Impurity-bearing ferrihydrite nanoparticle precipitation/deposition on quartz and corundum†
Chong
Dai
a,
Juanjuan
Liu
ab and
Yandi
Hu
 *a
*a
aDepartment of Civil & Environmental Engineering, University of Houston, Houston, TX 77004, USA. E-mail: yhu11@ uh.edu; Web: http://www.cive.uh.edu/faculty/hu Fax: +(713)743 4260; Tel: +(713)743 4285
bCollege of Natural Resources and Environment, Northwest A&F University, Yangling, Shanxi 712100, China
First published on 14th November 2017
Abstract
During ferrihydrite precipitation, metal ions can be sequestered in it to form impurity-bearing ferrihydrite (IBF). Using grazing-incidence small-angle X-ray scattering (GISAXS), heterogeneous precipitation/deposition of pure and IBF nanoparticles on quartz (SiO2) and corundum (Al2O3) was quantified in 0.1 mM Fe3+ solutions in the absence and presence of 1 mM Mn2+ or Al3+ (pH = 3.8 ± 0.1). The impurity ions (Mn and Al) greatly affected ferrihydrite nanoparticle precipitation/deposition on substrates. On SiO2, ferrihydrite nanoparticle precipitation/deposition was promoted in the presence of Mn but was inhibited in the presence of Al. On Al2O3, Mn- and Al-bearing ferrihydrite nanoparticle precipitation/deposition was slower than for pure ferrihydrite. Compared with on SiO2, pure and IBF nanoparticle precipitation/deposition on Al2O3 was significantly inhibited. To understand the mechanisms, interactions among impurity ions, substrates, and precipitates were explored. Surface enrichment of Mn and Al on precipitates was found to increase the zeta potential of ferrihydrite nanoparticles. The changes in surface charges of the precipitates and substrates affected heterogeneous IBF precipitation/deposition significantly. The rates and mechanisms of heterogeneous IBF precipitation/deposition provided here can help predict pollutant transport and design catalyst synthesis.
Environmental significanceThe importance of impurity-bearing ferrihydrite (IBF) nanoparticle precipitation/deposition cannot be overstated. In many natural soil and aquatic systems, IBF nanoparticle precipitation/deposition controls the fate of many aqueous contaminants. Also, IBF nanoparticles, as an economic and environmentally friendly material with large specific surface area and high reactivity, have been used widely for the removal and degradation of pollutants. To improve their performance, impurity ions are usually doped in ferrihydrite nanoparticles and the nanoparticles are usually coated on supporting substrates. Understanding the fundamentals of interactions among impurity ions, IBF nanoparticles, and substrates, which controls the heterogeneous precipitation/deposition of IBF nanoparticles, is of essential importance. |
Introduction
Ferrihydrite is abundant as colloids or coatings on substrates in many natural and engineered aqueous environments, and always contains various impurities (e.g., Al, Mn, Cr, etc.).1–4 In natural systems, impurity ions can be sequestered in ferrihydrite during its precipitation, and the precipitation of impurity-bearing ferrihydrite (IBF) nanoparticles controls the fate and transport of many aqueous inorganic and organic contaminants.5–9 Contaminant sequestration during IBF precipitation has been reported at field sites of acid mine drainage (AMD), managed aquifer recharge (MAR), and geologic CO2 sequestration (GCS).10,11 For example, at AMD sites, the contaminated water contains high concentrations of Fe3+, Al3+, and Mn2+, which can coprecipitate with ferrihydrite.10,1213 The IBF nanoparticles can precipitate in solution (homogeneous precipitation) and carry aqueous contaminants (e.g., As) to downstream through colloidal transport, or be precipitated/deposited on rocks (heterogeneous precipitation/deposition) and retard the transport of aqueous contaminants. Impure iron (hydr)oxide nanoparticles have also garnered considerable interest in the fields of material science and chemical engineering. For example, Al, Cr-doped Fe(III) (hydr)oxide nanoparticles have been synthesized to degrade the organic compound hydroquinone and to catalyze water gas shift reactions.14–19The homogeneous precipitation of IBF has been studied widely, and impurity ions have been reported to be sequestered in ferrihydrite through surface adsorption, surface precipitation, and structural incorporation.1–4 Impurity ions have also been reported to affect the morphology, structure, and reactivity of IBF nanoparticles significantly.14,16,20–22 For example, the amount of aluminum doping in ferrihydrite can affect the sizes of the nanoparticles significantly, as well as their adsorption capacities for As(III) and As(V) and their redox reactivity for hydroquinone degradation.14,20,23,24
However, the fundamental knowledge of heterogeneous precipitation/deposition of IBF nanoparticles is much less well understood, and the controlling mechanisms are not clear.25–28 In our recent studies, different adsorption behaviors of impurity metal ions (e.g., Pb, Cu, Al and Cr) onto quartz (SiO2) were observed, which affected heterogeneous IBF precipitation/deposition on SiO2 significantly.13,29,30 In the work of Legg et al. and Tosco et al., pure ferrihydrite deposition under different ionic strength (IS) on SiO2 was measured, and the controlling mechanisms were explored.31,32 IBF precipitation/deposition on different substrates has not been reported.
Also, it is important to quantify the initial IBF precipitation (i.e., nucleation and growth) and deposition from solution. In reactive transport models, the rates of mineral precipitation are predicted as a function of the rate constant (k), solution's saturation ratio (Ω), and mineral surface area (A).33 The latter is changed by nucleation and growth, and in particular, the initial surface areas of the newly precipitated minerals are calculated based on nucleation rates. Currently, due to a lack of data on initial nucleation rates, great inaccuracy has blighted estimation of the initial surface areas of minerals, leading to inaccuracies in these reactive transport models.
Here, we aimed to fill such important information gaps. The objectives of the present study were to: (1) quantify initial heterogeneous precipitation/deposition kinetics (within 30 min) of Al- and Mn-bearing ferrihydrite on SiO2 and corundum (Al2O3) using grazing incidence small angle X-ray scattering (GISAXS) under acidic conditions (pH = 3.8 ± 0.1); (2) understand fundamental mechanisms by exploring the atomic-level interactions among impurity ions, substrates, and IBF nanoparticles with an integration of interfacial characterization methods and aquatic chemistry measurements.
Materials and methods
Substrate and solution preparation
Here, SiO2 and Al2O3 were chosen as the substrates for the precipitation/deposition experiments because Si–O and Al–O are the main components of minerals on earth, and because they are used widely as substrates for coating nanomaterials in engineered systems. Also, SiO2 and Al2O3 have quite different surface properties (e.g., dielectric constant, pHiep), which might affect heterogeneous precipitation/deposition processes.34 Single-crystal SiO2 (10 × 10 × 0.5 mm, cut along the 10–10 plane, with roughness <5 Å, density of 2.68 g cm−2, and pHiep of 3.8) and Al2O3 (10 × 10 × 1 mm, cut along the 0001 plane, with roughness <5 Å, density of 3.97 g cm−2, and pHiep of 11.58) were purchased from MTI (Salt Lake City, UT, USA). Before the precipitation/deposition experiments, SiO2 and Al2O3 substrates were cleaned, and the detailed cleaning procedures can be found in our previous publications.13,29,30,35–37All chemicals (Fe(NO3)3·9H2O, Al(NO3)3·9H2O, Mn(NO3)3·4H2O, and NaNO3) were purchased from Sigma-Aldrich (Saint Louis, MO, USA) and ultrapure water (conductivity <18 mΩ) was used to prepare all solutions (Table 1). Impure ferrihydrite precipitation experiments were conducted with 0.1 mM Fe(III) and 1 mM Al(III) or Mn(II), and the solutions were labeled as “FeAl” or “FeMn”, respectively. Pure ferrihydrite precipitation experiments were also conducted with 0.1 mM Fe(III) and the solution was labelled as “Fe only”. The IS of all solutions was adjusted to be the same, with 5.9 and 2.9 mM NaNO3 added into Fe only and FeMn solutions, respectively. Using Geochemist's Workbench (GWB; Release 9.0; Aqueous Solutions, Champaign, IL, USA), the pH, IS, and saturation indices (SI) of solutions with respect to Fe(OH)3 were calculated (Table 1). The FeAl and FeMn solutions were also calculated to be undersaturated with respect to gibbsite (Al(OH)3) and amorphous Mn(OH)2.
| Sample name | Mn2+, mM | Al3+, mM | Fe3+, mM | NaNO3, mM | IS,a mM | pHb | SIc |
|---|---|---|---|---|---|---|---|
| Note:a IS: Ionic strength.b pH: GWB calculated pH values, which were consistent with measured pH (3.8 ± 0.1).c SI: Saturation indices with respect to Fe(OH)3. SI = log(Q/Ksp), where Q is the actual dissolved composition, and Ksp(Fe(OH)3) = 10–7.22 at 20 °C was used for SI calculations based on GWB database. | |||||||
| Fe only | 0 | 0 | 0.1 | 5.9 | 6.2 | 3.8 | 0.36c |
| FeMn | 1 | 0 | 0.1 | 2.9 | 6.2 | 3.8 | 0.36c |
| FeAl | 0 | 1 | 0.1 | 0 | 6.2 | 3.7 | 0.23c |
In situ GISAXS measurements
Before each GISAXS measurement, a cleaned SiO2 and Al2O3 substrate was placed in our self-designed GISAXS cell, and the substrate surface aligned vertically towards the center of the X-ray beam. To achieve the optimal incident angle for the strongest scattering signal, X-ray reflectivity at the substrate–water interface was considered. With a density value of 2.68 and 3.97 g cm−3 for SiO2 and Al2O3, the critical angles for total external reflection of X-rays (with an energy of 14 keV) at SiO2–water and Al2O3–water interfaces were calculated to be 0.105° and 0.136°, respectively.38 Therefore, in our measurements, an incident angle of 0.10° was chosen, and only scattering from particles on substrates was collected.38 More detailed information of calculations of critical angles can be found in Supplementary Information.After alignment, 0.7 mL of a freshly prepared solution (Fe only, FeMn, or FeAl, Table 1) was injected immediately into the cell and in situ GISAXS measurement started. The deposition and precipitation of IBF nanoparticles on substrates occurred simultaneously immediately after the reaction had started. During the measurements, GISAXS scattering signals caused by particles precipitating and depositing on substrates were collected every 1 min for 30 min. At the end of each 30 min in situ GISAXS experiment, the cell was moved horizontally for 1 mm and a GISAXS image collected at a fresh spot. The GISAXS image collected at the fresh spot was similar to the last image of each in situ GISAXS measurement, indicating no X-ray damage during our measurements. GISAXS experiments were done at Beamline 12 ID-B, Advanced Photon Source at the Argonne National Laboratory (Argonne, IL, USA).
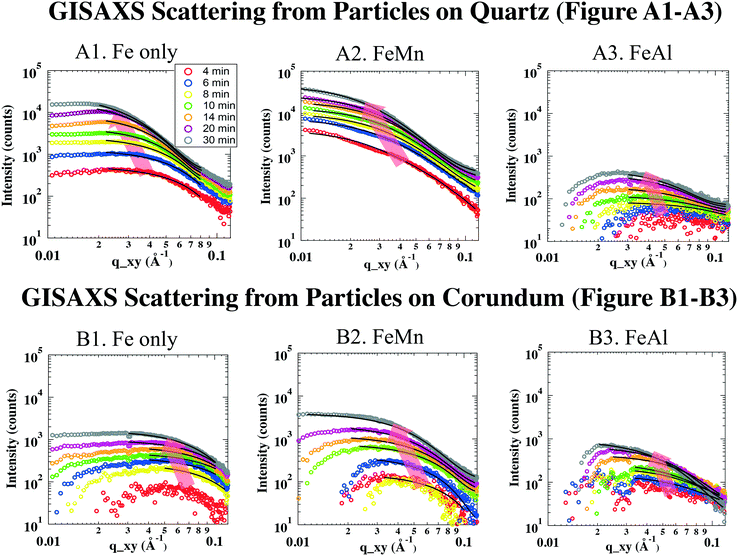
All data analyses were conducted using IRENA, GISAXS SHOP macro, and Igor Pro 6.34 (WaveMetrics, Lake Oswego, OR, USA). Background subtraction was undertaken using the first image of each in situ GISAXS experiment, where no discernible particle scattering was detected. In two-dimensional (2D) GISAXS images, no obvious scattering patterns were shown along vertical directions, indicating that the particles on substrates were not well ordered. Therefore, for simplicity, we assumed the shape of the disordered particles to be a low-resolution and highly symmetric shape, such as a sphere. The subtracted 2D scattering images were then deducted to 1D scattering intensity curves by cutting the long Yoneda wing, where the X-ray scattering signal is the strongest because of the Vineyard effect.39,40 The deducted 1D GISAXS scattering curves were plotted as scattering intensity (I) vs. scattering vector (q, with units of Å−1, reciprocally related to size) (Fig. 2).
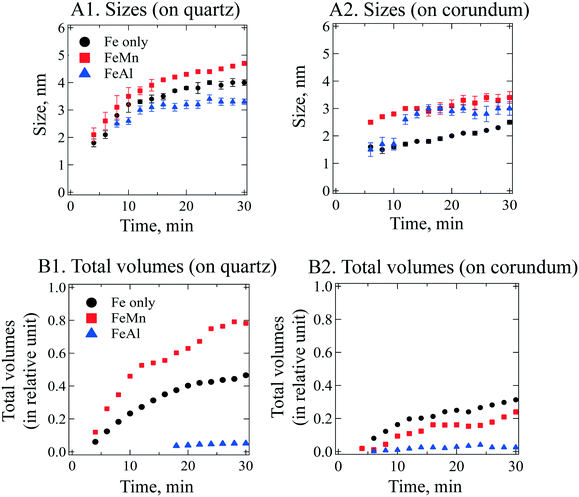
To obtain size information of the particles on substrates, a lognormal model of non-interacting spherical particles was used to fit all GISAXS scattering curves. Good fittings were obtained (the black lines in Fig. 2 are the fitted curves, and the colored dots are the measured data), and the size evolutions of particles (as radius, R, in nm) forming on SiO2 and Al2O3 are plotted in Fig. 3A1 and A2, respectively. Lorentz-corrected intensity curves (i.e., I × q2vs. q) were also plotted (Fig. S1 in ESI†), and the total particle volumes (V, in relative units) on SiO2 and Al2O3 were calculated as the so-called invariant 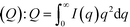 , as shown in Fig. 3B1 and B2, respectively.41 By assuming a spherical shape for the particles on substrates, using the total particle volume (V) and the mean particle radius (R), the numbers of particles on substrates (N) could be calculated using N = V/R.336
, as shown in Fig. 3B1 and B2, respectively.41 By assuming a spherical shape for the particles on substrates, using the total particle volume (V) and the mean particle radius (R), the numbers of particles on substrates (N) could be calculated using N = V/R.336
Mineral phase and chemical composition characterization of the precipitates
Because the amount of precipitates in solution was not sufficient for regular X-ray diffraction (XRD) measurement, synchrotron-based high-resolution X-ray diffraction (HRXRD; Fig. 1) measurements were conducted at Beamline 11-BM, Advanced Photon Source, Argonne National Laboratory. For each experimental condition listed in Table 1, 500 mL solution was freshly prepared and reacted for 30 min. Then, the precipitates were collected using centrifuge filter units (molecular weight, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000; Millipore, Burlington, MA, USA), and dried in a desiccator under room temperature. Then, the particles were filled in Kapton capillaries of diameter 0.8 mm and shipped to Advanced Photon Source for measurements. HRXRD results indicated that the precipitates from Fe only, FeMn and FeAl solutions were poorly crystallized 6-line ferrihydrite (Fig. 1).
000; Millipore, Burlington, MA, USA), and dried in a desiccator under room temperature. Then, the particles were filled in Kapton capillaries of diameter 0.8 mm and shipped to Advanced Photon Source for measurements. HRXRD results indicated that the precipitates from Fe only, FeMn and FeAl solutions were poorly crystallized 6-line ferrihydrite (Fig. 1).
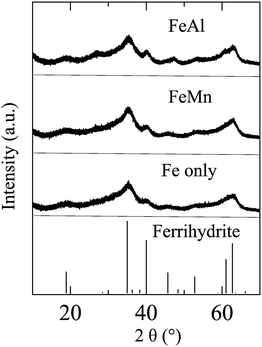 | ||
| Fig. 1 HRXRD patterns of the precipitates formed in 0.1 mM Fe3+ solutions in the absence (Fe only) and presence of 1 mM Mn2+ (FeMn) and 1 mM Al3+ (FeAl). | ||
The chemical compositions of precipitates in solutions were investigated by strong acid soak (pH = 0.5 HNO3) and consecutive dilute acid wash (pH = 3 HNO3) experiments.29,30,42,43 Solutions (500 mL) (Table 1) were freshly prepared and let to stand for 30 min. Then, precipitates were collected on the centrifugal filters (Millipore). To obtain the total amounts of impurity ions in the precipitates, particles on centrifugal filters were fully dissolved by soaking in 5 mL of 2% nitric acid (pH = 0.5) overnight. The concentrations of impurity ions (Al and Mn) and Fe were measured by inductively coupled plasma-mass spectrometry (ICP-MS), and the total atomic ratios of Mn/Al over Fe (Rt,M/Fe) in the precipitates calculated.
Studies have reported that impurity ions can be sequestered in ferrihydrite through structural incorporation and surface enrichment.1–3 To explore the sequestration mechanisms of Al or Mn in the precipitates, consecutive dilute acid wash experiments (pH = 3, HNO3) were undertaken. Initially, 5 mL of dilute HNO3 (pH = 3) was used to soak the collected particles on the filter for 10 min, and to dissolve the surface layer of the precipitates. Based on previous studies, dilute acid can desorb the metal ions adsorbed on ferrihydrite surfaces without significant dissolving ferrihydrite particles (<1%) within 30 min, which was also observed here.43 The concentrations of dissolved impurity ions (Mn/Al) and Fe ions were measured by ICP-MS, and the surface atomic ratios of Mn or Al over Fe (Rs,M/Fe) were calculated. Then, consecutively, 5 mL of pH = 3 HNO3 solutions were added to soak the remaining particles for a longer duration (i.e., 30, 60, and 360 min), until the measured atomic ratios of Mn or Al over Fe did not change significantly, which represented the lattice atomic ratios of Mn or Al over Fe (Rl,M/Fe). For the heterogeneous precipitates formed on SiO2 and Al2O3 surfaces, their amounts were too few for phase identification and chemical composition analyses.
Dynamic light scattering (DLS) measurements
To understand the interactions among metal ions, substrates, and precipitates, DLS using a Zetasizer Nano ZS system (Malvern Instruments, Malvern, UK) was employed to measure the zeta potential. Two sets of experiments were conducted: the first set was to measure the zeta potential of the precipitates, and the second set was to measure the zeta potential of the substrates in the presence of metal ions. In the first set, to measure the zeta potential of pure and IBF colloids, a freshly prepared solution (Table 1) was injected into a zeta cell (DTS1070 folded capillary cell; Malvern Instruments). In the second set, to investigate the potential effects of aqueous Mn2+ and Al3+ on the surface charges of substrates, the zeta potential of SiO2 and Al2O3 powders was measured at our experimental pH condition (3.8 ± 0.1, adjusted using HNO3) in 5.9 mM NaNO3, 1 mM Mn2+ (with 2.9 mM NaNO3), and 1 mM Al3+, without the addition of Fe3+. All zeta-potential data were collected continuously every 1 min for 1 h at 20 °C, and the mean values and standard deviations were calculated (Table 2). The detailed information on zeta-potential measurements of SiO2 and Al2O3 in the presence of metal ions can be found in our previous publications.36| Sample name | M(OH)xn−x,a, % | R s,bM/Fe | Rl,bM/Fe | Rt,bM/Fe | ζ,c mV | ζ_SiO2,d mV | ζ_Al2O3,d mV |
|---|---|---|---|---|---|---|---|
| Note:a M(OH)xn−x, %: percentages of metal ions in hydrolyzed states (e.g. Al(OH)2+, Al(OH)2+, Al(OH)3), which were calculated using GWB under our experimental conditions.b Rs,M/Fe, Rl,M/Fe, and Rt,M/Fe: surface, lattice, and total atomic ratios of impurity ions (Mn or Al) over Fe in the precipitates.c ζ: zeta potentials of pure or impurity-bearing ferrhydrite nanoparticles.d ζ_SiO2 and ζ_Al2O3: zeta potentials of quartz and corundum powders in the presence of 1 mM Mn2+ or Al3+ (pH adjusted to 3.8 ± 0.1), respectively. | |||||||
| Fe only | N/A | N/A | N/A | N/A | 29.1 ± 5.7 | −22.4 ± 2.6 | 20.5 ± 2.8 |
| FeMn | 0.5 | 4.14 ± 1.81 | 0.0003 ± 0.0001 | 0.04 ± 0.02 | 39.9 ± 2.7 | −25.1 ± 1.9 | 27.4 ± 6.8 |
| FeAl | 4.4 | 4.36 ± 0.08 | 0.03 ± 0.01 | 0.07 ± 0.01 | 40.6 ± 2.3 | 18.1 ± 3.9 | 34.3 ± 6.4 |
Results and discussion
Hydrolysis of impurity ions affects their structural incorporation in homogenously precipitated ferrihydrite
The chemical compositions of the homogeneously precipitated IBF nanoparticles in solutions were measured by ICP-MS, and the total atomic ratios of the impurity ions (Mn and Al) over Fe (Rt,M/Fe) were calculated. For Mn- and Al-bearing ferrihydrite nanoparticles, the total atomic ratios of Mn (Rt,Mn/Fe, 0.04 ± 0.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Table 2) and Al (Rt,Al/Fe, 0.07 ± 0.01
1, Table 2) and Al (Rt,Al/Fe, 0.07 ± 0.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Table 2) over Fe were low, indicating that small amounts of Mn and Al were sequestered in ferrihydrite precipitates.
1, Table 2) over Fe were low, indicating that small amounts of Mn and Al were sequestered in ferrihydrite precipitates.
Different sequestration mechanisms of impurity ions in ferrihydrite have been reported in experimental and modeling studies.44–47 Mn and Al ions can substitute Fe(III) ions in the lattice and be sequestered in Fe(III) (hydr)oxides as structural incorporation.1–3,44–46 Also, Mn and Al ions can be sequestered through surface adsorption and surface precipitation, resulting in their enrichment on ferrihydrite surfaces.1–3,46,47 Here, the sequestration mechanisms of Mn and Al in IBF were investigated by comparing the surface (Rs,M/Fe) and lattice (Rl,M/Fe) atomic ratios of the metal ions over Fe. If Rs,M/Fe ≤ Rl,M/Fe, then only structural incorporation occurred. Conversely, if Rs,M/Fe > Rl,M/Fe, then surface enrichment also occurred. As shown in Table 2, Rs,M/Fe (Rs,Mn/Fe = 4.14 ± 1.81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rs,Al/Fe = 4.36 ± 0.08
1, Rs,Al/Fe = 4.36 ± 0.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were much higher than Rl,M/Fe (Rl,Mn/Fe = 0.0003 ± 0.0001
1) were much higher than Rl,M/Fe (Rl,Mn/Fe = 0.0003 ± 0.0001![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Rl,Al/Fe = 0.03 ± 0.01
1, Rl,Al/Fe = 0.03 ± 0.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for FeMn and FeAl, indicating that Mn and Al were enriched on the surfaces of the precipitates. This is because Fe (hydr)oxides have high affinity to various metal ions, including Al and Mn.47,48
1) for FeMn and FeAl, indicating that Mn and Al were enriched on the surfaces of the precipitates. This is because Fe (hydr)oxides have high affinity to various metal ions, including Al and Mn.47,48
Lattice Rl,M/Fe ratios, which are indicative of structurally incorporated impurity ions in ferrihydrite, showed a ∼100-fold difference for the precipitates formed in FeAl (Rl,Al/Fe = 0.03 ± 0.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and FeMn (Rl,Mn/Fe = 0.0003 ± 0.0001
1) and FeMn (Rl,Mn/Fe = 0.0003 ± 0.0001![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solutions. To understand the different incorporation behaviors of the impurity ions in ferrihydrite, we first considered the size of these impurity ions. The ionic radii of impurity ions have been reported widely to affect their structural incorporation behaviors into Fe (hydr)oxide nanoparticles at neutral pH conditions. If the ionic radius of the impurity ion is similar to that of Fe3+, it is easier for the impurity ion to substitute for the Fe3+ and cause less stress in the lattice structure of Fe (hydr)oxide nanoparticles, resulting in easier structural incorporation.49 The radii of Al3+, Mn2+, and Fe3+ have been reported to be 0.0535, 0.0645, and 0.0645 nm, respectively.50–53 If the ionic radius was a major controlling factor, then Rl,Mn/Fe should be much higher than Rl,Al/Fe. However, our measurements showed the opposite result: Rl,Al/Fe was ∼100-fold higher than Rl,Mn/Fe. With regard to the effects of ferrihydrite precipitation rates on incorporation of impurity ions, Cismasu et al. synthesized two series of Al-bearing ferrihydrite particles under different precipitation rates, and the chemical compositions of Al-bearing ferrihydrite were similar.27 For Mn-bearing ferrihydrite, the effects of precipitation rates on Mn incorporation were not reported.
1) solutions. To understand the different incorporation behaviors of the impurity ions in ferrihydrite, we first considered the size of these impurity ions. The ionic radii of impurity ions have been reported widely to affect their structural incorporation behaviors into Fe (hydr)oxide nanoparticles at neutral pH conditions. If the ionic radius of the impurity ion is similar to that of Fe3+, it is easier for the impurity ion to substitute for the Fe3+ and cause less stress in the lattice structure of Fe (hydr)oxide nanoparticles, resulting in easier structural incorporation.49 The radii of Al3+, Mn2+, and Fe3+ have been reported to be 0.0535, 0.0645, and 0.0645 nm, respectively.50–53 If the ionic radius was a major controlling factor, then Rl,Mn/Fe should be much higher than Rl,Al/Fe. However, our measurements showed the opposite result: Rl,Al/Fe was ∼100-fold higher than Rl,Mn/Fe. With regard to the effects of ferrihydrite precipitation rates on incorporation of impurity ions, Cismasu et al. synthesized two series of Al-bearing ferrihydrite particles under different precipitation rates, and the chemical compositions of Al-bearing ferrihydrite were similar.27 For Mn-bearing ferrihydrite, the effects of precipitation rates on Mn incorporation were not reported.
Then, we recalled the pathways of ferrihydrite formation. First, Fe3+ were hydrolyzed to form Fe(OH)3 monomers (Fe3+ + 3H2O → Fe(OH)3 + 3H+). Then, polymerization occurred through continuous olation (2[Fe(H2O)5OH2+] → [(H2O)4Fe-(OH)2-Fe(H2O)4]4+ + 2H2O) and oxolation (2[Fe(H2O)5OH2+] → [(H2O)4Fe–O–Fe(H2O)5]4+ + 2H2O).54–56 In our previous study, ferrihydrite precipitation experiments were also conducted at pH = 3.7 ± 0.2 with aqueous Al/Fe ratios of 1 and 5, and the total Al/Fe ratios in the precipitates were <1%, much lower than the aqueous Al/Fe ratios.13 Under acidic pH conditions (pH = 3.8 ± 0.1) in our previous and current study, ∼95% Al ions were present as unhydrolyzed Al3+.13 Considering the hydrolysis and polymerization reactions during ferrihydrite formation, we hypothesized that only hydrolyzed metal ions (e.g., Al(OH)n3−n) could be incorporated in the lattice of ferrihydrite through oxolation and olation reactions with Fe(OH)3 monomers and polymers. Hydrolysis reactions for As metal were instantaneous, much faster than those for ferrihydrite precipitation. Therefore, the hydrolyzed ion concentrations at equilibrium conditions, calculated by GWB, were used to explain their structural incorporation in ferrihydrite. Under our experimental pH condition (3.8 ± 0.1), 4.4% of Al ions were in their hydrolyzed states (Al(OH)2+, Al(OH)2+, Al2(OH)24+, Al3(OH)45+, Al(OH)4−, and Al(OH)3), whereas only 0.5% of Mn ions were hydrolyzed. The lower amount of hydrolyzed Mn ions resulted in the small percentage of lattice Mn/Fe ratio in the precipitates, a result which supported our hypothesis. This hypothesis is also supported by studies of Al incorporation during ferrihydrite precipitation at neutral pH conditions.57,58 For example, Cismasu et al. synthesized a series of Al-bearing ferrihydrite particles in solutions (pH = 7.5 ± 0.2) with a total metal ion concentration of 2 mM and various aqueous Al/Fe ratios (0.10–0.67).58 Under the neutral pH condition, as >99% of Fe and Al ions were hydrolyzed, the atomic ratios of Al/Fe in the precipitates were similar to their aqueous ratios.58
Our hypothesis was further supported by the reported Cr incorporation in ferrihydrite during ferrihydrite precipitation at neutral and acidic pH conditions.2,59,60 For example, in the work of Tang et al., a series of Cr-bearing ferrihydrite nanoparticles was synthesized with a total metal ion concentration of 0.1 M and different Cr/Fe ratios (0.1–9) at pH ∼ 7.2 Under this neutral pH condition, because >99% Fe and Cr ions were hydrolyzed, the atomic Cr/Fe ratios in the Cr-bearing ferrihydrite particles were similar to the initial aqueous Cr/Fe ratios.2 Conversely, under an acidic condition (pH = 3.7 ± 0.1), because only ∼36% of aqueous Cr ions were hydrolyzed, the atomic ratios of Cr/Fe (0.06–0.18) in the precipitates were much lower than the aqueous Cr/Fe ratios (0.2–2.5).30
To summarize, all previous studies of Al and Cr incorporation in ferrihydrite under acidic and neutral pH conditions confirmed our hypothesis: i.e., the hydrolysis of impurity ions affected their structural incorporation in ferrihydrite.2,13,30,57–60 IBF precipitation experiments have been conducted,2,13,30,57–60 and the ionic radii of metal ions were considered to be the controlling factors for their structural incorporation during IBF precipitation. Here, the hydrolysis of impurity ions, which is highly dependent upon pH, was reported, for the first time, to affect their structural incorporation in IBF nanoparticles greatly. The fundamental information provided here can be used to better understand and control incorporation of impurity ions in ferrihydrite during its precipitation under different pH conditions. Also, it would be an interesting future direction to develop a correlation equation of incorporation of impurity ions in ferrihydrite as a function of the radius, oxidation states, and hydrolysis reactions of impurity ions.
Surface enrichment of Mn on ferrihydrite promoted its precipitation/deposition on SiO2
The GISAXS scattering curves caused by ferrihydrite nanoparticles precipitated/deposited on SiO2 are shown in Fig. 2A1–A3. Under all of our experimental conditions, the GISAXS scattering intensities increased with reaction time, indicating continuous precipitation/deposition of ferrihydrite nanoparticles on SiO2. As the reaction proceeded, the peak positions of the GISAXS scattering curves (indicated by red arrows in Fig. 2) shifted from high q range to low q range, indicating an increase in particle size by growth. By fitting the GISAXS scattering curves, the size evolutions of particles on SiO2 were plotted (Fig. 3A1). At the end of our 30 min precipitation/deposition experiments, the mean radius of pure, Mn- and Al-bearing ferrihydrite nanoparticles was 4.0 ± 0.1, 4.7 ± 0.1, and 3.3 ± 0.1 nm, respectively. The evolution of total particle volumes (indicative of precipitation/deposition rates) is shown in Fig. 3B1. Compared with pure ferrihydrite on SiO2, the total volume of Mn-bearing ferrihydrite increased to 160%, whereas the total volume of Al-bearing ferrihydrite decreased to 11%.To understand the different effects of impurity ions on heterogeneous ferrihydrite precipitation/deposition, the electrostatic interactions between the precipitates and substrates were considered. The zeta potential of Mn- and Al-bearing ferrihydrite nanoparticles was measured to be 39.9 ± 2.7 and 40.6 ± 2.3 mV, higher than that of pure ferrihydrite (29.1 ± 5.7 mV). Impurity ions, adsorbed or precipitated on ferrihydrite surfaces, have been reported to change the pHpzc of ferrihydrite nanoparticles.58 With a pHpzc of 8.5 for pure ferrihydrite, the pHpzc of Si-bearing ferrihydrite nanoparticles decreased due to the low pHpzc of silica (2–4) on ferrihydrite surfaces. In contrast, the pHpzc of Mn(OH)2 and Al(OH)3 was higher (∼10) than that of ferrihydrite.61,62 Therefore, the Mn and Al enriched on ferrihydrite surfaces resulted in the increased zeta potential of Mn- and Al-bearing ferrihydrite nanoparticles.
The zeta potential of SiO2 powder in the absence and presence of NaNO3 was similar, indicating no adsorption of Na+ onto SiO2 powder under our experimental conditions (pH = 3.8 ± 0.2). The SiO2 surfaces in the absence and presence of 1 mM Mn(II) ions under our experimental pH conditions (3.8 ± 0.1) were both negatively charged, with a zeta potential of −22.4 ± 2.6 and −25.1 ± 1.9 mV, respectively. Conversely, in the presence of 1 mM Al(III) ions, the SiO2 surfaces became positively charged (zeta potential = 18.1 ± 3.9 mV). The different effects of impurity ions on the surface charges of SiO2 may be related to the different behaviors of metal ion adsorption onto substrates, which is an important topic that we are investigating now, but it is not the focus of the present study. The surface zeta potential of the precipitates and substrates, which determined their electrostatic interactions, explained well the observed trend of heterogeneous ferrihydrite precipitation/deposition. In the presence of Mn, stronger attractive forces existed between the more positively charged Mn-bearing ferrihydrite (39.9 ± 2.7 mV) and negatively charged SiO2 surfaces (−25.1 ± 1.9 mV), resulting in the promoted Mn-bearing ferrihydrite precipitation/deposition on SiO2. Conversely, in the presence of Al, repulsive forces existed between the positively charged Al-bearing ferrihydrite precipitates (40.6 ± 2.3 mV) and SiO2 surfaces (18.1 ± 3.9 mV), thereby hindering Al-bearing ferrihydrite precipitation/deposition on SiO2. The changes in zeta potential of the substrates in FeMn and FeAl solutions could be caused by adsorption of Mn2+ and Al3+ or heterogeneous precipitation of Mn(OH)2 and Al(OH)3 on substrates. Further discussion about possible adsorption of Mn2+ and Al3+vs. Mn(OH)2 and Al(OH)3 precipitation on substrates can be found in ESI.†
Inhibited heterogeneous IBF precipitation/deposition on Al2O3
The GISAXS scattering curves due to pure and IBF nanoparticles precipitated/deposited on Al2O3 surfaces are shown in Fig. 2B. Compared with those for SiO2, the precipitation/deposition of pure, Mn- and Al-bearing ferrihydrite nanoparticles were all inhibited on Al2O3. Compared with the precipitates on SiO2 from Fe only, FeMn, and FeAl solutions, at the end of the 30-min experiments, the particle sizes on Al2O3 were smaller (2.5 ± 0.1, 3.4 ± 0.2, and 3.0 ± 0.2 nm, respectively, Fig. 3A2), and the total particle volumes were lower (67%, 31%, and 50% of the particle volumes for pure, Mn- and Al-bearing ferrihydrite precipitates on SiO2, respectively). Using the total particle volume and mean particle radius, the numbers were calculated. Under Fe only, FeMn, and FeAl solution conditions, the numbers of nanoparticles on Al2O3 were calculated to be ∼3 ± 1-, ∼0.8 ± 0.4- and ∼0.7 ± 0.4-fold those on SiO2. Hence, compared with on SiO2, the mean sizes of particles on Al2O3 were smaller under all three solution conditions; whereas the total particle number was larger on Al2O3 under a Fe-only solution. Heterogeneous precipitation (including heterogeneous nucleation and growth) and deposition on substrates can occur simultaneously, and growth controls particle size. The growth of ferrihydrite on substrates was controlled by the attachment of Fe hydroxide polymers onto the substrates, which is affected by the electrostatic forces between the polymers and substrates.13,36 To understand the reason for the smaller sizes of ferrihydrite nanoparticles on Al2O3 than on SiO2, electrostatic interactions between the polymers and Al2O3/SiO2 surfaces were considered. In Fe-only and FeMn solutions, the Al2O3 surfaces were positively charged (zeta potential = 20.5 ± 2.8 and 27.4 ± 6.8 mV), whereas SiO2 surfaces were negatively charged (zeta potential = −22.4 ± 2.6 and −25.1 ± 1.9 mV). In FeAl solution, the surface of Al2O3 was more positively charged (zeta potential = 34.3 ± 6.4 mV) than that of SiO2 (zeta potential = 18.1 ± 3.9 mV). All precipitates were positively charged so, assuming that the Fe hydroxide polymers carried similar charges to those of the precipitates, the stronger electrostatic repulsive forces between the polymers and Al2O3 surfaces inhibited the heterogeneous growth of IBF on Al2O3, resulting in smaller particles on Al2O3.Heterogeneous nucleation and deposition can increase the total particle number on substrates. The deposition behavior can be predicted by the Derjaguin, Landau, Vervey, and Overbeek (DLVO) theory. For a Fe-only solution, stronger electrostatic repulsive forces were present between more positively charged Al2O3 surfaces and ferrihydrite surfaces, which should have inhibited ferrihydrite deposition onto Al2O3 to a greater extent than on SiO2. Therefore, if deposition was the dominant process, the number of ferrihydrite particles (N) on Al2O3 should be less than that on SiO. However, the number of pure ferrihydrite particles on Al2O3 was calculated to be ∼2-fold higher than that on SiO2. Therefore, heterogeneous nucleation, instead of deposition, should be the dominant pathway for the pure ferrihydrite nanoparticle formation on Al2O3.
The promoted nucleation of pure ferrihydrite on Al2O3 rather than on SiO2 has been reported in our previous work of pure ferrihydrite precipitation on SiO2 and Al2O3.36 The latter has higher hydrophobicity, which indicates higher substrate–water interfacial energy than for SiO2,36 so the interfacial energy barrier for heterogeneous ferrihydrite nucleation on Al2O3 was lower than that on SiO2 according to classic nucleation theory.36 Therefore, heterogeneous ferrihydrite nucleation was promoted on Al2O3.36
The total volume of nanoparticles (representing precipitation/deposition rates) on Al2O3 surfaces was also compared (Fig. 3B2). Compared with pure ferrihydrite precipitation on Al2O3, Al- and Mn-bearing ferrihydrite precipitation/deposition was inhibited. Such inhibition can be explained well from the zeta-potential differences of the substrates and nanoparticles in the absence and presence of impurity ions. In the presence of Al and Mn, the zeta potential of Al2O3 and precipitate surfaces was more positively charged, and the stronger electrostatic repulsive forces between the substrate and IBF nanoparticles resulted in slower IBF precipitation/deposition on Al2O3. In the presence of Al and Mn, the increase in the zeta potential of IBF nanoparticles was caused by Al/Mn enrichment on the particle surfaces, as explained above. The increase in the zeta potential of Al2O3 might have been caused by adsorption of Mn and Al ions onto Al2O3. Further understanding of the mechanisms controlling ion adsorption onto substrates is not the focus of the present paper, but is a very interesting research topic which we are currently exploring.
In summary, the hydrolysis of impurity ions affected their structural incorporation into ferrihydrite lattices. Also, enrichment of impurity ions on IBF surfaces can affect the zeta potential of IBF nanoparticles. Furthermore, the surface charges of substrates can be altered greatly in the presence of impurity ions. Through all these three mechanisms, impurity ions can affect the heterogeneous precipitation/deposition of IBF on mineral surfaces significantly.
Conclusions
The present study provided new insights on the rates and mechanisms of heterogeneous IBF precipitation/deposition on different substrates. The hydrolysis of impurity ions, which is affected by pH, was found to impact their structural incorporation in IBF nanoparticles. The information provided here can help predict and control the incorporation of metal ions in IBF nanoparticles under different pH conditions. Also, the interactions of the impurity ions and IBF nanoparticles with Al2O3 were observed to be totally different to those of SiO2, which altered the zeta potential of the precipitates and substrates, thus affecting heterogeneous IBF precipitation/deposition considerably. The fundamental information provided here can be used to better predict the fate and transport of metal ions in natural and engineered aqueous systems. It can also be used to better design metal-doped ferrihydrite nanoparticles as adsorbents or precursors of Fe oxide catalysts for industrial applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Chong Dai and Juanjuan Liu were supported by a Faculty Start-up Fund from the University of Houston to conduct the GISAXS, DLS, and ICP-MS experiments. Chong Dai was also supported by the US Department of Energy, Office of Science, Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division, to analyze the experimental data and prepare the manuscript. We thank Dr. Xiaobing Zuo for valuable discussion on GISAXS experiments and data analyses at beamline 12-ID-B, Dr. Saul H. Lapidus for HRXRD analyses at beamline 11-BM, Advanced Photon Source, Argonne National Laboratory, and Dr. James D. Kubichi and Dr. Nadine Kabengi for valuable discussion of ion adsorption onto substrates. Use of the facilities at beamlines sectors 12-ID-B and 11-BM at Advanced Photon Source was supported by the US Department of Energy, Office of Science, Office of Basic Energy Science, under contract number DE-AC02-06CH11357.References
- C. E. Martinez and M. B. McBride, Environ. Sci. Technol., 1998, 32, 743–748 CrossRef CAS.
- Y. Tang, F. M. Michel, L. Zhang, R. Harrington, J. B. Parise and R. J. Reeder, Chem. Mater., 2010, 22, 3589–3598 CrossRef CAS.
- A. C. Vajpel, A. Rousset, K. Uma, I. Chandra, P. Saraswat and V. K. Mathur, Solid State Ionics, 1989, 32–33(Part 2), 741–748 CrossRef.
- C. Gu, Z. Wang, J. D. Kubicki, X. Wang and M. Zhu, Environ. Sci. Technol., 2016, 50, 8067–8076 CrossRef CAS PubMed.
- H. Zeng, B. Fisher and D. E. Giammar, Environ. Sci. Technol., 2007, 42, 147–152 CrossRef.
- H. Zeng, A. Singh, S. Basak, K.-U. Ulrich, M. Sahu, P. Biswas, J. G. Catalano and D. E. Giammar, Environ. Sci. Technol., 2009, 43, 1373–1378 CrossRef CAS PubMed.
- Z. Wang, K.-U. Ulrich, C. Pan and D. E. Giammar, Environ. Sci. Technol. Lett., 2015, 2, 227–232 CrossRef CAS.
- M. Tong, S. Yuan, Z. Wang, M. Luo and Y. Wang, J. Hazard. Mater., 2016, 305, 41–50 CrossRef CAS PubMed.
- M. A. Hinkle, Z. Wang, D. E. Giammar and J. G. Catalano, Geochim. Cosmochim. Acta, 2015, 158, 130–146 CrossRef CAS.
- G. Lee, J. M. Bigham and G. Faure, Appl. Geochem., 2002, 17, 569–581 CrossRef CAS.
- C. W. Neil, Y. J. Yang and Y.-S. Jun, J. Environ. Monit., 2012, 14, 1772–1788 RSC.
- P. Wu, C. Tang, C. Liu, L. Zhu, T. Pei and L. Feng, Environ. Geol., 2009, 57, 1457–1467 CrossRef CAS.
- Y. Hu, Q. Li, B. Lee and Y.-S. Jun, Environ. Sci. Technol., 2013, 48, 299–306 CrossRef PubMed.
- T. L. Jentzsch and R. L. Penn, J. Phys. Chem. B, 2006, 110, 11746–11750 CrossRef CAS PubMed.
- G. Doppler, A. X. Trautwein, H. M. Ziethen, E. Ambach, R. Lehnert, M. J. Sprague and U. Gonser, Appl. Catal., 1988, 40, 119–130 CrossRef CAS.
- J. Moutinho, T. D. Souza and M. D. C. Rangel, React. Kinet. Catal. Lett., 2004, 83, 93–98 CrossRef.
- S. Natesakhawat, X. Wang, L. Zhang and U. S. Ozkan, J. Mol. Catal. A: Chem., 2006, 260, 82–94 CrossRef CAS.
- A. L. C. Pereira, G. J. P. Berrocal, S. G. Marchetti, A. Albornoz, A. O. D. Souza and M. D. C. Rangel, J. Mol. Catal. A: Chem., 2008, 281, 66–72 CrossRef CAS.
- C. Ratnasamy and J. P. Wagner, Catal. Rev.: Sci. Eng., 2009, 325–440 CAS.
- T. L. Jentzsch, C. L. Chun, R. S. Gabor and R. L. Penn, J. Phys. Chem. C, 2007, 111, 10247–10253 CAS.
- E. B. Quadro, M. D. Dias, A. Maria, M. Amorium and M. D. Rangel, J. Braz. Chem. Soc., 1999, 10, 51–59 CrossRef CAS.
- J. Liu, C. Liang, H. Zhang, Z. Tian and S. Zhang, J. Phys. Chem. C, 2012, 116, 4986–4992 CAS.
- Y. Masue-Slowey, R. H. Loeppert and S. Fendorf, Geochim. Cosmochim. Acta, 2011, 75, 870–886 CrossRef CAS.
- G. Waychunas, B. Rea, C. Fuller and J. Davis, Geochim. Cosmochim. Acta, 1993, 57, 2251–2269 CrossRef CAS.
- S. Krehula and S. Musić, J. Alloys Compd., 2006, 426, 327–334 CrossRef CAS.
- K. Rout, A. Dash, M. Mohapatra and S. Anand, J. Environ. Chem. Eng., 2014, 2, 434–443 CrossRef CAS.
- A. C. Cismasu, F. M. Michel, J. F. Stebbins, C. Levard and G. E. Brown, Geochim. Cosmochim. Acta, 2012, 92, 275–291 CrossRef CAS.
- B. M. Sass and D. Rai, Inorg. Chem., 1987, 26, 2228–2232 CrossRef CAS.
- C. Dai and Y. Hu, Environ. Sci. Technol., 2015, 49, 292–300 CrossRef CAS PubMed.
- C. Dai, X. Zuo, B. Cao and Y. Hu, Environ. Sci. Technol., 2016, 50, 1741–1749 CrossRef CAS PubMed.
- B. A. Legg, M. Zhu, L. R. Comolli, B. Gilbert and J. F. Banfield, Environ. Sci. Technol., 2014, 48, 13703–13710 CrossRef CAS PubMed.
- T. Tosco, J. Bosch, R. U. Meckenstock and R. Sethi, Environ. Sci. Technol., 2012, 46, 4008–4015 CrossRef CAS PubMed.
- C. I. Steefel and P. Van Cappellen, Geochim. Cosmochim. Acta, 1990, 54, 2657–2677 CrossRef CAS.
- J. Liu, X. Wu, Y. Hu, C. Dai, Q. Peng and D. Liang, J. Chem., 2016, 2016, 11 Search PubMed.
- Y. Hu, B. Lee, C. Bell and Y.-S. Jun, Langmuir, 2012, 28, 7737–7746 CrossRef CAS PubMed.
- Y. Hu, C. Neil, B. Lee and Y.-S. Jun, Environ. Sci. Technol., 2013, 47, 9198–9206 CrossRef CAS PubMed.
- C. Dai, A. G. Stack, A. Koishi, A. Fernandez-Martinez, S. S. Lee and Y. Hu, Langmuir, 2016, 32, 5277–5284 CrossRef CAS PubMed.
- B. L. Henke, E. M. Gullikson and J. C. Davis, At. Data Nucl. Data Tables, 1993, 54, 181–342 CrossRef CAS.
- B. Lee, S. Seifert, S. J. Riley, G. Tikhonov, N. A. Tomczyk, S. Vajda and R. E. Winans, J. Chem. Phys., 2005, 123, 074701 CrossRef PubMed.
- G. Renaud, R. Lazzari, C. Revenant, A. Barbier, M. Noblet, O. Ulrich, F. Leroy, J. Jupille, Y. Borensztein and C. R. Henry, Science, 2003, 300, 1416–1419 CrossRef CAS PubMed.
- Y. Li, T. Gao and B. Chu, Macromolecules, 1992, 25, 1737–1742 CrossRef CAS.
- R. G. Ford, K. Kemner and P. M. Bertsch, Geochim. Cosmochim. Acta, 1999, 63, 39–48 CrossRef CAS.
- M. Edwards and M. M. Benjamin, J. - Water Pollut. Control Fed., 1989, 61, 481–490 CAS.
- A. Manceau and W. Gates, Clay Miner., 2013, 48, 481–489 CrossRef CAS.
- M. Alvarez, E. E. Sileo and E. H. Rueda, Chem. Geol., 2005, 216, 89–97 CrossRef CAS.
- R. Cornell and R. Giovanoli, Clays Clay Miner., 1987, 35, 11–20 CAS.
- C. Hansel, D. Learman, C. Lentini and E. Ekstrom, Geochim. Cosmochim. Acta, 2011, 75, 4653–4666 CrossRef CAS.
- X. Wang, M. Zhu, S. Lan, M. Ginder-Vogel, F. Liu and X. Feng, Chem. Geol., 2015, 415, 37–46 CrossRef CAS.
- R. M. Cornell and U. Schwertmann, The iron oxides: structure, properties, reactions, occurrences and uses, John Wiley & Sons, 2003 Search PubMed.
- B. Singh, D. Sherman, R. Gilkes, M. Wells and J. Mosselmans, Clay Miner., 2002, 37, 639–649 CrossRef CAS.
- D. Schulze, Clays Clay Miner., 1984, 32, 27–39 Search PubMed.
- W. Stiers and U. Schwertmann, Geochim. Cosmochim. Acta, 1985, 49, 1909–1911 CrossRef CAS.
- A. Y. Ramos, H. C. N. Tolentino, J. Enzweiler, S. M. Netto and M. D. C. M. Alves, Am. Mineral., 2003, 88, 876–882 CrossRef.
- C. M. Flynn, Chem. Rev., 1984, 84, 31–41 CrossRef CAS.
- A. L. Rose and T. D. Waite, Environ. Sci. Technol., 2003, 37, 3897–3903 CrossRef CAS PubMed.
- M. Zhu, C. Frandsen, A. F. Wallace, B. Legg, S. Khalid, H. Zhang, S. Mørup, J. F. Banfield and G. A. Waychunas, Geochim. Cosmochim. Acta, 2016, 172, 247–264 CrossRef CAS.
- M. S. Massey, J. S. Lezama-Pacheco, F. M. Michel and S. Fendorf, Environ. Sci.: Processes Impacts, 2014, 16, 2137–2144 CAS.
- A. C. Cismasu, C. Levard, F. M. Michel and G. E. Brown, Geochim. Cosmochim. Acta, 2013, 119, 46–60 CrossRef CAS.
- J. E. Amonette and D. Rai, Clays Clay Miner., 1990, 38, 129–136 CAS.
- N. Papassiopi, Z. Gaitanarous and A. Xenidis, Fresenius Environ. Bull., 2012, 21, 2399–2405 CAS.
- J. Rosenqvist, P. Persson and S. Sjöberg, Langmuir, 2002, 18, 4598–4604 CrossRef CAS.
- H. Jung and Y.-S. Jun, Environ. Sci. Technol., 2015, 50, 105–113 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of GISAXS calculations of critical angles, XPS measurements, and discussion on adsorption of Mn2+ and Al3+ ion vs. Mn(OH)2 and Al(OH)3 precipitation on substrates, as well as GISAXS Lorentz-corrected curves (Fig. S1) and XPS measurements of Mn oxidation states (Fig. S2). See DOI: 10.1039/c7en00835j |
| This journal is © The Royal Society of Chemistry 2018 |