weeks using the Von Frey hair test.
At the end of the study, and after 16 hours of fasting, the abdominal circumference and body length (nose-to-anus length) were determined in all studied animals.
The rats were anaesthetized with an intraperitoneal injection of ketamin (87 mg kg−1) and xilacin (13 mg kg−1) and sacrificed by decapitation. Blood was collected into tubes containing lithium heparin as an anticoagulant. These samples were centrifuged at 500g for 20 minutes at 4 °C to obtain plasma, which was divided into aliquots and kept frozen at −80 °C until analysis. Epididymal adipose tissue, liver and tibia were immediately excised. Adipose tissue and liver were weighed and the tibia length was registered.
2.3. Diabetic neuropathy evaluation – Von Frey test
The development of peripheral neuropathy was evaluated with the Von Frey hair test. In this test, a significant decrease in Von Frey hair withdrawal threshold evoked by tactile–mechanical stimuli is suggestive of mechanical allodynia (increased sensitivity to non-noxious stimuli).Mechanical sensitivity was assessed at week 0, 6, 12 and 18. Rats were placed individually on an elevated iron mesh in a clear plastic cage and were allowed to adapt to the testing environment for at least 10 min. Habituation to this environment was also performed on the day before assessment. Calibrated Von Frey hairs ranging from 4 to 60 g (4, 8, 10, 15, 26 and 60 g) were applied to the plantar aspect of each hind paw, from below the mesh floor. This protocol was repeated five times with 3 s intervals. Withdrawal responses to the stimulus were recorded. A positive result was considered when at least three of five responses were obtained with each filament, and this value was considered as the tactile threshold. When less than three positive responses were detected with any of the hair trials, the process was repeated with the next higher force hair.
2.4. Plasma leptin and adiponectin
Plasma leptin and adiponectin concentrations were determined using rat ELISA kits (Cusabio, bioNova científica S.L., Spain) according to the manufacturer's instructions. Results were expressed as ng leptin per mL plasma and as μg adiponectin per mL plasma.2.5. Oxidative stress biomarkers
2.6. Glucose metabolism determinations
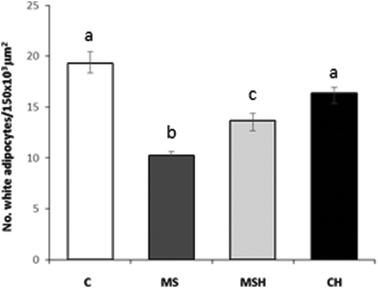
Plasma glucose levels were analyzed using a glucose-oxidase enzymatic commercial kit (Spinreact SAU, Spain). Plasma glucose concentrations were determined spectrophotometrically at a wavelength of 540 nm by using a microplate reader (Biotek HT Synergy, USA). In addition, plasma insulin concentration was spectrophotometrically quantified at 450 nm by using an ultrasensitive rat insulin enzyme immunoassay commercial kit (Mercodia AB, Sweden) with a microplate reader (Biotek HT Synergy).Moreover, plasma concentrations of both glucose and insulin were used to calculate the insulin resistance index (homeostasis model assessment [HOMA]-IR) with the following formula:23
2.7. Lipid metabolism
Plasma cholesterol and triglycerides (TG) were assayed using enzymatic and colorimetric methods with commercial kits (Spinreact S.A/S.A.U, Spain). The concentrations were determined at 450 nm with a spectrophotometer (Biotek HT Synergy, USA). Results were expressed as mg cholesterol per mL plasma and mg TG per mL plasma.2.8. Histopathological analysis
White adipose tissue and liver were fixed in buffered 10% formalin and embedded in paraffin. Tissues were cut in sections of 5 μm and stained with hematoxylin–eosin (HE) for general analysis. They were studied under a Zeiss Axioskop 2 microscope (Zeiss International, USA) equipped with the image analysis software package AxioVision 4.6 (Zeiss International). A qualitative analysis was made in 2 to 4 slices of adipose tissue per animal. Besides, adipocyte size was measured counting the number of cells per field under a 20× objective.2.9. Statistical analysis
The results were expressed as mean values ± S.E.M. for a minimum of 6 rats, and were analyzed by the Student t test and one or two-way analysis of variance (ANOVA), using the GraphPad Prism 5 software (GraphPad, USA). Differences between the groups were assessed by the Bonferroni post-hoc test. Differences between the means were considered to be significant when P < 0.05.3. Results
3.1. Effects on food and fluid intakes and body composition
Food intake was significantly lower in rats consuming the high-fat/high-dextrose diet (MS and MSH) compared to those consuming the standard diet (C and CH). No differences were observed in this parameter in rats consuming hydrolysate (C vs. CH and MS vs. MSH) (Fig. 1A). Although there were no differences in fluid intakes between groups before hydrolysate administration (before week 10), MSH rats drank significantly more fluids than the other groups when they started consuming the hydrolysate (Fig. 1B). As a consequence, energy intake was significantly higher in MSH rats when they started the hydrolysate consumption, compared to C and MS rats (Fig. 1C).As shown in Fig. 1D, rats consuming the high-fat/high-dextrose diet (MS and MSH) showed a significant body weight gain increase than rats consuming the standard diet (C and CH). When the hydrolysate consumption started, MSH rats significantly decreased their body weight gain until values were similar to C and CH rats. No differences in this parameter were observed in CH vs. C rats.
Regarding body composition parameters (Table 1), at the end of the study the abdominal circumference was significantly higher in the MS than in the C group, and it was significantly lower in MSH rats when compared to MS animals. Body length was also significantly higher in MS rats when compared to C rats, but no differences in this parameter were observed in MSH rats when compared to both C and MS rats.
| Variable | C | MS | MSH | CH |
|---|---|---|---|---|
| Body composition | ||||
| Abdominal circumference, cm (n = 7–10) | 22.01 ± 0.34a | 25.51 ± 0.44b | 23.77 ± 1.10c | 21.96 ± 0.31a |
| Body length, cm (n = 7–10) | 24.94 ± 0.24a | 25.65 ± 0.69b | 25.25 ± 1.32ab | 26.11 ± 1.21b |
| BMI (n = 7–10) | 0.87 ± 0.03a.b | 0.92 ± 0.01b | 0.89 ± 0.02a.b | 0.80 ± 0.04a |
| Tissue and organ wet weights, g cm −1 tibial length (n = 7–10) | ||||
| Epididymal adipose tissue | 3.65 ± 0.24a | 6.10 ± 0.47b | 5.03 ± 0.16c | 3.52 ± 0.30a |
| Liver | 2.89 ± 0.09a.b | 3.04 ± 0.1b | 2.74 ± 0.09a | 3.05 ± 0.11b |
| Lipid metabolism | ||||
| Triglycerides, mg dl−1 (n = 7–10) | 38.76 ± 2.07a | 57.97 ± 7.15b | 65.90 ± 3.67b | 43.97 ± 3.03a |
| Cholesterol, mg dl−1 (n = 7–10) | 53.45 ± 4.00a | 51.18 ± 2.84a | 56.49 ± 2.72a | 56.63 ± 3.73a |
| HDL, mg dl−1 (n = 7–10) | 8.92 ± 1.09a | 6.63 ± 1.64a | 6.55 ± 1.42a | 13.06 ± 0.38b |
| Glucose metabolism | ||||
| Glucose, mg dl−1 (n = 7–10) | 226.4 ± 18.9a | 327.7 ± 28.3b | 277.4 ± 16.1a.b | 249.6 ± 25.7a.b |
| Insulin, μmol ml−1 (n = 7–10) | 4.15 ± 1.27a | 4.44 ± 0.65a | 4.36 ± 0.98a | 4.02 ± 0.76a |
| HOMA-IR (n = 7–10) | 0.025 ± 0.009a | 0.036 ± 0.015a | 0.028 ± 0.008a | 0.025 ± 0.013a |
Relative epididymal adipose tissue weight (Table 1) was significantly higher in MS rats compared to C rats. This parameter was significantly reduced in MSH animals when compared to the MS group. However, the relative epididymal adipose tissue weight in the MSH group did not reach the values of the C and CH groups. Although no significant differences were observed, relative liver weight was slightly increased in MS rats compared to C rats (Table 1). This parameter was significantly reduced in MSH animals compared to MS animals, reaching control values.
3.2. Effects on lipid and glucose metabolism
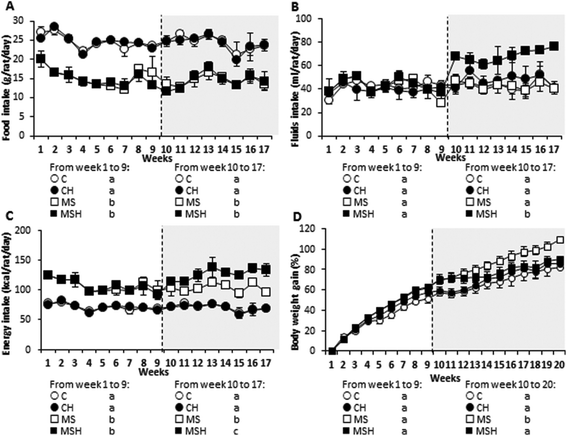
As shown in Table 1, plasma TG levels were significantly higher in high-fat/high-dextrose fed rats (MS and MSH) compared to standard diet fed rats (C and CH). No differences between all groups were observed in plasma cholesterol levels. Regarding HDL cholesterol, no significant differences were shown in MS or MSH animals compared to C animals. However, it is important to note that the rats of the CH group presented significantly higher HDL levels than the animals of the C group. Similarly, the size of adipocytes was significantly higher in the MS and MSH groups compared to the C animals. This hypertrophy significantly decreases in the MSH rats compared to MS animals (Fig. 2).Plasma glucose levels were significantly higher in MS, compared to C rats (Table 1). Although there were no significant differences, this parameter was partially reduced in MSH animals when compared to the MS group. No differences were observed in the CH group compared to the C group. Differences in plasma insulin levels were not observed between the experimental groups (Table 1). The HOMA-IR index was slightly increased in the MS group compared to the C group and it was slightly decreased in MSH compared to MS rats, but no significant differences were observed in any experimental group (Table 1).
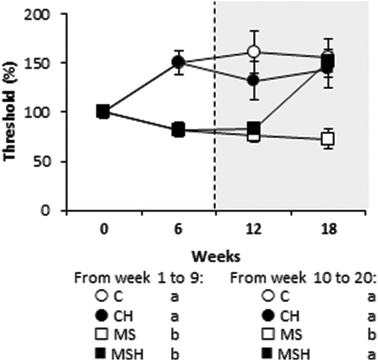
The presence of tactile allodynia was also evaluated (Fig. 3). Before hydrolysate consumption, rats consuming the high-fat/high-dextrose diet (MS and MSH) presented a significantly lower mechanical threshold than rats consuming the standard diet (C and CH). This situation was significantly reversed in MSH after hydrolysate consumption.
3.3. Effects on oxidative stress and inflammation
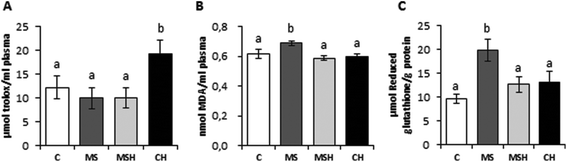
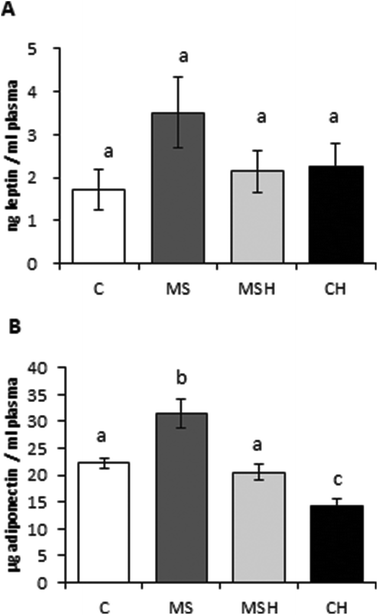
Regarding the plasma antioxidant capacity of the animals (Fig. 4A), no differences were observed between the control group (C) and diet-induced MetS groups (MS and MSH). However, rats of the CH group presented significantly higher radical scavenging capacity of plasma compared to C rats. On the other hand, plasma MDA levels (Fig. 4B) were significantly increased in MS animals when compared to C animals, and this parameter was significantly reduced in MSH, reaching levels similar to the C and CH groups. No differences were observed in CH vs. C rats regarding plasma MDA levels. Liver GSH levels (Fig. 4C) were increased in MS rats compared to C rats. These values were reduced in the MSH group when compared to the MS group, and no differences were observed when MSH rats were compared to controls.No significant differences were observed in plasma leptin levels (Fig. 5A). However, this parameter was slightly increased in MS rats compared to the rest of the experimental groups. Plasma adiponectin levels (Fig. 5B) were significantly increased in the MS group compared to the C group. MSH rats showed adiponectin values significantly lower than the MS group, reaching control-like values. In addition, plasma adiponectin levels were significantly decreased in CH rats compared to control rats.
4. Discussion
In this study Wistar rats were fed a high-fat/high-dextrose diet for 20 weeks to induce MetS. Body weight gain, abdominal obesity, adipose tissue and liver weight were significantly increased in diet-induced MetS rats. Some metabolic parameters, such as plasma glucose, TG, MDA, GSH, and adiponectin also became worse in MS animals compared to the C group animals.MSH rats attenuated their body weight gain when they started to consume the hydrolysate, in comparison to the MS group, without affecting food intake. Despite the satiating effect that proteins have shown,24 specially egg white proteins,25 the consumption of EWH did not produce satiety in the animals of this study. Moreover, Garcés-Rimón et al. did not observe this effect in Zucker fatty rats.18 In addition, fluid intake, and therefore dextrose intake, was increased in MSH rats, thereby increasing energy intake, which enhances the importance of the effects seen on body weight gain in this experimental group. The results found on body composition correspond to those found in body weight gain. The group of MS rats presented abdominal obesity, which is one of the major risk factors in the development of MetS. Visceral fat presents a higher activity in adipokine secretion compared to subcutaneous fat. Some of these adipokines, such as TNF-α or leptin, play key roles in the development of hypertension, insulin resistance, and inflammation, related with MetS.26,27 At the end of the experimental period, those MetS induced animals which consumed EWH significantly reduced their waist circumference compared to MS rats, decreasing their abdominal fat and therefore, the risk to develop MetS. The reduction in fat mass in MSH rats was also confirmed regarding their adipose tissue weight. Epididymal adipose tissue doubled its size in MS rats compared to C rats, and this increase was partially reduced in MSH animals. Although there were no significant differences, liver weight was also increased in MS animals compared to C rats, which may be related to inflammation and lipid accumulation28 leading to nonalcoholic liver steatosis in this experimental model over time, similar to human metabolic syndrome development. As expected, MSH rats significantly reduced liver weight, reaching values similar to the C group. Garcés-Rimón et al. already observed an important prevention in liver steatosis developed in Zucker fatty rats when they consumed this pepsin EWH.18 All these results suggest a modification in lipid and/or carbohydrate absorption and its metabolism, which leads to a reduction in fat accumulation. Other researchers have also observed similar results using food-derived peptides. Soybean-derived peptides showed to inhibit Fatty Acid Synthase (FAS) activity in vitro and to upregulate fatty acid oxidation in vivo in mice and diabetic models.29 Some peptides derived from egg white digested with pepsin have already demonstrated to alter the intestinal lipid uptake by inhibiting their solubilization into micelles.29
Regarding lipid metabolism, no differences were observed in cholesterol levels between groups. However, MS rats presented an increase in TG levels, an indicator of abnormal lipid metabolism, which could lead to dyslipidemia, a risk factor of cardiovascular disease.29 EWH did not seem to have any effect on this alteration developed in MS rats; Garcés-Rimón et al. did not observe an effect in TG or cholesterol levels in Zucker fatty rats,18 despite the hypocholesterolemic activity that this hydrolysate showed in vitro.17 On the other hand, an important increase in HDL levels in CH rats was observed, which could imply a protective effect caused by the hydrolysate in healthy animals.
Regarding glucose metabolism, MS rats significantly increased plasma glucose levels, compared to C animals. No changes in plasma insulin levels were observed between different experimental groups. The HOMA-IR index was slightly increased in the MS group, which could indicate an early state of insulin resistance. In addition, MS rats presented tactile allodynia, which is a symptom of sensory neuropathy, and appears in the early stages of diabetes.3 This alteration is also described in the MetS situation in rats fed high-energy/high-sucrose diets.21 According to these results, we conclude that MS rats developed an early insulin resistance, with an important sensory neuropathy. This pre-diabetes stage is reverted after EWH consumption. MSH rats presented slightly reduced glycaemia compared to MS animals, and diabetic neuropathy was also reversed when the animals started to consume the EWH. There are studies which describe hypoglycemic food hydrolysates and peptides which act as dipeptidyl peptidase IV (DPP-IV) inhibitors both in vitro and in vivo30 from different sources, including egg white.31 The EWH used in this study had previously demonstrated a DPP-IV inhibitory activity in vitro17 as well as lowering plasma insulin properties in Zucker fatty rats (data not published); in this study we have not observed a reduction in plasma insulin levels, as this model of MetS did not present increased insulin plasma levels. However, we could observe an important hypoglycemic activity by reduction in plasma glucose levels, which could not be observed in Zucker fatty rats due to the normoglycemic characteristics of this genetic MetS model. Therefore, these results obtained in glucose metabolism complement those obtained by our research group (data not published) and suggest that this EWH could be used to control alterations of glucose metabolism associated with MetS.
The key role of oxidative stress in the development and maintenance of MetS and related complications is well known.32 Antioxidant foods have been suggested as a strategy to reduce or ameliorate this pathology or some of its complications.33 Moreover, oxidative stress has been also related with diabetic neuropathy, being a possible target in pharmaceutical intervention, especially using peptide-based antioxidants.34 By this way, an improvement in the oxidative stress could help to ameliorate most of the complications related to MetS. In this study, high-fat/high-dextrose fed rats (MS and MSH groups) did not show differences in their plasma antioxidant capacity compared to C animals. However, there was a significant improvement in the plasma ORAC values of CH rats compared to the C group. This result could be related to the increase in HDL cholesterol levels, also observed in this group. HDL is considered an important plasma antioxidant defense system, which acts by preventing the oxidation of LDL.35 It suggests that the consumption of EWH can produce an increase of the antioxidant defense in healthy situations to provide high protection in the presence of oxidative stress related diseases.
MDA is used as a biomarker of lipid peroxidation. Its determination in plasma is one of the most useful methods to predict oxidative stress levels.36 Plasma MDA levels were significantly increased in MS rats; so we can confirm the presence of increased oxidative stress in this animal model. This parameter was, on the contrary, significantly decreased in the plasma of MSH animals at the end of the study. These results agree with those obtained by Garcés-Rimón et al. in Zucker fatty rats18 and confirm that the EWH studied could reduce the oxidative stress associated with MetS conditions. It is worth emphasizing that EWH exhibited antioxidant properties and hydroxyl radical scavenging capacity in previous in vitro studies.17 In addition, we found an increase in liver GSH levels in MS animals. This increase has been also observed in high fructose-fed Sprague Dawley rats.37 It has been suggested that increased GSH levels in liver may be an early tissue defense mechanism against oxidative stress. This alteration in liver GSH levels was attenuated in MSH rats, which indicates the presence of a compensatory mechanism in the oxidative stress situation after consumption of EWH. These results suggest that the intake of EWH could have a protective role not only in subjects with MetS and/or oxidative damage conditions but also in non-pathological situations, keeping a prepared antioxidant defense that can act rapidly in punctual oxidative stress situations.
The relationship between obesity and inflammation has been extensively studied. White adipose tissue has been recognized as an endocrine organ which mediates the development of MetS in obese subjects due to the secretion of adipokines.38 Leptin, one of the most studied adipokine, plays an important role in the regulation of satiety and food intake. Obese patients typically present high circulating leptin levels due to the development of leptin resistance;26 it has been also shown that leptin can directly modulate the immune system, acting as a pro-inflammatory factor.38 No significant differences were observed in circulating leptin levels between different experimental groups. MS rats showed a tendency to increase their plasma circulating leptin levels but, however, this increase was not observed in MSH obese animals after the consumption of EWH. This result suggests a leptin sensitization caused by the EWH consumption that, together with a less fat mass in these animals, could lead to a decreased leptin levels in plasma. It would also mean a reduction of the inflammation signaling in MSH animals compared to MS. On the other hand, adiponectin is recognized as an anti-inflammatory adipokine with protective effects against MetS.26 However, the MS group increased circulating adiponectin levels in plasma. This unexpected adiponectin increase has been also observed in palatable diet-fed C57BL/6 mice39 and in Zucker fatty rats.18 Garcés-Rimón et al. associated high levels of this adipokine with adiponectin resistance.18 Actually, less expression of adiponectin receptors in hyperinsulinemic and hyperglycemic states has also been observed.40 MSH rats showed adiponectin levels similar to C rats, and much lower than MS animals. This suggests that the consumption of EWH reverted or protected against adiponectin resistance developed in the MS group during the study.
In conclusion, the present study showed that the oral administration of a pepsin EWH could be used as a functional ingredient to improve some complications associated with MetS induced by unhealthy diets. More research is required to delve in depth into the mechanisms and molecular pathways involved in their beneficial effects, and human clinical studies are necessary to consider its use to prevent or treat these complications in MetS patients.
Conflicts of interest
All authors certify that there is no conflict of interests in this study.Acknowledgements
Authors would like to acknowledge the financial support of the Spanish Ministry of Economy, Industry and Competitiveness (MINECO) (under the projects AGL2012-32387 and CSIC – Intramural 201570I028 and under grant number BES-2013-065684).References
- S. K. Panchal and L. Brown, Rodent models for metabolic syndrome research, J. Biomed. Biotechnol., 2011 DOI:10.1155/2011/351982.
- S. Y. Ren and X. Xu, Role of autophagy in metabolic syndrome-associated earth disease, Biochim. Biophys. Acta, 2015, 1852, 225–231 CrossRef CAS PubMed.
- S. Lupachyk, P. Watcho, N. Hasanova, U. Julius and I. G. Obrosova, Triglyceride, nonesterified fatty acids, and prediabetic neuropathy: role for oxidative-nitrosative stress, Free Radical Biol. Med., 2012, 52, 1255–1263 CrossRef CAS PubMed.
- J. Carillon, C. Romain, G. Bardy, G. Fouret, C. Feillet-Coudray, S. Gaillet, D. Lacan, J. P. Cristol and J. M. Rouanet, Cafeteria diet induces obesity and insulin resistance associated with oxidative stress but not with inflammation: improvement by dietary supplementation with a melon superoxide dismutase, Free Radical Biol. Med., 2013, 65, 254–261 CrossRef CAS PubMed.
- K. L. Sweazea, Compounding evidence implicating Western diets in the development of metabolic syndrome, Acta Physiol., 2014, 211, 471–473 CrossRef CAS PubMed.
- I. Heinonen, P. Rinne, S. T. Ruohonen, S. Ruohonen, M. Ahotupa and E. Savontaus, The effects of equal caloric high fat and western diet on metabolic syndrome, oxidative stress and vascular endothelial function in mice, Acta Physiol., 2014, 211, 515–527 CrossRef CAS PubMed.
- Y. Tominaga, M. Kitano, T. Mae, S. Kakimoto and K. Nakagawa, Effect of licorice flavonoid oilon visceral fat in obese subjects in the United States, Nutrafoods, 2014, 13, 35–43 CrossRef CAS.
- N. D. Yuliana, H. Korthout, C. H. Wijaya, H. K. Kim and R. Verpoorte, Plant-derived food ingredients for stimulation of energy expenditure, Crit. Rev. Food Sci. Nutr., 2014, 54, 373–388 CrossRef CAS PubMed.
- S. S. Ko and Y. J. Jeon, Marine peptides for preventing metabolic syndrome, Curr. Protein Pept. Sci., 2013, 14, 183–188 CrossRef CAS PubMed.
- M. Miguel, I. Recio, J. A. Gomez-Ruiz, M. Ramos and R. Lopez-Fandiño, Angiotensin I-converting enzyme inhibitory activity of peptides derived from egg white proteins by enzymatic hydrolysis, J. Food Prot., 2004, 67, 1914–1920 CrossRef CAS PubMed.
- M. Miguel, M. Manso, A. Aleixandre, M. J. Alonso, M. Salaices and R. López-Fandiño, Vascular effects, angiotensin I-converting enzyme (ACE)-inhibitory activity, and antihypertensive properties of peptides derived from egg white, J. Agric. Food Chem., 2007, 55, 10615–10621 CrossRef CAS PubMed.
- A. Dávalos, M. Miguel, B. Bartolomé and R. López-Fandiño, Antioxidant activity of peptides derived from egg white proteins by enzymatic hydrolysis, J. Food Prot., 2004, 67, 1939–1944 CrossRef.
- M. Miguel, Y. Alvarez, R. López-Fandiño, M. J. Alonso and M. Salaices, Vasodilator effects of peptides derived from egg white proteins, Regul. Pept., 2007, 140, 131–135 CrossRef CAS PubMed.
- M. Miguel, R. López-Fandiño, M. Ramos and A. Aleixandre, Short-term effect of egg-white hydrolysate products on the arterial blood pressure of hypertensive rats, Br. J. Nutr., 2005, 94, 731–737 CrossRef CAS PubMed.
- M. Miguel, R. López-Fandiño, M. Ramos and A. Aleixandre, Long-term intake of egg-white hydrolysate attenuates the development of hypertension in spontaneously hypertensive rats, Life Sci., 2006, 78, 2960–2966 CrossRef CAS PubMed.
- M. A. Manso, M. Miguel, J. Even, R. Hernández, A. Aleixandre and R. López-Fandiño, Effect of the long-term intake of an egg white hydrolysate on the oxidative status and blood lipid profile of spontaneously hypertensive rats, Food Chem., 2008, 109, 361–367 CrossRef CAS PubMed.
- M. Garcés-Rimón, I. Lopez-Exposito, R. López-Fandiño and M. Miguel, Egg white hydrolysates with in vitro biological multiactivities to control complications associated with the metabolic syndrome, Eur. Food Res. Technol., 2016, 242, 61–69 CrossRef.
- M. Garcés-Rimón, C. González, J. A. Uranga, V. Lopez-Miranda, R. Lopez-Fandiño and M. Miguel, Pepsin egg white hydrolysate ameliorates obesity-related oxidative stress, inflammation and steatosis in Zucker Fatty Rats, PLoS One, 2016 DOI:10.1371/journal.pone.0151193.
- S. K. Panchal, H. Poudyal, A. Iyer, R. Nazer, M. A. Alan, V. Diwan, K. Kauter, C. Sernia, F. Campbell, L. Ward, G. Gobe, A. Fenning and L. Brown, High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats, J. Cardiovasc. Pharmacol., 2011, 57, 611–624 CrossRef PubMed.
- C. B. Mobley, R. C. Toedebush, C. M. Lockwood, A. J. Heese, C. Zhu, A. E. Krieger, C. L. Cruthirds, J. C. Hofheins, J. M. Company, C. E. Wiedmeyer, D. Y. Kim, F. W. Booth and M. D. Roberts, Herbal adaptogens combined with protein fractions from bovine colostrum and hen egg yolk reduce liver TNF-α expression and protein carbonylation in western diet feeding rats, Nutr. Metab., 2014, 11, 19 Search PubMed.
- F. Xie, H. Fu, J. F. Hou, K. Jiao, M. Costigan and J. Chen, High energy diets-induced metabolic and prediabetic painful polyneuropathy in rats, PLoS One, 2013 DOI:10.1371/journal.pone.0057427.
- H. Kamencic, A. Lyon, P. G. Paterson and B. H. J. Juurlink, Monochlorobimane fluorometric method to measure tissue glutathione, Anal. Biochem., 2000, 286, 35–37 CrossRef CAS PubMed.
- E. Ferrannini and A. Mari, How to measure insulin sensitivity, J. Hypertens., 1998, 16, 895–906 CrossRef CAS PubMed.
- D. König, K. Muser, A. Berg and P. Deibert, Fuel selection and appetite-regulating hormones after intake of a soy protein-based meal replacement, Nutrition, 2012, 28, 35–39 CrossRef PubMed.
- J. Ratliff, J. O. Leite, R. de Ogburn, M. J. Puglisi, J. VanHeest and M. L. Fernandez, Consuming eggs for breakfast influences plasma glucose and ghrelin, while reducing energy intake during the next 24 hours in adult men, Nutr. Res., 2010, 30, 96–103 CrossRef CAS PubMed.
- K. Nakamura, J. J. Fuster and K. Walsh, Adipokines: A link between obesity and cardiovascular disease, J. Cardiol., 2014, 63, 250–259 CrossRef PubMed.
- S. Golbidi and I. Laher, Exercise induced adipokine changes and the metabolic syndrome, J. Diabetes Res., 2014 DOI:10.1155/2014/726861.
- S. C. L. Sanches, L. N. Z. Ramalho, M. J. Augusto, D. M. Da Silva and F. Silva Ramalho, Nonalcoholic steatohepatitis: A search for factual animal models, BioMed Res. Int., 2015 DOI:10.1155/2015/574832.
- C. C. Udenigwe and K. Rouvinen-Watt, The role of food peptides in lipid metabolism durin dyslipidemia and associated health conditions, Int. J. Mol. Sci., 2015, 16, 9303–9313 CrossRef CAS PubMed.
- O. Power, A. B. Nongonierma, P. Jakeman and R. J. FitzGerald, Food protein hydrolysates as a source of dipeptidyl peptidase IV inhibitory peptides for the management of type 2 diabetes, Proc. Nutr. Soc., 2014, 73, 34–46 CrossRef PubMed.
- A. Van Amerongen, M. J. C. Beelen-Thomissen, L. A. M. Van Zeeland-Wolbers, W. H. Van Gils, J. H. Buikema and J. W. P. M. Nelissen, Egg protein hydrolysates, Int. Pat, WO2009128713, 2009 Search PubMed.
- F. Bonomini, L. F. Rodella and R. Rezzani, Metabolic syndrome, aging and involvement of oxidative stress, Aging Dis., 2015, 6, 109–120 CrossRef PubMed.
- C. Andersen and M. L. Fernandez, Dietary strategies to reduce metabolic syndrome, Rev. Endocr. Metab. Disord., 2013, 14, 241–254 CrossRef CAS PubMed.
- M. A. Babizhayev, I. A. Strokov, V. V. Nosikov, E. L. Savel'yeva, V. F. Sitnikov, T. E. Yegorov and V. Z. Lankin, The role of oxidative stress in diabetic neuropathy: Generation of free radical species in the glycation reaction and gene polymorphisms encoding antioxidant enzymes to genetic susceptibility to diabetic neuropathy in population of type I diabetic patients, Cell Biochem. Biophys., 2015, 71, 1425–1443 CrossRef CAS PubMed.
- T. M. T. Avelar, A. S. Storch, L. A. Castro, G. V. M. M. Azevedo, L. Ferraz and P. F. Lopes, Oxidative stress in the pathophysiology of metabolic syndrome: which mechanisms are involved?, J. Bras. Patol. Med. Lab., 2015, 51, 23–239 Search PubMed.
- Z. Singh, I. P. Karthigesu, P. Singh and R. Daur, Use of malondialdehyde as a biomarker for assessing oxidative stress in different disease pathologies: a review, Iran. J. Public Health, 2014, 3, 7–16 Search PubMed.
- N. M. Shawky, G. S. G. Hehatou, M. A. Rahim, G. M. Suddek and N. M. Gameil, Levocetirizine ameliorates high fructose diet-induced insulin resistance, vascular dysfunction and hepatic steatosis in rats, Eur. J. Pharmacol., 2014, 740, 353–363 CrossRef CAS PubMed.
- A. Aguilar-Valles, W. Inoue, C. Rummel and G. N. Luheshi, Obesity, adipokines and neuroinflammation, Neuropharmacology, 2015, 96, 124–134 CrossRef CAS PubMed.
- M. J. Morris, H. Chen, R. Watts, A. Shulkes and D. Cameron-Smith, Brain neuropeptide Y and CCK and peripheral adipokine receptors: temporal response in obesity induced by palatable diet, Int. J. Obes., 2008, 32, 249–258 CrossRef CAS PubMed.
- A. Tsuchida, T. Yamauchi, Y. Ito, Y. Hada, T. Maki, S. Takekawa, J. Kamon, M. Kobayashi, R. Suzuki, K. Hara, N. Kubota, Y. Terauchi, P. Froguel, J. Nakae, M. Kasuga, D. Accili, K. Tobe, K. Ueki, R. Nagai and T. Kadowaki, Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity, J. Biol. Chem., 2004, 279, 30817–30822 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |