Development and application of a digestion-Raman analysis approach for studying multiwall carbon nanotube uptake in lettuce†
Abstract
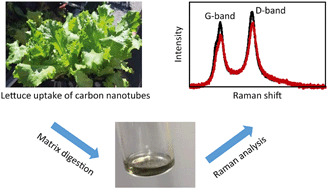
With increasing production and use of carbon nanotubes (CNTs) and their inevitable release during the life cycle of CNT-based products, these engineered nanomaterials are likely to accumulate in environmental compartments such as wastewater and biosolids, sediments, and biosolids-amended soils. Subsequent uptake of CNTs by agricultural crops could increase the risk of human exposure through the food chain. Unambiguous detection of CNTs in crop plants is essential for food safety assessment. In this study, we developed a method for the detection of multiwall CNTs (MWCNTs) in tissues of lettuce (Lactuca sativa L.), coupling digestion and Raman analysis. Five digestion reagents, including sulfuric acid, hydrochloric acid, nitric acid, ammonium hydroxide, and hydrogen peroxide, were examined. Nitric acid showed the best performance, removing 98–99% leaf/stem/root biomass (dry weight) and minimizing matrix background signals that can interfere with MWCNT Raman signals. Application of nitric acid digestion-Raman analysis to spiked lettuce tissues suggested a detection limit of 25 mg kg−1 dry weight or lower. We then applied this method to lettuce plants grown hydroponically with 0, 5, 10, and 20 mg L−1 pristine (p-) or carboxyl-functionalized (c-) MWCNT. Both p-MWCNT and c-MWCNT were detected in the root, stem, and leaf tissues of most exposed lettuce plants, indicating uptake and translocation of both MWCNTs in this edible plant. Comparisons of the plants grown with 20 mg L−1 p-MWCNT or c-MWCNT suggested that carboxylation facilitated uptake and translocation of MWCNT in lettuce. Our results demonstrated that nitric acid digestion in conjunction with Raman analysis is an effective approach for detecting MWCNTs in food crops, contributing to the potential development of new analytical platforms for studying the environmental fate of CNTs in the soil–plant system and human exposure through the food chain.
- This article is part of the themed collection: Best Papers 2018 – Environmental Science: Nano


 Please wait while we load your content...
Please wait while we load your content...