An exploratory study of red raspberry (Rubus idaeus L.) (poly)phenols/metabolites in human biological samples
Abstract
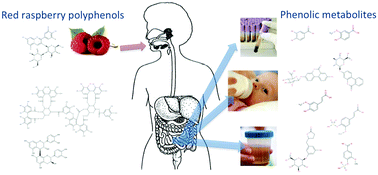
Red raspberry (Rubus idaeus L.) contains a variety of polyphenols including anthocyanins and ellagitannins. Red raspberry polyphenols absorbed in different forms (parent compounds, degradants or microbial metabolites) are subject to xenobiotic metabolism in the intestine, liver, and/or kidney, forming methylate, glucuronide, and sulfate conjugated metabolites. Upon acute exposure, (poly)phenol/metabolite presence in the blood depends mainly on intestinal absorption, enterohepatic circulation, and metabolism by resident microbiota. However, chronic exposure to red raspberry polyphenols may alter metabolite patterns depending on adaptions in the xenobiotic machinery and/or microbiota composition. Understanding the metabolic fate of these compounds and their composition in different biological specimens relative to the exposure time/dose will aid in designing future health benefit studies, including the mechanism of action studies. The present exploratory study applied ultra-high performance liquid chromatography (UHPLC) coupled with quadrupole time-of-flight (QTOF) and triple quadrupole (QQQ) mass spectrometries to characterize red raspberry polyphenols in fruit and then their appearance, including metabolites in human biological samples (plasma, urine and breast milk) after the chronic intake of red raspberries. The results suggested that the most abundant polyphenols in red raspberries included cyanidin 3-O-sophoroside, cyanidin 3-O-glucoside, sanguiin H6 and lambertianin C. Sixty-two (poly)phenolic compounds were tentatively identified in the plasma, urine and breast milk samples after the intake of red raspberries. In general, urine contained the highest content of phenolic metabolites; phase II metabolites, particularly sulfated conjugates, were mainly present in urine and breast milk, and breast milk contained fewer parent anthocyanins compared to urine and plasma.
- This article is part of the themed collections: Around the Supermarket: Staple Foods and Berry Health Benefits Symposium


 Please wait while we load your content...
Please wait while we load your content...