Open Access Article
Open Access ArticleExpert perspectives on potential environmental risks from nanomedicines and adequacy of the current guideline on environmental risk assessment†
Indrani
Mahapatra
 *a,
Julian R. A.
Clark
a,
Peter J.
Dobson
bc,
Richard
Owen
d,
Iseult
Lynch
*a,
Julian R. A.
Clark
a,
Peter J.
Dobson
bc,
Richard
Owen
d,
Iseult
Lynch
 a and
Jamie R.
Lead
e
a and
Jamie R.
Lead
e
aSchool of Geography, Earth and Environmental Sciences, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK. E-mail: i.mahapatra@bham.ac.uk
bThe Queen's College, Oxford OX1 4AW, UK
cWarwick Manufacturing Group, University of Warwick, Coventry, CV4 7AL, UK
dDepartment of Management, University of Bristol, The Priory Road Complex, Priory Road, Clifton, Bristol, BS8 1TZ, UK
eCenter for Environmental Nanoscience and Risk, Department of Environmental Health Sciences, Arnold School of Public Health, University of South Carolina, Columbia, South Carolina 29208, USA
First published on 11th July 2018
Abstract
In the next couple of decades, nanotechnology-enabled healthcare applications will significantly influence the diagnosis, prevention and treatment of human diseases. Since pharmaceutical products (PPs) have been detected in various environmental compartments, and low-level chronic exposure to PPs has induced adverse and sometimes unexpected effects on non-target organisms, the question of potential environmental risks from increased usage of nanomedical products arises. The risks and benefits to patients from nanomedicines are the focus of exhaustive evaluation by regulatory agencies; by contrast, risks to the environment from nanomedicines are only briefly considered by regulators and are rarely discussed by nanoscientists. To start to fill this gap, 66 experts from nanomedicine R&D, representatives of research funding agencies and of institutions involved in safeguarding public health and the environment were interviewed using a semi-structured questionnaire regarding possible hazards and risks from nanomedicine and on the adequacy of current the environmental risk assessment (ERA) framework for medicines. The interview recordings were transcribed verbatim and analysed via qualitative content analysis. Experts interviewed commented that hazards were possible but risks were unlikely from nanomedicines due to expected minimal exposure. They qualified their statements by comparing risks from nanomedicine with risks from nanomaterials in other industries, conventional pollutants and larger global issues like climate change. Regarding adequacy of the current risk framework for assessment of environmental risks from nanomedicines, perceptions of experts were more varied; some argued that complete overhaul of the ERA framework was required including changes in toxicity endpoints, whereas others suggested that the framework was adequate, though some adjustments were needed.
Environmental significanceThe possible environmental concentrations of nanomedicines containing gold nanoparticles for high-use and worst-case scenario was modelled to be in the range 4–468 pg L−1 in surface waters, and 130–150 μg kg−1 in sewage sludge. Low level chronic exposure to pharmaceuticals can induce adverse and sometimes unexpected effects on non-target organisms in the environment, raising concerns regarding the potential environmental risks from nanomedicines. In this article, the opinions of 66 experts regarding possible risks arising from nanomedicines and the suitability of current environment risk assessment (ERA) procedures for nanomedicines is presented. Experts noted that hazards were possible but that risks were unlikely from nanomedicines. Recommendations regarding ERA for nanomedicines ranged from customisation of existing frameworks to complete overhaul. |
Introduction
Nanomedicines are designed with new properties to enhance their bio-availability and are enabled to be activated by external stimuli.1,2 They have the potential to be of immense benefit for improving human health, despite their current high cost and the technical challenges in mass manufacture. It is believed that in the next couple of decades, nanotechnology-enabled health care applications will have a significant influence on diagnosis, prevention, and treatment of diseases.3,4 Due to their unique chemical and physical properties, such as superparamagnetism and increased luminescence and optical scattering because of plasmonic effects, metal nanoparticles can be used for in vitro and in vivo diagnostics5,6 and for cancer treatment.7 Carbon-based nanoparticle types – micellar, liposomal, polymeric – can be used as drug delivery vehicles for poorly soluble pharmaceuticals (e.g. albumin nanoparticles for delivery of paclitaxel for treatment of metastatic breast cancer),8 peptides, nucleotides and genes,9,10 and can be used in implants,11 vaccines,12 and in regenerative medicine.13 Reformulation of approved drugs with nanoparticles can limit systemic toxicities by improved targeting and cellular uptake.14 The many potential applications of nanomedicine (including medical devices) have recently been reviewed by Pelaz et al.15 Scientists at the Center of Drug Evaluation Research (CDER) of the United States Food and Drug Administration (USFDA) found an increase in drug applications of medicines containing nanomaterials over 45 years (1970 to 2015) and reported 359 nanomedicine applications submitted for approval. Their study showed that 40% of the drug applications containing nanomaterials submitted between 2010 and 2015 were for cancer treatment, followed by treatments for immune, pain and inflammation indications (18%) and for infectious diseases (14%).16 However, in addition to these positive uses, there is the possibility that nanomedicine use could result in toxicities via different modes of action – for example, different toxicodynamics or pharmacodynamics, different organ and cellular distributions, or altered clearance rates (toxicokinetics or pharmacokinetics). Additionally, the diversity of nanomaterial compositions, sizes, surface coatings, and functionalities, can result in varied toxic potentials (e.g. across different cell types and organisms).Patient safety, efficacy and quality resulting from use of nanomaterials in drug products are addressed by the responsible agencies, such as the USFDA,17 the European Medicines Agency (EMA),18 and national medicinal agencies‡ in Europe. However, the risks to the environment from such medicines after use are seldom discussed (possibly due to the high perceived benefits from nanomedicines outweighing potential risks) despite the fact that pharmaceuticals products (PPs) have been detected in various environmental compartments – soil, biosolids, surface water and ground water.19,20 PPs have been detected in surface waters of 41 countries,21 and it has been found that low-level chronic exposure to PPs can have adverse and sometimes unexpected effects on non-target organisms.22,23 Surface water concentrations of iron oxide nanoparticle (IONP) based MRI contrast agents, modelled using 25% and 50% market penetration (i.e., replacement of gadolinium-based MRI contrast agents), were estimated to be 3.1 ng L−1 and 8.4 ng L−1 for the years 2015 and 2020 respectively in Germany.24 Annual surface water concentrations for select gold nanoparticle-based medical applications were modelled to be 5 and 470 pg L−1 for the US and UK respectively.25 Many drug delivery and targeting applications in the nanomedicine field have polymeric coatings, such as polyethylene glycol (PEG) which is used for its stealth properties and stability in biological fluids,26 however, their very stability and ability to escape an organism's biological barriers and immune system raises the question as to whether nanomedicines could pose risks to organisms in the environment when they get released without any transformation, metabolism or degradation. Very few studies have reported on the interaction of nanomedicines with non-biotic components of the environment and some (limited) ecotoxicology studies have been done with nanomaterials representative of nanomedicines25,27 although it is important to note that these studies have not utilised the actual nanomedicine formulations approved or in clinical trials.
Expert judgement is the preferred method to evaluate and characterise risks in cases where uncertainties and data gaps exist,28,29 which is largely the case with nanomaterials. Indeed, many government agencies and national committees with statutory or advisory responsibility for protecting human health and the environment seek views from established scientists or are composed from them; therefore, it was decided to gather the perspectives of applied scientists, social scientists, regulators, policymakers, and representatives of industries along the nanomedicine R&D spectrum on the potential environmental impacts from nanomedicine. Also, for this study, perceptions on the suitability of the current environment risk assessment framework was explored by conducting semi structured interviews with sixty-six respondents (see Methodology below).
There are varied views on the risks posed by nanomedicine. In a study by Petersen and Anderson,30 experts considered human health risks from medical applications to be high by reasoning that injecting nanoparticles into human bodies can cause unknown effects due to increased bioaccumulation of active ingredients when compared to conventional medicines, whereas in a survey-based study by Siegrist et al.,31 it was found that experts rated risks from use of nanomaterials in medicines to be low compared to risks associated with nanomaterials in other areas such as food packaging and sunscreens. This could perhaps be due to the perceived beneficial effects of medicines, as noted above. Similarly, Capon et al.32 showed that use of nanomaterials in medicines was considered less risky by academics and industry representatives when compared to their use in food, cosmetics, and pesticides. Moreover, one of the reasons for using nanomaterials in medicine is to use smaller and highly targeted doses, therefore reducing systemic toxicity. To ensure safe and sustainable propagation of new and emerging technologies, such as nanotechnologies, all possible impacts from the applications of the new technology should be identified and described with respect to a particular application sector. For example, comprehensive identification and description of benefits, risks, and uncertainties related to nanotechnology and food, and nanotechnology and pharmaceutical products should be evaluated, rather than implications of nanotechnology as a whole. Descriptions of these impacts may be theoretical or hypothesis-based, but they need to be explored so that concerns are assessed, and to enable policymakers to agree on, and develop, future research priorities.
To date, there have been no studies gathering expert viewpoints across nanomedicine R&D regarding the environment risks from nanomedicine. However, one study from Portugal, intended to understand how nanomedicine researchers perceived ethical issues in nanomedicine, reported that of those researchers (17 out of 22) who expressed potential risks from nanomedicine, 6 (of the 17) mentioned environmental risks of nanomedicine,33 although no further elaborations on environment risks were done by the authors of this study. Therefore in this paper, we discuss responses of experts (from research, industry, regulatory/policy perspectives) on the two key research questions: (1) the potential environmental hazards and risks from nanomedicines, and (2) the adequacy of existing risk assessment framework for assessing environmental risks from nanomedicines.
Before describing the study methodology we define the terms used in the paper. Althaus34 describes the concept of risk used in various disciplines (e.g., sociology, history, and medicine). For example, logic and mathematics see risk as a calculable phenomenon, applied science and medicine see risk as an objective reality, whereas sociology views risk as a socially constructed phenomenon, and psychology as a behavioural and cognitive experience. This study elicits expert responses on the type of risk assessment which is used within the applied science and medicine fields as discussed by Althaus,34 with special emphasis on environmental risk assessment (ERA). Environmental and human health risk expressed simply is hazard multiplied by exposure, where hazard and exposure are assessed under laboratory conditions using standard protocols and methods, and quantification is done using certain assumptions and postulates. Therefore, risk is the likelihood or probability of occurrence of an event which is not desired. ERA conducted primarily for chemicals (e.g. pesticides) helps decision-makers to set environmental quality standards (EQS).
For medicinal products for human use, the European Medicines Agency (EMA) needs an ERA as part of the marketing authorisation application submitted to the agency for any new medicine, generic, hybrid product, fixed combination or similar biological product. An ERA is also required if a change is made in terms of the marketing authorisation, for example, addition of a new disease indication, or increase in dosage (known as type II variation). Justification for not submitting a complete ERA is acceptable if it is shown that there is no increase in environmental exposure. The ERA has two distinct phases: phase I: the screening phase, in which the surface water concentrations of the active pharmaceutical ingredient (API) is calculated and a persistence, bioaccumulation, toxicity (PBT) assessment is done if the octanol–water partition coefficient is greater than 4.5, and phase II: biodegradation, transformation and toxicity tests based on OECD guidelines (e.g., OECD 301, 308, 201) on test organisms specific to the environment compartment. The details of the tiered approach for ERA of medicinal products for human use and lack of environment risk assessment framework for medical devices in the EU have been discussed elsewhere.27,35 However, two important points of note are that: (1) the octanol–water partition coefficient is not suitable for assessing behaviour of nanomaterials as it is based on equilibrium partitioning36 (rather than kinetics) as a result of nanomaterials accumulating at the interface rather than effectively separating,37 and (2) the OECD test guidelines are currently undergoing extensive revision for nanomaterials38 to account for non-equilibrium behaviour, active receptor mediated uptake driven by surface interactions with biomolecules, agglomeration of nanomaterials in the standardised (non-environmentally representative) test media and many other limitations. Using the current test protocols could lead to significant errors in both fate and hazard predictions and thus overall risk assessment.
Risk assessment helps regulatory agencies to take decisions based on evidence and “objective” analysis.§ In the case of pharmaceuticals and medical devices, regulatory agencies need to take into account the benefit–risk ratio of the product. Also, potential environmental harm is not sufficient at present to stop or prevent approval of a drug39 in the EU. In cases where environmental harm is foreseen, proper information and communication of risk management steps is advised by the USFDA.40,41 Our study aims to add clarity to the exploratory discussions on environmental risks from nanomedicine by presenting expert perspectives on this issue, which has not been attempted previously. We conclude by confirming, using Stirling's42 arguments, that in the case of uncertainty and ignorance, risk assessment is not fool-proof and may indeed be irrational since it does not fulfil the mandate of evidence-based policy, leading to the conclusion that a different approach to the governance of nanomedicine is required.
Methodology
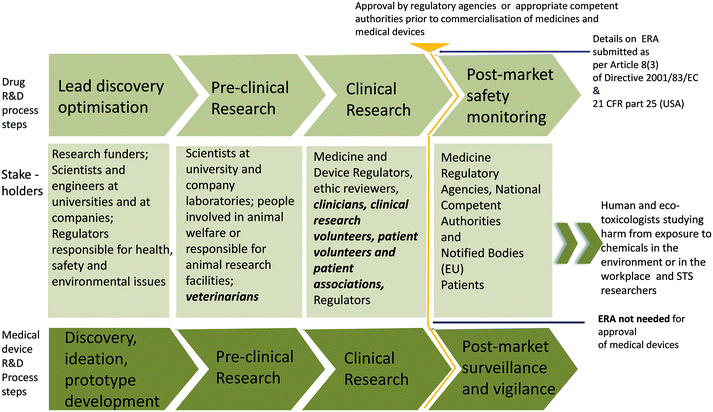
For this exploratory research study, in-depth interviews with 66 experts were conducted using a semi-structured questionnaire (customised for the expert stakeholder type, i.e., academic, industry, regulatory/policy or funder) shared in advance. The questions were informed by the theoretical debates in the field of nanomedicine R&D, safety and regulatory studies, nanotoxicology and nanotechnology risk assessment. Therefore, questions about the challenges in nanomedicine R&D, intellectual property matters, gaps in knowledge, challenges in the nanotoxicology domain, and upstream public engagement were discussed, although the results of analysing these questions are outside the scope of this paper (they are partly reported in Mahapatra et al.43). Only the qualitative analyses of questions related to potential environmental risks from nanomedicine and adequacy of the current risk framework for assessing environmental risks from nanomedicines are presented here. Since risk is constructed in specific sociotechnical contexts, both environmental hazards and exposures were explored. The open-ended questions asked are presented in the ESI.† Consent to be interviewed was gained via email and permission from the expert was sought before recording the conversation. The experts were assured of the confidentiality and anonymity of their responses and were given the option of reviewing interview transcripts. Table 1 lists the profiles of the experts interviewed, categorised under broader stakeholder groups and research focus, while Fig. 1 maps the stakeholders along the medicine and medical device research and commercialisation pathway.| Stakeholder group | Number |
|---|---|
| Academics researching on nanomedicine | 20 |
| Academics researching on human health implications of nanomaterials | 4 |
| Academics researching on environmental implications of nanomaterials | 5 |
| Academics researching on research and innovation policy, science-technology-society (STS) studies and regulatory studies | 9 |
| Representatives from EMA, MHRA, Department for Environment Food & Rural Affairs (DEFRA), Environment Agency (EA), Health and Safety Executive (HSE), Health Protection Agency (HPA) and notifying bodies | 12 |
| Representative of UK Research Councils (MRC, BBSRC, NERC, EPSRC, ESRC) | 8 |
| Representatives of pharmaceutical and medical device companies | 8 |
| Total | 66 |
To initiate the academic interviews, the scientists contacted were principal investigators (PIs) of the EPSRC¶ nanotechnology grand challenge health care call.|| Other than EPSRC-funded PIs, other scientists researching in nanomedicine were identified from published literature (last authors, corresponding authors or well-established and leading scientists in their domains; suggestions regarding nanomedicine scientists from the initial interviewees helped confirm these selections), from information available online on various conferences, and from lists of speakers or of members of the advisory boards for such conferences. Scientists researching on human and eco-toxicological aspects of nanomaterials, and social scientists researching on science, technology and society studies who were considered relevant to the study, were identified from the literature and were contacted to check whether they would be willing to take part. The identified regulatory agencies were the agencies involved in the pharmaceutical and medical device approval and agencies involved in the protection of human health and the environment. The industry representatives were identified from the list of industries funded by Innovate UK (formerly the Technology Strategy Board, UK) under the call for proposals titled ‘Nanoscale technology enabled healthcare: building the supply chain competition for collaborative R&D funding’. Some industries were identified from the meetings organised by the UK Nanotechnology Knowledge Transfer Network.
The interviewees were anonymised by grouping them into categories. An alphanumeric code was assigned on the basis of their area of expertise and the sequence of conducting the interviews. For example, NMS 1 meant a scientist doing research in nanomedicine and the first to be interviewed in that group, and NMS 13 meant another nanomedicine scientist and the thirteenth in that group to be interviewed. The abbreviations used for the different areas of expertise/stakeholders are: NMS, nanomedicine scientist; NMEn, nanomedicine entrepreneur; HTOC, human toxicologist; ETOC, ecotoxicologist; RC, research council; SS, social scientists; PP, policy makers. Representatives from regulatory bodies and industry were named ‘Regulators’ and ‘Industry’ respectively. Table S1 of ESI† provides the details of interviewees and dates of interviews.
50 of the 66 interviews were conducted by the first author alone. The remaining 12 were conducted by the first author with both JRL and JRAC or with either JRL or JRAC. The interviews were transcribed verbatim and a general inductive approach44 was adopted to analyse the data in order to form a link between the research objective and the qualitative data. Repeated readings of the transcripts, systematic and rigorous coding, and then combination of these codes into thematic categories were performed to arrive at the findings discussed here. These findings have been explained by frequent reference to studies in the field of nanotechnology and chemicals where experts' opinions have been surveyed. For example, the findings are related to outcomes from quantitative studies on expert judgements on risks from chemicals,45–49 expert perception on nanomaterial regulations, risks, and benefits,50–55 and surveys related to perceptions of risks related to nanomaterials and nanotechnology by industry and other stakeholders.56–58
Given our objectives, a purely qualitative research design was deemed most appropriate for our study, and the findings presented here reflects this approach. Generally, qualitative inquiries are an entry when a field is emerging and little research has been done and there are very few hypothesis to enable influence research policies.59 In qualitative research, individual attitudes and opinions are crucial. Moreover, research attempts to provide a more nuanced account of everyday human practices and understandings that goes beyond the limitations imposed by binary ‘yes’/‘no’ categories. Hence the research questions evolved during the interview process to accommodate different expertise areas and to extract the maximum value from each interview. Discussions on environmental hazards and risks from nanomedicines are still evolving, and at this stage it was considered important to capture the diverse range of expert viewpoints, which we hope can inform future large-n quantitative survey work. In fact, we believe that the methodology and research design used could be adopted to explore expert opinions in other emerging technologies (such as synthetic biology). The empirical qualitative data was analysed keeping in mind these aspects and the results of the analysis is presented in the next section. The quotes used here are verbatim (with only very minor edits for clarity and brevity), but explanations and their contextualisation are our own.
Results
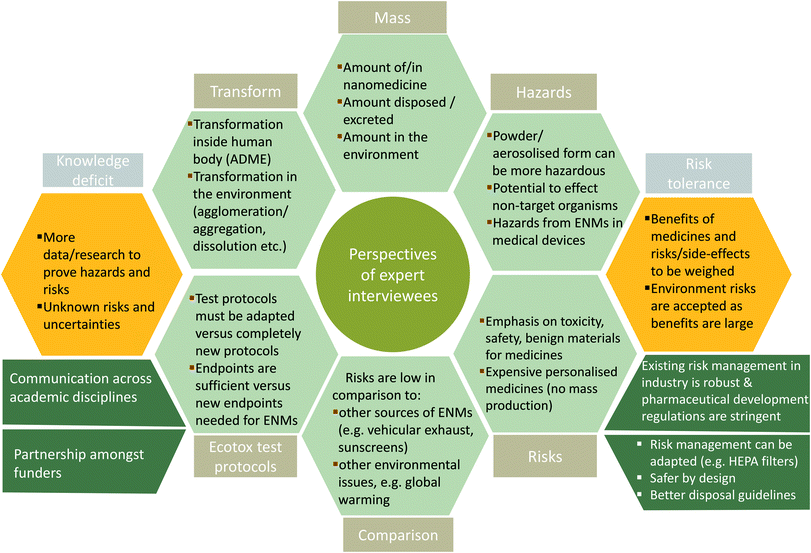
Findings are presented in broad thematic categories that emerged from the interview transcripts through coding (keeping in mind the research questions). These thematic categories were to understand experts' opinions on possible hazards and risks from nanomedicine and medical devices to the organisms in the environment and the adequacy of the existing environment risk assessment framework and protocols. From the analysis of the interview transcripts, two major groups of responses emerged, which are analysed here: 1) interviewees pointed out that the benefit–risk that is an inherent part of research in medicines, i.e., the benefit of preserving human health versus environmental or even human health risks, needs to be considered; and 2) interviewees compared insignificant risks posed by nanomedicines in the environment (due to very small amounts) to more pervasive chemical pollutants and global issues, such as climate change. The results from the analysis are structured under four broad headings: importance of assessing risk–benefit profile, views on environmental hazards and risks from nanomedicines, views on environmental hazards and risks from nano-enabled medical devices (as the regulatory pathway is distinct for medical devices), and views on the adequacy of existing regulations to protect the environment from possible harms from medicines. All interviews were conducted with experts in the EU and hence the interpretation of their responses has been contextualised in the frame of existing EU regulations for medicines and medical device commercialisation and environmental risk assessment of medicines for human use. Fig. 2 provides a summary of the main responses of the experts interviewed in this study.Importance of assessing risk–benefit profile
Some scientist interviewees researching nanomedicine and representatives of medicine regulatory agencies emphasised that benefits versus risk of medicines should be assessed. Thus, before starting to answer a question on environmental risks, a regulator dealing with approval of medicines explicitly mentioned the importance of looking at both risks and benefits and explained that they have the mandate to assess the benefits to public health from new medicines: “I just have a question and it's always the same question. Why do you look at the hazards and risks? Why don't you make a note in your dissertation also about the potential benefits.....for us, it is very difficult to look into this aspect of risks and hazards without looking also at aspects like the expected benefits.” [Regulator 2]They continued “I mean biodegradable, liposomal particles or block polymer micelles....if you look at the benefits for example you are reducing 100s of fold the level of doxorubicin, paclitaxel and other chemotherapeutic agents.......this is, to me, already a big advantage. On top [of that] you have the patients with less mucositis, less carditis, so you need less of other medications.”
They also said that nanomedicines might result in reduced environmental concentrations of medicines because of higher efficacy and targeting capacity in comparison to conventional drugs.
“Nanoparticles are not the only pollutants, so maybe in the future you can have minute portions releasing into the environment instead of big quantities of aluminum, big quantities of other materials which are equally polluting. So I think it is important to keep the perspective. Nanostructures might at the end of the day reduce pollution from medicines in many ways, in addition to potential benefit to public health.” [Regulator 2]
Viewpoints of expert interviewees on environmental hazards and risks from nano-therapeutics
“I would say yes [to both hazards and risks]. For instance, concerns over oestrogen – whether it comes from birth control pills, or whether it comes from the farming industries that get into waste streams – has affected apparently some fish populations. And is there any reason to expect that just because it's a nanomaterial and inorganic that somehow it might not behave as a drug? Things that interact with biological systems as drugs [do] can obviously interact with biological systems of other animals, there's no reason [to believe] that it might not also affect things at a mechanical level in the environment.” [Industry 04]
Several experts expressed the view that risks to the environment would be negligible due to low volumes and dilution, and a few were confident that nanomedicines would not cause any risks in near future. However, many experts gave more cautious (and reflective) responses. Thus an expert researching on health risks identified the problem with pollution from pharmaceutical industry:
“So for the industries that are making the drugs, it's how efficiently I suppose these materials are actually going to be used, what sort of waste there is, because where industry is concerned it's likely that waste is going be dumped and so, I think disposal of waste in the manufacturing industries that are making the drugs would be the primary environmental hazard.” [HTOC3]
This interviewee went on to add that from medicines used in hospitals or in clinics, the hazards would be negligible; however, if use of the therapeutic is widespread and frequent then there could be likely exposures and risk.
“With regard to actual clinical use, I think it's going to be a lot lower as far as environmental hazard is concerned. If it's a material that's excreted from the body then you have got hazard as far as the water ways are concerned but again, it goes back to the level of usage. If it's a very minimal use then I think that the impact is not going to be that heavy. If it's something that would be used, for example, to treat everyday cold and which everybody would be taking on a regular basis and it is excreted from the body then you've got a much more substantial problem. So it's similar I suppose to women taking contraceptive pills and there have been concerns about the hormones that are going into the waterways and how that affects feminization of fish but you've got millions of women taking it on a daily basis and so that's where you have the problem.”
This interviewee emphasised the potential of hazards and risks from discharge from pharmaceutical manufacturing plants as follows:
“I think unless nanomedicines are actually used to that extent, then I think that that's not going to be quite as much of a problem. In the near future, it's very least but the manufacturing side of things, I think is more problematic.” [HTOC3]
A typical response common to all expert groups (i.e., scientists, industry representatives, and toxicologists) on the question of potential environmental hazards and risks of nanomedicines followed the line that nanomedicines can cause hazards, but the amounts are negligible and they can be biodegraded or transformed in the body and the environment, and then compared it with other industries or natural nanoparticles. For example an industry representative commented:
“It's very difficult to say that some of them won't get into the environment. Whether they get in in the same form that they went into the body is another matter. The body can do an awful lot of metabolism. So there could be changes there. The whole environment issue is a bit strange because there are nanoparticles out there, all around us, and nano seems to scare some people.” [Industry 08]
This interviewee went on to say that the quantities are likely to be insignificant and that medical products go through rigorous toxicity testing for humans, however, adding later in the interview that the toxicity is not tested on environmental organisms like blue-green algae. They concluded that:
“I think its [hazards and risks are] very unlikely and the environment is a huge place, there is a massive dilutional effect....you can't say no [to hazard and risk], but I think it would be very unlikely.” [Industry 08]
A social scientist emphasised the phrasing of the question and said risk potential would be there, even if minimal, so the possibility of no risk is unobtainable:
“Yes of course, it has the potential. This question says do you think nanomedical applications might have the potential to pose environmental health risks. But it would be very, very odd to say no to that question, wouldn't it?” [SS02]
This expert asked what other interviewees' answers had been and when told that majority had said that as the quantities are likely to be minute, risk is unlikely the interviewee remarked: “So, it would be reduced risk but still some risk.”
“Nanomedicines I think is probably an absolutely tiny component compared to what else has been chucked out from other industries.” [NMS 16]
However, despite the suggestion of negligible concentrations of nanomedicines in the environment, some industry representatives and academics researching nanomedicine expressed concern about the health and consequently environmental risks of engineered nanomaterials, which can be aerosolised or are in powder form. The concern of hazards and risks from powder or aerosolised nanomedicine forms may be due to the many publications on health risks from CNTs (e.g. reviewed by Donaldson et al. and Aschberger et al.60,61) and historic and epidemiological studies on inhalation of fine particulates and worker exposure to air contaminants in the workplace. Other studies, for example,31,51 have reported that experts and industry representatives generally view inhalation exposure or dry powders of nanomaterials to be highly hazardous. Another probable reason for considering potentially higher hazard (and risk) from aerosolised forms of ENMs could be that risk science has matured in the case of human health impacts of airborne pollutants and acceptability of the risk assessment methodology has also been achieved for such exposures and hence experts from academia and industry managers drew upon their knowledge to explain the possible risks of nanomedicine if in powder form, or if nanomedicines are administered through the nasal route. However, a key question here is whether this is “folk theory?**”62 Experts emphasised that the nanomedicines they were working on are generally in liquid or are nanoparticles only when in aqueous media. For example, an academic expert involved in developing nanomedical applications who has also established a company said:
“First of all the nanoparticles that we make, they are nanoparticles only once they are in contact with aqueous media....The nanoparticles that we should be concerned about are those that can be aerosolised, those that can be in the atmosphere. You can breathe them in. Most of the NPs under development are not those types so they are not being made as dusts and fine powders. They usually are made as an aqueous dispersion....and they will only cross the biological barriers once they are introduced into the body and they are normally introduced by ingestion or by injection; those are the two main routes.” [NMEn07]
Moreover, no novel environmental risks from nanomedicine were suggested by most experts and hence no specific nanomedicine related change in regulations was required, a finding similar to Silva Costa's34 study where nanomedical researchers did not think that ethical issues specific to nanomedicine need to be considered.
Views of expert interviewees on environmental hazards and risks from medical devices
Most experts mentioned that medical devices made with nanotechnology would not be hazardous to the environment as the nanomaterials would be embedded within a non-nanomaterial and consequently there would not be any direct exposure. Hansen et al. (2008)63 developed a categorisation framework for nanomaterials which classified consumer products where nanomaterials are suspended in solids as having no exposure. This conviction that embedded or bound nanomaterials would not cause harm or raise less concerns has been reported by Weil in her survey of 22 firms in the US Midwest64 and by Capon et al.32 in their survey of Australian scientists, representatives from industry and government, and lay persons. However, some experts mentioned that general wear and tear can cause some exposure. The possibility of human health risks from wear debris of medical implants such as replacement hip-joints have been reported and novel mechanisms of effects elucidated (for example, DNA damage caused by influencing the cellular signalling pathway without compromising the structural integrity of cells or the cell barrier).65,66Interestingly, some experts developing nanomedicines and representatives from regulatory bodies mentioned designing medical drugs and devices in such a manner that they have minimal negative environmental implications, the so-called safer-by-design or benign-by-design considerations.67 For example, one expert from a regulatory agency said regarding design of devices:
“Scientists are, for example, coating those nonbiodegradable devices in a way that once they go into the environment they become susceptible to light. So the beauty of the nanoparticles, even the activatible implantable ones, is that you can play with the physical properties, the optical properties whereby as soon as they go out of the body they can be self-destroyed....if it was [that] the cumulative amount in the environment would pose a hazard, you can find a way to make them vulnerable to the environment, so difference in temperatures, for example, can break the particle or light exposure can break the particle.” [Regulator 02]
On the other hand, high attrition rates of drug candidates (e.g. approximately 40% of drugs fail in the preclinical phase due to non-clinical toxicity68) and the need to make drugs with desired pharmacokinetics and efficacy pushes considerations regarding environmental biodegradability lower down in the priority list. For example, in a study by Doerr-MacEwen and Haight,69 where experts from academia, industry, and government from North America and Europe were consulted to gain their perceptions on environmental risk management from pharmaceuticals, it was reported that incentives for green drug manufacturing strategies were ranked low both in terms of effectiveness and feasibility (7th and 8th rank respectively out of 8 environmental risk management strategies suggested in the study) for risk management. Moreover, industry representatives in that study mentioned that the function of a pharmaceutical was wholly dependent on its structure and informed about the high attrition rates of drugs in the drug research and development process, and further pointed out that commercialisation of medicines would become more complicated if drugs need to be biodegradable in the environment.69
Using biodegradable substrates (though substances after biodegradation can give rise to toxic products) and not using plastic casings for lab-on-chip devices was another design feature mentioned by interviewees. A social scientist mentioned that rather than having end-of-pipe solutions and more regulations, it is better to have a technological fix to make a product less risky to the environment. The case of small amounts of nanomaterials present in medical devices was also discussed by the interviewees. They also noted that contaminated medical devices are incinerated at their end-of-life and hence are not likely to pose hazards and risks (nanomaterials are distributed majorly between bottom ash and fly ash when products containing nanomaterials are incinerated, though the distribution depends on the nanomaterial type and size, combustion conditions and design of the incinerators70,71). However, whether the experts meant hazards and risks to human health or the environment was not explored further, because mostly these responses were preceded by discussions regarding safety to human health. Many experts mentioned the need to have proper disposal instructions, but had confidence in the current disposal guidelines of medical waste and their implementation success. However, the poor state of reporting of medical waste in the UK and US have been mentioned elsewhere.25
Views of experts on adequacy and adaptation of the current environment risk assessment framework for nanomedicines
Experts researching the topics of human toxicity and ecotoxicity, i.e., downstream experts, and a few experts researching nanomedicines (upstream experts) commented that in principle the current risk assessment procedure is applicable to nanomedicines and that only some adaptation is required for test systems, standards, and protocols for hazard identification, characterisation and exposure assessment. A need for modification of current test media and protocols has been shown in some studies, see for example, Park et al.72 and Casey et al.,73 and establishment of suitable dose metrics has been discussed for conducting various exposure assessment studies.74–77 However, the majority of experts interviewed, including some experts researching the toxic effects of pollutants on human health and the environment, were unaware of the ERA guidelines for pharmaceuticals for human use or the need for ERA as a step (if needed) in the drug approval process.Responses to the question on adequacy of the current ERA for medicinal products for human use (the guidance document was shared with experts with the questionnaire) elicited contradictory viewpoints. An eco-toxicologist aired their disagreement with the current broader scientific consensus about the inadequacy of current regulatory toxicity endpoints to reflect chronic effects of nanomaterials. The expert emphasised that new toxicity endpoints specific to nanomaterials are not needed and that current endpoints meant for bulk chemicals are sufficient:
“I think the endpoints are fine....I think probably the tests are fine as long as they're performed in the right way....I really don't think we need new endpoints for nanomaterials. We just need to think about refining the test so that we are able to do them with nanomaterials and that the results are meaningful in the natural environment.” [ETOC2]
This expert continued:
“I know a lot of academics say that we need new ecotox for nanomaterials, but I think the endpoints have been established for years and they are there to protect different taxonomic groups. There's no reason why we should say a biochemical endpoint or histological endpoint for a nanomaterial and not do it for other chemicals. So, I really struggle with some of the academics that are really trying to push subtle [toxicity] endpoints.” [ETOC2]
The excerpts above indicate one extreme end of the spectrum of opinions regarding adequacy of existing risk assessment frameworks for nanomaterials. At the other end another extreme viewpoint from an eco-toxicologist was that risk assessment of nanomaterials should be viewed through a completely new lens if novelty of nanomaterials is the key issue.
“....I have formulated another hypothesis saying that it is not possible to adapt. I think we need to start on a clean sheet of paper and that's because as far as I read most of the guidelines, now it is [for] ecotoxicity, our underlying assumption is mainly that we are dealing with dissolved chemicals....” [ETOC1]
These contrasting views and the divergence regarding testing methodologies, standards, and protocols are not new in the “young science of risk assessment”.45 A survey of UK toxicologists showed that these scientists disagreed on the extrapolation of animal models to sufficiently predict risks to human health.47 In the case of nanomaterial risk assessment, consensus expert opinion is that the risk assessment framework for bulk conventional chemicals can be used for nanomaterials; however, it has been discussed that the properties of nanomaterials (especially for nanomaterials less than 10 nm) are different than those of bulk materials and hence the procedures for conducting risk assessment will need customisation.78–80
Some nanomedicine experts and industry representatives mentioned that as a rule of thumb they treat the nanomaterials that they work with as hazardous, since they do not have complete understanding or knowledge of their toxicity, and hence follow the necessary rules of managing and handling hazardous waste. The representatives from industry and some of the nanomedicine scientists in academic institutions indicated that they are using whatever knowledge they have to deal with risks, and proactively engage with the Health and Safety Executive (HSE) to sort out issues.†† Similarly, some [NMS 09 and NMS 19] emphasised the difficulty of filling the existing COSHH‡‡ forms (designed for traditional chemicals) and the need to adapt the forms for nanomaterials. This indicates a certain amount of concern and care on the part of the researchers, a kind of moral responsibility as presented by Ladd81 which is necessary in the context of the knowledge gaps, uncertainties and lack of regulation or specific prescriptions for behaviour which is pervasive in the nanotechnology field, indicating a strong alliance with, for example, the EC's code of conduct for responsible nanoscience and nanotechnology.82
Only one expert (an eco-toxicologist) mentioned the threshold or trigger value of predicted environmental concentration (PEC) to do an environmental risk assessment as not being correct for nanomedicines, because of the value being a mass-based metric and hence not taking into account the unique functionality of the nanomedicines, and indeed the ongoing debate in the broader nanomaterials safety community of the appropriate dose metrics being either surface area or particle number.74
When asked about the applicability of the partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow values) to assess the persistence and bioaccumulation potential of nanomaterials, most of the experts (except eco-toxicologists) initially interviewed acknowledged their lack of knowledge regarding the question and asked for further explanation. It was interesting to note that human toxicologists interviewed did not know about the use of log
Kow values) to assess the persistence and bioaccumulation potential of nanomaterials, most of the experts (except eco-toxicologists) initially interviewed acknowledged their lack of knowledge regarding the question and asked for further explanation. It was interesting to note that human toxicologists interviewed did not know about the use of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow, indicating strong disciplinary orientations. So, it was decided that this will be asked only to those experts who would be able to answer. The experts who were posed the question on applicability of log
Kow, indicating strong disciplinary orientations. So, it was decided that this will be asked only to those experts who would be able to answer. The experts who were posed the question on applicability of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow for ERA of nanomaterials unanimously responded that log
Kow for ERA of nanomaterials unanimously responded that log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow was not a good surrogate and they stated that it was not even fully applicable to conventional pharmaceuticals for assessing persistence. For nanomedicines, they suggested that finding log
Kow was not a good surrogate and they stated that it was not even fully applicable to conventional pharmaceuticals for assessing persistence. For nanomedicines, they suggested that finding log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow value is complicated and they responded by saying it will need to be assessed on a case-by-case basis. Experts (all eco-toxicologists and representatives of a regulatory agency) who had an understanding of the concept expressed uncertainty about estimating it in the laboratory. Three eco-toxicologists [ETOC1, ETOC2, ETOC3] stressed that octanol–water partition coefficient was not the right proxy for bioaccumulation and one further added that the distribution coefficient is not even the right predictor of bioconcentration: “I don't think we should be thinking about the log
Kow value is complicated and they responded by saying it will need to be assessed on a case-by-case basis. Experts (all eco-toxicologists and representatives of a regulatory agency) who had an understanding of the concept expressed uncertainty about estimating it in the laboratory. Three eco-toxicologists [ETOC1, ETOC2, ETOC3] stressed that octanol–water partition coefficient was not the right proxy for bioaccumulation and one further added that the distribution coefficient is not even the right predictor of bioconcentration: “I don't think we should be thinking about the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) Kowfor nano materials or Dow.” [ETOC2]. The challenges of, and issues with, measuring octanol–water partition coefficient for nanomaterials have been discussed elsewhere.27,35
Kowfor nano materials or Dow.” [ETOC2]. The challenges of, and issues with, measuring octanol–water partition coefficient for nanomaterials have been discussed elsewhere.27,35
Interestingly, some experts researching on nanomedicine said that they need to consult with their colleagues who might be able to tell them how to go about measuring log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow. The medicines regulatory agency experts indicated that the criterion of log
Kow. The medicines regulatory agency experts indicated that the criterion of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow for medicines is one area that is being looked into. One of the ecotoxicity experts interviewed got back later after discussing the issue with a material scientist:
Kow for medicines is one area that is being looked into. One of the ecotoxicity experts interviewed got back later after discussing the issue with a material scientist:
“The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) Kowmeasurement is a bit of a moot point in terms of nanomaterials – specifically if they are dissolved they are no longer nanomaterials and should be therefore treated as a normal chemical. Instead there should be a measurement of the affinity of the surface of the nanomaterial to a polar or non-polar liquid....” [ETOC5]
Kowmeasurement is a bit of a moot point in terms of nanomaterials – specifically if they are dissolved they are no longer nanomaterials and should be therefore treated as a normal chemical. Instead there should be a measurement of the affinity of the surface of the nanomaterial to a polar or non-polar liquid....” [ETOC5]
Several experts from academia and the policy makers were unaware of the regulatory requirements regarding ERA of pharmaceuticals. Lack of awareness of regulations has been reported by Marquis et al.,83 where they conducted a thought experiment with bench scientists researching on nanomedicines to understand the requirements to get approval for a medical product from the USFDA.
Five scientists researching nanomedicines mentioned safety to patients and health care staff responsible for administering nanomedicines to patients in their responses, and described their own practice in the laboratory regarding following the necessary safety rules, indicating that risk framing by scientists is based on their subject expertise or institutional affiliations, as has also been observed elsewhere.49,84 This might be due to the difficulty in imagining that questions on environmental risks of nanomedicine could be asked, although the aim of the research was detailed in the introductory mail and the questionnaire and associated documents were sent in advance, or was perhaps because stress and time limitations being part and parcel of academic life,85 meant that they had not had the time to go through the questions.
The novel properties of nanomaterials and hence new risks, the lack of knowledge of the fate and behaviour of nanomaterials in the human body as well as the environment, and the associated uncertainties have been widely discussed in the nanotechnology literature. Below is an excerpt from a detailed discussion:
“The first part of any risk assessment is probably the formulation of exposure assessment. So, some of the assumptions in exposure assessment don't fit the way the nanomaterial would behave for they don't capture the fact that they're dynamic, that they change in time.” [ETOC3]
This expert, and some others, believed that the current risk assessment framework for chemicals was applicable for nanomaterials; however, they detailed the uncertainties and challenges involved in conducting an environmental risk assessment of nanomaterials:
“The basic principles of risk assessment are fine. The devil is in the detail. So, transformation is important....for example, do you want to risk-assess materials even though they're modified in the environment or do you want to assess the modified materials? You can do the hazard tests, but as in any form of risk assessment, what those mean in terms of real affect is less easy to interpret because of this dynamic nature change.” [ETOC3]
A social scientist clearly indicated the need for communicating uncertainties and knowledge gaps: “I think it's important not to just focus on the risks but also to take seriously the prospect of uncertainties and areas of ignorance as well.” [SS03]
Discussion
Use of nanomaterials in health care is perceived to be beneficial by experts (as expressed by the experts interviewed in the current research and in other studies) as well as the public.86 In contrast, environmental pollution risks from nanomaterials are generally of lower concern to experts than animal and human health risks54 due to expected minimal exposure. They reasoned that nanomedicines would undergo transformation in the body as well as in the environment, which could make them less hazardous (although this is not currently tested for nanomedicines, for nanomaterials used in other applications, transformations in media have been shown to increase87 or decrease toxicity88,89). The presence of traditional PPs (e.g. oestrogens, antibiotics, painkillers) in surface waters suggests this is too simplistic an argument as evidenced by feminisation of fish and behavioural changes.90–92 Pharmaceutical products are considered to be micro-pollutants/emerging contaminants, and hence these substances are not controlled by emission limits. Therefore, in the UK, discharges of PPs from waste water treatment plants are not under regulatory control and neither are PPs being monitored from an enforcement perspective in surface waters, although the increasing awareness of environmental impacts of PPs has led to the addition of diclofenac and hormones 17β-estradiol and 17α-ethinylestradiol to the watch list of substances monitored in surface waters under Directive 2008/105/EC (as amended by Directive 2013/39/EU) of the European Parliament “for the purpose of facilitating the determination of appropriate measures to address the risk posed by those substances”.93 It is likely that these pollutants will come under the remit of the Industrial Emissions Directive (2010/75/EU) and Environmental Quality Standards Directive (2013/39/EU) in due course.With regard to the prevalent perception on GMP followed in pharmaceutical manufacturing sites, studies have reported the presence of PPs downstream of pharmaceutical industries in developed countries.94 For example, a recent study reported that the monitored amount of PPs in effluents from waste water treatment plants (WWTPs) receiving waste water from pharmaceutical industries was 10 to 1000 times higher than typical WWTP effluents (where generally the concentration of PPs is less than 1 μg L−1) which don't receive discharge from pharmaceutical industry waste water.95 Hence discharge from pharmaceutical industries can be an important source of PPs into the environment,§§ contrary to the viewpoint of the experts interviewed in this study. Interestingly, literature published by pharmaceutical industries themselves report estimated concentrations (lower μg to ng L−1 range) in effluent from their manufacturing and formulation units that are lower than what has been reported in the academic literature. For example, by using a mass balance approach, Roche (Basel, Switzerland) estimated the concentrations of active pharmaceutical ingredient (APIs) to be in the range 0.01 to 38 μg L−1 in their effluent,96 and excipients were estimated to be in the range ∼38 μL−1 to 22.5 ng L−1.97 While production data from plants manufacturing pharmaceuticals and data from pharmaceutical formulation units are generally not available to the public and estimated and monitored concentrations show wide divergences, nevertheless it is clear that the pollution aspect from pharmaceutical manufacturers exists. Moreover, it is well established that non-human stakeholders, i.e., the environment, are rarely paid much attention in risk assessment.98 Furthermore, risk and responsibility are defined and perceived in a particular socio-cultural-economic situation and are highly contextual to a particular sector.31,99 In some sectors like health, tolerability and acceptability of risk100,101 could be different when compared to other sectors such as food or transportation.
In a few instances, a lengthy discussion took place between the interviewee and the interviewer(s) with regard to risk aversion, especially in the UK context. It was explained how the risk averse attitude is thwarting innovation and making the life of academic scientists more challenging. These same experts expressed unhappiness with the current health and safety guidelines due to their sometimes ‘unnecessary’ cautiousness (precaution), and preferred not to be burdened with more new rules and regulations regarding health and safety of nanomaterials. Some experts explained the hazardous substances handled in chemistry laboratories and in comparison expressed the possible benign nature of nanomaterials that they were using. Most scientists developing medicines, and industry representatives, explained the concept of possible risks and hazards by citing their own research work and the materials they use. For example, the nanomaterials they were using were either abundant in nature, e.g. silica, or were considered to be biodegradable, like polymers (can be persistent if not biodegradable), proteins, and lipids. Robichaud et al.102 used an insurance industry risk quantification protocol and applied it to industries manufacturing chemicals and compared them with nanomaterial manufacturing and found that environmental risks from manufacturing nanomaterials were less than or equal to those from other chemicals such as manufacture of aspirin and petroleum refining. However, novel properties of nanomaterials, their persistence, and potential unique modes of action can cause environmental risks and remain to be studied. The nano(eco)toxicology field is very new and filled with uncertainty.103
Additionally, detailed discussions took place on the complexity of nanomaterial systems, the immense possibilities to create a plethora of nanomaterials, their varied properties which would make it challenging to put them in a particular class or category, the current inability to detect them both in the body and the environment, their unpredictability in the human body and environmental systems, their dynamic nature, toxicity, biodegradability or biopersistence, bioaccumulation and excretion being dependant on shape, size, surface functionality, and surface chemistry, and associated uncertainties and knowledge gaps.
Generally, it is accepted that scientists researching risks from pollutants to human health and the environment, or experts involved in risk assessment, will have strong views about risks,84 however, in this research it was found that none of the experts were overly concerned about the hazards and risks from nanomedical applications. They agreed there was the possibility of hazards but expressed their reservation about environmental risks from nanomedicine. However, some of them expressed their satisfaction that research was being done to explore environmental risks from nanomedicine and that it was not a neglected area. For example, SS 01 (well-known scientist involved in the deliberations on science and innovation governance) was asked: are the current [environmental] risk assessment test methodologies and protocols fit-for-purpose for nanomedicine? Are you aware of them? To which the response was that the person was not aware of them and could not comment on their applicability, not being an expert in environmental risk assessment, adding “I would be worried if there weren't any such protocols. I would be worried if there were no researchers researching into it. It's important that those things are done.”
All experts were enthusiastic about applications of nanotechnology in human health. Some interviewees included disclaimers that benefits and risks need to be compared and also that comparison should be made with other conventional chemicals (e.g. endocrine disruptors) and other global environmental problems like climate change.
Overall, the interviewed experts in this study were not very concerned about environmental risks from nanomedicine and prided themselves in leading the way with respect to health and safety in their laboratories and workplaces. Some experts gave examples of their proactiveness in engaging with the HSE to discuss ways to handle the nanomaterials/nanomedicines which they were manufacturing. They gave the impression that they were very diligent with respect to health and safety and designing safety into products. Industry representatives talked about the risk management controls which they already have in place. Generally industries have indicated they know the best health, safety, and environment measures that need to be taken in a given scenario.51,64 Ability to work with radioactive materials was frequently cited as an achievement or a triumph against hazardous materials and hence the confidence that risks can be managed or controlled (ability to control risk generally results in lower perception of risks) with increasing knowledge and technical know-how.
All but one of the UK research councils shifted the responsibility of assessing the environmental risks to the Natural Environment Research Council (NERC), whose remit includes funding research on environmental issues. Experts from this research council in turn informed that the regulatory agencies dealing with medicines would be most appropriate to be approached on environmental risk from nanomedicines. Even the regulatory agencies with the responsibility to deal with environment issues suggested that the regulatory agency dealing with medicines would be in the best position to answer questions related to adequacy of regulatory frameworks for ERA. This indicates that the pharmaceutical sector is a very distinct sector with regard to downstream implications with very few overlaps between various governance agencies (both funding and oversight) and it seems responsibility can be easily attributed (or passed to) someone else. However, an interesting point is that research on pollution from traditional pharmaceuticals is funded by NERC and environmental pollution issues are addressed by the environment agencies.
It was intriguing to note the admiration that scientists had for the pharmaceutical industry sector and its regulatory bodies, and the confidence they had in them to follow proper health and safety protocols. Generally, experts have been shown to have more confidence¶¶ in government agencies dealing with risks,31 perhaps due to the knowledge that regulatory agencies have experts who are qualified in their subject areas (i.e., PhDs) whereas the public appears to have less trust in government regulatory agencies and pharmaceutical industries.32,104 The experts interviewed here repeatedly mentioned the stringent regulations in the pharmaceutical sector, the ‘smartness and intelligence’ of the pharmaceutical industry, and the regulatory preparedness, which contrasts with the poor reputation of pharmaceutical companies.105,106 However, regulators did not see themselves as being prepared for handling the regulatory challenges that would be posed by nanotechnology products and expressed the need for interaction across disciplines. This observation is very much aligned with what other investigators have reported. Beaudrie et al.52 in their survey of 254 US-based scientists, decision-makers, and environmental health and safety (EHS) scientists reported that regulatory scientists did not consider themselves fully prepared to manage risks from nanotechnology applications whereas scientists and EHS experts perceived regulators to be more prepared (than the regulators' self-assessment) for managing risks from nanotechnologies. A similar uncertainty on the part of the regulators was reported in their survey of experts from both the US and Canada,53 and also by Helland et al.,58 who gathered perceptions of experts from academic, health and safety agency and industry.
Another interesting outcome observed from the interviews across the spectrum of expertise was that the questions from one area could promote discussion or even follow-up questions to colleagues from different disciplines. For example, a team of scientists developing a nanomedical application started discussing the implications of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow for their product, and a scientist researching environmental impacts of nanomaterials was intrigued when they were shared examples of studies which found excretion of the nano-form in the urine and/or faeces. This indicates that an interview process can also prompt reflexivity thereby promoting modesty and pluralism in viewpoints of experts.107
Kow for their product, and a scientist researching environmental impacts of nanomaterials was intrigued when they were shared examples of studies which found excretion of the nano-form in the urine and/or faeces. This indicates that an interview process can also prompt reflexivity thereby promoting modesty and pluralism in viewpoints of experts.107
Conclusions
Discussions on nanomedicine to date have focused on regulations,108,109 public perception of applications of nanotechnology and nanomedicine,110,111 public engagement for defining nanomedicine funding strategy,112 or on ethical issues of nanomedicine.113 Very few studies have explored the environmental risks from nanomedicine, especially none on expert's perceptions on environmental risks from nanomedicine, which is exactly what this study has done.The instinctive and spontaneous discussion on possible human health risks from nanomedicine shows that the concept of environmental risk assessment seems to be distant and distinct (except for specialist eco-toxicologists). However it was heartening to know that health and safety issues have become mainstream and habitual throughout a product value chain, although potentially with some naiveté and over-confidence from nanomedicine-developing academics. Nonetheless, our research highlights a significant gap in terms of awareness of environmental regulations as well as a lack of orientation towards an ecosystem perspective. Thus, a significant conclusion from this paper is a call for effective communication and deliberation strategies to reduce this gap, and to raise awareness regarding the environmental aspects of risk assessment of nanomedicines, and more broadly of pharmaceuticals. More collaborative working across disciplines which includes social scientists can be made mandatory by funding agencies (e.g., the way EPSRC funded the grand challenge for nanotechnology for health care), especially since social scientists are aware of the subjective nature of risk and that risk can never be nullified. Furthermore, EPSRC's strategy of funding a public engagement exercise before the grand challenge call, to identify and prioritise the funding areas in nanomedicine, is also a useful strategy to get stakeholders involved in research topics.112
Risk perception is not unidimensional in the sense that risk is not an ‘objective’ fact described only by the probability of harm; rather, risk is a multidimensional socially constructed concept and is dependent on many factors, thereby making risk assessment subjective in nature and hence not value-free, even though guidance documents on risk assessment state that risk assessment is a scientific process. For example, the EMA did a study to assess what influences medicinal assessors|||| regarding decisions on approval of medical products which found that variables such as gender and number of years as an assessor can influence perception of risks and benefits. Science is assumed to be objective in its positivist philosophy; however scientific ‘facts’ are contested often enough, for example, dietary fat and cholesterol and their link to coronary heart disease114 and the recent controversy on the carcinogenic potential of glyphosphate.115 Also, social science scholars have shown the influence of micro- and macro-social interests in shaping research and its outcomes.116–118 Moreover, Stirling42 has argued as to why, in conditions of uncertainty, ambiguity, and ignorance, risk assessment – a reductive technique – is neither science based nor rational. Therefore, anticipatory risk governance needs to be conceptualised upstream of technology or product development and commercialisation.
Special risk governance of active and complex nanosystems was called for by Renn and Roco.119 Furthermore, Renn120 proposed a “risk escalator”, where he suggests involving various stakeholders to resolve risk issues induced by complexity, ambiguity, and uncertainty. Similarly, the precautionary principle can deal with risks in conditions of uncertainty. However, the precautionary principle is rarely discussed with respect to pharmaceutical development because risks from pharmaceuticals to humans are more individualised and can be controlled, and are not as diffuse as environmental risks for which the principle was developed.121 Some other related anticipatory risk governance approaches suggested are multicriteria decision analysis and weight of evidence approach for nanomedicine research and development.67,122 Linkov et al. recently made a strong case on collaborative, adaptive, and integrative risk governance for early assessment of emerging technologies.123 Furthermore, such risk governance approaches is a step towards responsible innovation.124 Environmental impacts of nanomedicine are likely to be negligible for some years. Moreover, robust data and evidence are needed to change existing policies and formalise regulatory criteria, which calls for further research to address basic conceptual problems, such as: (1) what are the trigger limits in the environment in terms of mass concentrations of nanomedicines; (2) how to establish bioaccumulation criteria and appropriate test assays; and (3) what to do in the case of complex nanomedicines. Specifically, we suggest that risk assessors consider inclusion of a specific test for the durability of the complex between a nanocarrier and the therapeutic agent in environmental media as a means to determine probability of release, as once the therapeutic agent is released from the carrier, the nano-specific concerns regarding mobility, enhanced or receptor mediated uptake etc. could disappear. Such an approach has also been proposed as a means to regulate nano-pesticides, for example.125 A point worth mentioning here is that the medicines regulatory agencies of the US and the EU have not been lagging much behind innovation in terms of deliberating on the impacts of new and emerging technologies which shows that they are trying to keep up with the science. For example, an International Workshop on Nanomedicine was organised by the EMA as early as September 2010 where the need to review environmental guidelines was discussed,126 (all the presentations of the workshop are on the EMA's website) and the recent publication “Determining the Need for and Content of Environmental Assessments for Gene Therapies, Vectored Vaccines, and Related Recombinant Viral or Microbial Products” from the USFDA41 discusses how the industry can go about preparing environmental assessments for these emerging therapeutics. The customisation of OECD methods for sediment and aquatic testing specific to nanomaterials is under way, which when completed can be incorporated in the guidance document for environmental risk assessment.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors thank the School of Geography, Earth and Environmental Sciences, University of Birmingham which funded the studentship which supported IM. Financial support from the SmartState Center for Environmental Nanoscience and Risk is acknowledged. This work was also part funded via the EU FP7 Marie Curie Career Integration grant EcofriendlyNano (PCIG14-GA-2013-631612).References
- T. L. Andresen, D. H. Thompson and T. Kaasgaard, Mol. Membr. Biol., 2010, 27, 353–363 CrossRef PubMed.
- A. Jhaveri, P. Deshpande and V. Torchilin, J. Controlled Release, 2014, 190, 352–370 CrossRef PubMed.
- ETP, Roadmap in Nanomedicine: Towards 2020, http://www.etp-nanomedicine.eu/public/press-documents/publications/etpn-publications, (accessed 10 January, 2012).
- ETP, Nanomedicine: Nanotechnology for Health (Strategic Research Agenda for Nanomedicine), http://www.etp-nanomedicine.eu/public/press-documents/publications/etpn-publications, (accessed 3 May, 2011).
- C. S. Thaxton, R. Elghanian, A. D. Thomas, S. I. Stoeva, J.-S. Lee, N. D. Smith, A. J. Schaeffer, H. Klocker, W. Horninger, G. Bartsch and C. A. Mirkin, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 18437–18442 CrossRef PubMed.
- M. Nahrendorf, E. Keliher, B. Marinelli, P. Waterman, P. F. Feruglio, L. Fexon, M. Pivovarov, F. K. Swirski, M. J. Pittet, C. Vinegoni and R. Weissleder, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 7910–7915 CrossRef PubMed.
- MagForce AG, The Nanomedicine Company, http://www.magforce.com/en/produkte/nanotherm-therapie.html, (accessed 30 June, 2018).
- R. Pita, F. Ehmann and M. Papaluca, AAPS J., 2016, 18, 1576–1582 CrossRef PubMed.
- R. Kanasty, J. R. Dorkin, A. Vegas and D. Anderson, Nat. Mater., 2013, 12, 967–977 CrossRef PubMed.
- J.-S. Lee, J. J. Green, K. T. Love, J. Sunshine, R. Langer and D. G. Anderson, Nano Lett., 2009, 9, 2402–2406 CrossRef PubMed.
- A. Mahapatro, Mater. Sci. Eng., C, 2015, 55, 227–251 CrossRef PubMed.
- J. Lisziewicz, N. Bakare, S. A. Calarota, D. Bánhegyi, J. Szlávik, E. Újhelyi, E. R. Tőke, L. Molnár, Z. Lisziewicz, B. Autran and F. Lori, PLoS One, 2012, 7, e35416 CrossRef PubMed.
- E.-S. Kim, E. H. Ahn, T. Dvir and D.-H. Kim, Int. J. Nanomed., 2014, 9, 1–5 CrossRef PubMed.
- Z. Yan, Z.-L. Zhu, Z.-Z. Qian, G. Hu, H.-Q. Wang, W.-H. Liu and G. Cheng, Acta Pharmacol. Sin., 2012, 33, 852–858 CrossRef PubMed.
- B. Pelaz, C. Alexiou, R. A. Alvarez-Puebla, F. Alves, A. M. Andrews, S. Ashraf, L. P. Balogh, L. Ballerini, A. Bestetti, C. Brendel, S. Bosi, M. Carril, W. C. W. Chan, C. Chen, X. Chen, X. Chen, Z. Cheng, D. Cui, J. Du, C. Dullin, A. Escudero, N. Feliu, M. Gao, M. George, Y. Gogotsi, A. Grünweller, Z. Gu, N. J. Halas, N. Hampp, R. K. Hartmann, M. C. Hersam, P. Hunziker, J. Jian, X. Jiang, P. Jungebluth, P. Kadhiresan, K. Kataoka, A. Khademhosseini, J. Kopeček, N. A. Kotov, H. F. Krug, D. S. Lee, C.-M. Lehr, K. W. Leong, X.-J. Liang, M. Ling Lim, L. M. Liz-Marzán, X. Ma, P. Macchiarini, H. Meng, H. Möhwald, P. Mulvaney, A. E. Nel, S. Nie, P. Nordlander, T. Okano, J. Oliveira, T. H. Park, R. M. Penner, M. Prato, V. Puntes, V. M. Rotello, A. Samarakoon, R. E. Schaak, Y. Shen, S. Sjöqvist, A. G. Skirtach, M. G. Soliman, M. M. Stevens, H.-W. Sung, B. Z. Tang, R. Tietze, B. N. Udugama, J. S. VanEpps, T. Weil, P. S. Weiss, I. Willner, Y. Wu, L. Yang, Z. Yue, Q. Zhang, Q. Zhang, X.-E. Zhang, Y. Zhao, X. Zhou and W. J. Parak, ACS Nano, 2017, 11, 2313–2381 CrossRef PubMed.
- S. R. D'Mello, C. N. Cruz, M.-L. Chen, M. Kapoor, S. L. Lee and K. M. Tyner, Nat. Nanotechnol., 2017, 12, 523–529 CrossRef PubMed.
- B. S. Zolnik and N. Sadrieh, Adv. Drug Delivery Rev., 2009, 61, 422–427 CrossRef PubMed.
- F. Ehmann, K. Sakai-Kato, R. Duncan, D. H. Pérez de la Ossa, R. Pita, J.-M. Vidal, A. Kohli, L. Tothfalusi, A. Sanh, S. Tinton, J.-L. Robert, B. Silva Lima and M. P. Amati, Nanomedicine, 2013, 8, 849–856 CrossRef PubMed.
- B. P. Chari and R. U. Halden, Water Res., 2012, 46, 4814–4824 CrossRef PubMed.
- A. D. McEachran, D. Shea, W. Bodnar and E. G. Nichols, Environ. Toxicol. Chem., 2016, 35, 898–905 CrossRef PubMed.
- S. R. Hughes, P. Kay and L. E. Brown, Environ. Sci. Technol., 2013, 47, 661–677 CrossRef PubMed.
- T. Porsbring, H. Blanck, H. Tjellström and T. Backhaus, Aquat. Toxicol., 2009, 91, 203–211 CrossRef PubMed.
- K. Volkova, N. Reyhanian Caspillo, T. Porseryd, S. Hallgren, P. Dinnétz and I. Porsch-Hällström, Horm. Behav., 2015, 73, 30–38 CrossRef PubMed.
- J. Filser, D. Arndt, J. Baumann, M. Geppert, S. Hackmann, E. M. Luther, C. Pade, K. Prenzel, H. Wigger, J. Arning, M. C. Hohnholt, J. Koser, A. Kuck, E. Lesnikov, J. Neumann, S. Schutrumpf, J. Warrelmann, M. Baumer, R. Dringen, A. von Gleich, P. Swiderek and J. Thoming, Nanoscale, 2013, 5, 1034–1046 RSC.
- I. Mahapatra, T. Sun, J. Clark, P. Dobson, K. Hungerbuehler, R. Owen, B. Nowack and J. Lead, J. Nanobiotechnol., 2015, 13, 93 CrossRef PubMed.
- J. S. Suk, Q. Xu, N. Kim, J. Hanes and L. M. Ensign, Adv. Drug Delivery Rev., 2016, 99, 28–51 CrossRef PubMed.
- I. Mahapatra, J. Clark, P. J. Dobson, R. Owen and J. R. Lead, Environ. Sci.: Processes Impacts, 2013, 15, 123–144 RSC.
- D. W. Engel and A. C. Dalton, CCSI Risk Estimation: An Application of Expert Elicitation, Pacific Northwest National Laboratory (PNNL), Richland, WA (US), 2012 Search PubMed.
- P. Krayer von Krauss Martin, A. Casman Elizabeth and J. Small Mitchell, Risk Anal., 2004, 24, 1515–1527 CrossRef PubMed.
- A. Petersen and A. Anderson, Nanoethics, 2007, 1, 243–256 CrossRef.
- M. Siegrist, C. Keller, H. Kastenholz, S. Frey and A. Wiek, Risk Anal., 2007, 27, 59–69 CrossRef PubMed.
- A. Capon, J. Gillespie, M. Rolfe and W. Smith, BMC Public Health, 2015, 15, 1–13 CrossRef PubMed.
- H. Silva Costa, S. Sethe, A. P. Pêgo and I. A. S. Olsson, Nanomedicine, 2011, 6, 681–691 CrossRef PubMed.
- C. E. Althaus, Risk Anal., 2005, 25, 567–588 CrossRef PubMed.
- S. Berkner, K. Schwirn and D. Voelker, Environ. Toxicol. Chem., 2016, 35, 780–787 CrossRef PubMed.
- A. Praetorius, N. Tufenkji, K.-U. Goss, M. Scheringer, F. von der Kammer and M. Elimelech, Environ. Sci.: Nano, 2014, 1, 317–323 RSC.
- P. Westerhoff and B. Nowack, Acc. Chem. Res., 2013, 46, 844–853 CrossRef PubMed.
- K. Hund-Rinke, A. Baun, D. Cupi, T. F. Fernandes, R. Handy, J. H. Kinross, J. M. Navas, W. Peijnenburg, K. Schlich, B. J. Shaw and J. J. Scott-Fordsmand, Nanotoxicology, 2016, 10, 1442–1447 CrossRef PubMed.
- EMA, Guideline on the Environmental Risk Assessment of Medical Products for Human Use, http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500003978.pdf, (accessed 10 June, 2012).
- USFDA, Guidance for Industry: Environmental Assessment of Human Drug and Biologics Applications, Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER), 1998 Search PubMed.
- USFDA, Determining the Need for and Content of Environmental Assessments for Gene Therapies, Vectored Vaccines, and Related Recombinant Viral or Microbial Products; Guidance for Industry, US Food and Drug Administration, 2015 Search PubMed.
- A. Stirling, EMBO Rep., 2007, 8, 309–315 CrossRef PubMed.
- I. Mahapatra, P. J. Dobson, J. R. Lead, I. Lynch, R. Owen and J. R. A. Clark, in Responsibility and Emerging Technologies: Experiences, Education and Beyond, ed. D. M. Bowman, A. Dijkstra, C. Fautz, J. Guivant, K. V. Konrad, H. van Lente and S. Woll, AKA Press, Berlin, 2016 Search PubMed.
- D. R. Thomas, Am. J. Eval., 2006, 27, 237–246 CrossRef.
- N. Neil, T. Malmfors and P. Slovic, Toxicol. Pathol., 1994, 22, 198–201 CrossRef PubMed.
- N. Kraus, T. Malmfors and P. Slovic, Risk Anal., 1992, 12, 215–232 CrossRef.
- C. K. Mertz, P. Slovic and I. F. H. Purchase, Risk Anal., 1998, 18, 391–404 CrossRef PubMed.
- P. Slovic, T. Malmfors, D. Krewski, C. K. Mertz, N. Neil and S. Bartlett, Risk Anal., 1995, 15, 661–675 CrossRef.
- P. Slovic, T. Malmfors, C. Mertz, N. Neil and I. F. Purchase, Hum. Exp. Toxicol., 1997, 16, 289–304 CrossRef PubMed.
- E. A. Corley, D. A. Scheufele and Q. Hu, J. Nanopart. Res., 2009, 11, 1573–1585 CrossRef PubMed.
- C. Engeman, L. Baumgartner, B. Carr, A. Fish, J. Meyerhofer, T. Satterfield, P. Holden and B. Harthorn, J. Nanopart. Res., 2012, 14, 1–12 CrossRef.
- C. E. Beaudrie, T. Satterfield, M. Kandlikar and B. H. Harthorn, PLoS One, 2013, 8, e80250 CrossRef PubMed.
- C. E. Beaudrie, T. Satterfield, M. Kandlikar and B. H. Harthorn, PLoS One, 2014, 9, e106365 CrossRef PubMed.
- J. C. Besley, V. L. Kramer and S. H. Priest, J. Nanopart. Res., 2008, 10, 549–558 CrossRef.
- D. A. Scheufele, E. A. Corley, S. Dunwoody, T.-J. Shih, E. Hillback and D. H. Guston, Nat. Nanotechnol., 2007, 2, 732–734 CrossRef PubMed.
- J. A. Conti, K. Killpack, G. Gerritzen, L. Huang, M. Mircheva, M. Delmas, B. H. Harthorn, R. P. Appelbaum and P. A. Holden, Environ. Sci. Technol., 2008, 42, 3155–3162 CrossRef PubMed.
- A. Helland, H. Kastenholz and M. Siegrist, J. Ind. Ecol., 2008, 12, 449–458 CrossRef.
- A. Helland, H. Kastenholz, A. Thidell, P. Arnfalk and K. Deppert, J. Nanopart. Res., 2006, 8, 709–719 CrossRef.
- M. Q. Patton, Qualitative research, John Wiley & Sons, Ltd, 2005 Search PubMed.
- K. Donaldson, R. Aitken, L. Tran, V. Stone, R. Duffin, G. Forrest and A. Alexander, Toxicol. Sci., 2006, 92, 5–22 CrossRef PubMed.
- K. Aschberger, H. J. Johnston, V. Stone, R. J. Aitken, S. M. Hankin, S. A. K. Peters, C. L. Tran and F. M. Christensen, Crit. Rev. Toxicol., 2010, 40, 759–790 CrossRef PubMed.
- A. Rip, Sci. Cult., 2006, 15, 349–365 CrossRef.
- S. Hansen, E. Michelson, A. Kamper, P. Borling, F. Stuer-Lauridsen and A. Baun, Ecotoxicology, 2008, 17, 438–447 CrossRef PubMed.
- V. Weil, Nanoethics, 2013, 7, 217–229 CrossRef.
- G. Bhabra, A. Sood, B. Fisher, L. Cartwright, M. Saunders, W. H. Evans, A. Surprenant, G. Lopez-Castejon, S. Mann, S. A. Davis, L. A. Hails, E. Ingham, P. Verkade, J. Lane, K. Heesom, R. Newson and C. P. Case, Nat. Nanotechnol., 2009, 4, 876–883 CrossRef PubMed.
- A. Sood, S. Salih, D. Roh, L. Lacharme Lora, M. Parry, B. Hardiman, R. Keehan, R. Grumme, E. Winterhager, P. J. Gokhale, P. W. Andrews, C. Abbott, K. Forbes, M. Westwood, J. D. Aplin, E. Ingham, I. PapageorgiouI, M. Berry, J. Liu, A. D. Dick, R. J. Garland, N. Williams, R. Singh, A. K. Simon, M. Lewis, J. Ham, L. Roger, D. M. Baird, L. A. Crompton, M. A. Caldwell, H. Swalwell, M. Birch Machin, G. Lopez Castejon, A. Randall, H. Lin, M. S. Suleiman, W. H. Evans, R. Newson and C. P. Case, Nat. Nanotechnol., 2011, 6, 824–833 CrossRef PubMed.
- T. Rycroft, B. Trump, K. Poinsatte-Jones and I. Linkov, J. Nanopart. Res., 2018, 20, 52 CrossRef.
- M. J. Waring, J. Arrowsmith, A. R. Leach, P. D. Leeson, S. Mandrell, R. M. Owen, G. Pairaudeau, W. D. Pennie, S. D. Pickett, J. Wang, O. Wallace and A. Weir, Nat. Rev. Drug Discovery, 2015, 14, 475 CrossRef PubMed.
- N. A. Doerr-MacEwen and M. E. Haight, Environ. Manage., 2006, 38, 853–866 CrossRef PubMed.
- H. R. Paur, W. Baumann, M. Hauser, I. Lang, N. Teuscher, H. Seifert and D. Stapf, J. Phys.: Conf. Ser., 2017, 838, 012012 CrossRef.
- T. Walser, L. K. Limbach, R. Brogioli, E. Erismann, L. Flamigni, B. Hattendorf, M. Juchli, F. Krumeich, C. Ludwig, K. Prikopsky, M. Rossier, D. Saner, A. Sigg, S. Hellweg, D. Gunther and W. J. Stark, Nat. Nanotechnol., 2012, 7, 520–524 CrossRef PubMed.
- S. Park, J. Woodhall, G. Ma, J. G. Veinot, M. S. Cresser and A. B. Boxall, Nanotoxicology, 2013, 1–30, DOI:10.3109/17435390.2013.818173.
- A. Casey, E. Herzog, M. Davoren, F. M. Lyng, H. J. Byrne and G. Chambers, Carbon, 2007, 45, 1425–1432 CrossRef.
- M. Simkó, D. Nosske and W. G. Kreyling, Int. J. Environ. Res. Public Health, 2014, 11, 4026–4048 CrossRef PubMed.
- G. Oberdorster, E. Oberdorster and J. Oberdorster, Environ. Health Perspect., 2005, 113, 823–839 CrossRef PubMed.
- C. J. E. Delmaar, W. J. G. M. Peijnenburg, A. G. Oomen, J. Chen, W. H. de Jong, A. J. A. M. Sips, Z. Wang and M. V. D. Z. Park, Environ. Toxicol. Chem., 2015, 34, 1015–1022 CrossRef PubMed.
- G. Oberdörster, J. Intern. Med., 2010, 267, 89–105 CrossRef PubMed.
- SCENIHR, Risk Assessment of Products of Nanotechnologies, Scientific Committee on Emerging and Newly-Identified Health Risks, 2009 Search PubMed.
- V. H. Grassian, A. J. Haes, I. A. Mudunkotuwa, P. Demokritou, A. B. Kane, C. J. Murphy, J. E. Hutchison, J. A. Isaacs, Y.-S. Jun, B. Karn, S. I. Khondaker, S. C. Larsen, B. L. T. Lau, J. M. Pettibone, O. A. Sadik, N. B. Saleh and C. Teague, Environ. Sci.: Nano, 2016, 3, 15–27 RSC.
- S. Rocks, S. Pollard, R. Dorey, L. Levy, P. Harrison and R. Handy, Comparison of risk assessment approaches for manufactured nanomaterials: Report compiled as part of Defra project (CB403), DEFRA, 2008 Search PubMed.
- J. Ladd, IEEE TECHNOL. SOC. MAG., 1982, 1, 3–10 Search PubMed.
- EC, Commission recommendation on A code of conduct for responsible nanosciences and nanotechnologies research & Council conclusions on Responsible nanosciences and nanotechnologies research, European Commission, Luxembourg, 2009 Search PubMed.
- B. Marquis, M. Maurer-Jones, Ö. Ersin, Y.-S. Lin and C. Haynes, J. Nanopart. Res., 2011, 13, 1389–1400 CrossRef.
- M. C. Powell, Health Risk Soc., 2007, 9, 173–190 CrossRef.
- R. Dowling, Prog. Hum. Geogr., 2008, 32, 812–820 CrossRef.
- M. Bottini, N. Rosato, F. Gloria, S. Adanti, N. Corradino, A. Bergamaschi and A. Magrini, Int. J. Nanomed., 2011, 6, 3473–3485 CrossRef PubMed.
- S. P. Yang, O. Bar-Ilan, R. E. Peterson, W. Heideman, R. J. Hamers and J. A. Pedersen, Environ. Sci. Technol., 2013, 47, 4718–4725 CrossRef PubMed.
- B. C. Reinsch, C. Levard, Z. Li, R. Ma, A. Wise, K. B. Gregory, G. E. Brown and G. V. Lowry, Environ. Sci. Technol., 2012, 46, 6992–7000 CrossRef PubMed.
- O. K. Choi and Z. Q. Hu, Water Sci. Technol., 2009, 59, 1699 CrossRef PubMed.
- S. Jobling and R. Owen, Ethinyl oestradiol in the aquatic environment, Report No. 1, vol. 13, European Environment Agency, Copenhagen, Denmark, 2013 Search PubMed.
- C. Minier, G. Caltot, F. Leboulanger and E. Hill, Analusis, 2000, 28, 801–806 CrossRef.
- T. Brodin, J. Fick, M. Jonsson and J. Klaminder, Science, 2013, 339, 814–815 CrossRef PubMed.
- EU, Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy Text with EEA relevance, 2013 Search PubMed.
- Y. Lester, H. Mamane, I. Zucker and D. Avisar, Water Res., 2013, 47, 4349–4356 CrossRef PubMed.
- P. J. Phillips, S. G. Smith, D. W. Kolpin, S. D. Zaugg, H. T. Buxton, E. T. Furlong, K. Esposito and B. Stinson, Environ. Sci. Technol., 2010, 44, 4910–4916 CrossRef PubMed.
- C. C. Hoerger, B. Dörr, C. Schlienger and J. O. Straub, Integr. Environ. Assess. Manage., 2009, 5, 331–337 CrossRef PubMed.
- K. C. Wirz, M. Studer and J. O. Straub, Sustainable Chem. Pharm., 2015, 1, 28–35 CrossRef.
- R. A. Phillips and J. Reichart, J. Bus. Ethics, 2000, 23, 185–197 CrossRef.
- M. Siegrist, N. Stampfli, H. Kastenholz and C. Keller, Appetite, 2008, 51, 283–290 CrossRef PubMed.
- M. Gibbons, C. Limoges, H. Nowotny, S. Schwartzman, P. Scott and M. Trow, The New Production of Knowledge. The dynamics of science and research in contemporary societies, Sage, London, 1994 Search PubMed.
- R. E. Löfstedt, D. Slavin and F. Bouder, The Tolerability of Risk: a new framework for risk management, Routledge, 2013 Search PubMed.
- C. O. Robichaud, D. Tanzil, U. Weilenmann and M. R. Wiesner, Environ. Sci. Technol., 2005, 39, 8985–8994 CrossRef PubMed.
- A. Kahru and A. Ivask, Acc. Chem. Res., 2013, 46, 823–833 CrossRef PubMed.
- P. Slovic, N. Kraus, H. Lappe and M. Major, Can. J. Public Health, 1991, 82, S15–S20 Search PubMed.
- R. E. Lofstedt, J. Health Comm., 2007, 12, 471–491 CrossRef PubMed.
- M. Kessel, Nat. Biotechnol., 2014, 32, 983–990 CrossRef PubMed.
- A. Stirling, in Reflexive governance for sustainable development, ed. J.-P. Voß, D. Bauknecht and R. Kemp, Edward Elgar Publishing Ltd, 2006, p. 225 Search PubMed.
- B. R. Dorbeck-Jung and N. Chowdhury, Law Policy, 2011, 33, 266–303 Search PubMed.
- R. Gaspar, in International Handbook on Regulating Nanotechnologies, ed. G. A. Hodge, A. D. Maynard and D. Bowman, Edward Elgar Publishing, UK, 2010, ch. Theraeutic products: regulating drugs and medical devices, pp. 291–390 Search PubMed.
- S. Priest, T. Lane, T. Greenhalgh, L. J. Hand and V. Kramer, Risk Anal., 2011, 31, 1718–1733 CrossRef PubMed.
- G. Sechi, D. Bedognetti, F. Sgarrella, L. V. Eperen, F. M. Marincola, A. Bianco and L. G. Delogu, Nanomedicine, 2014, 9, 1475–1486 CrossRef PubMed.
- R. Jones, Nat. Nanotechnol., 2008, 3, 578–579 CrossRef PubMed.
- G. Khushf, Nanomedicine, 2007, 2, 511–521 CrossRef PubMed.
- K. Garrety, Soc. Stud. Sci., 1997, 27, 727–773 CrossRef PubMed.
- R. E. Tarone, Eur. J. Cancer Prev., 2018, 27, 82–87 CrossRef PubMed.
- B. Martin and E. Richards, Handbook of science and technology studies, 1995, pp. 506–526 Search PubMed.
- S. Jasanoff, Soc. Stud. Sci., 1987, 17, 195–230 CrossRef.
- S. Jasanoff, The Fifth Branch: Science Advisers as Policymakers, Harvard Univ Press, 1990 Search PubMed.
- O. Renn and M. Roco, J. Nanopart. Res., 2006, 8, 153–191 CrossRef.
- O. Renn, in Global risk governance, Springer, 2008, pp. 3–73 Search PubMed.
- T. Callreus, Drug Saf., 2005, 28, 465–471 CrossRef PubMed.
- I. Linkov, F. K. Satterstrom and L. M. Corey, Nanomed.: Nanotechnol., Biol. Med., 2008, 4, 167–171 CrossRef PubMed.
- I. Linkov, B. D. Trump, E. Anklam, D. Berube, P. Boisseasu, C. Cummings, S. Ferson, M.-V. Florin, B. Goldstein, D. Hristozov, K. A. Jensen, G. Katalagarianakis, J. Kuzma, J. H. Lambert, T. Malloy, I. Malsch, A. Marcomini, M. Merad, J. Palma-Oliveira, E. Perkins, O. Renn, T. Seager, V. Stone, D. Vallero and T. Vermeire, Environment Systems and Decisions, 2018, 38, 170–176 CrossRef.
- R. Owen, J. Bessant and M. Heintz, Responsible Innovation: managing the responsible emergence of science and innovation in society, John Wiley & Sons, 2013 Search PubMed.
- R. S. Kookana, A. B. A. Boxall, P. T. Reeves, R. Ashauer, S. Beulke, Q. Chaudhry, G. Cornelis, T. F. Fernandes, J. Gan, M. Kah, I. Lynch, J. Ranville, C. Sinclair, D. Spurgeon, K. Tiede and P. J. Van den Brink, J. Agric. Food Chem., 2014, 62, 4227–4240 CrossRef PubMed.
- EMA, First International Workshop on Nanomedicine (2–3 September 2010), http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/events/2009/12/event_detail_000095.jsp&mid=WC0b01ac058004d5c3, (accessed 4 May, 2012).
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8en00053k |
| ‡ For example, Medicines & Healthcare products Regulatory Agency (MHRA) in the UK. See website for details: https://www.gov.uk/government/groups/mhra-innovation-office. |
| § A recent study by the EMA showed that even medicinal assessors are influenced by demography and their own attitudes. For details see: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2012/02/WC500123226.pdf. Moreover, risks are generally socially constructed. Though risk assessment is said to be objective and a scientific process and hence is claimed to be positivist, risk assessment or any scientific process is value laden and subjective. |
| ¶ Engineering and Physical Sciences Research Council (EPSRC) is the UK's main agency for funding research in engineering and the physical sciences. |
| || The Research Councils UK had identified (2007–2008) nanoscience and nanotechnology as one of the priority themes of research and within which they funded three areas related to the challenges facing society in the 21st century: nanotechnology for healthcare; nanotechnology for the environment and nanotechnology for energy. |
| ** A point to note is that very few conventional medicines (e.g. medicines for asthma, migraine) are administered through nasal or inhalation routes and hence the question arises whether this could be a “folk theory”, as per Arie Rip, i.e. a “pattern that evolves in ongoing practices, and serves the purposes of the members of the various practices”; however, these claims are not systematically checked. |
| †† An interesting point to note here is the absence of any mention of the Environment Agency in the discussions. |
| ‡‡ COSHH: control of substances hazardous to health. |
| §§ The monitored concentrations of PPs in the receiving waters of pharmaceutical manufacturing units in both developed and developing countries have been reviewed by Larsson (2014). D. G. J. Larsson, Philos. Trans. R. Soc., B, 2014, 369. |
| ¶¶ It is important here to note that in the risk perception literature trust and confidence have been nuanced. Earle (2010) mentions ‘confidence’ as calculative trust where trust is based on past behaviour or knowledge about a process. T. C. Earle, Risk Anal., 2010, 30, 541–574. |
| |||| http://www.ema.europa.eu/docs/en_GB/document_library/Report/2012/02/WC500123226.pdf. |
| This journal is © The Royal Society of Chemistry 2018 |