Open Access Article
Open Access ArticleChallenges in characterizing the environmental fate and effects of carbon nanotubes and inorganic nanomaterials in aquatic systems
Peter
Laux
 *a,
Christian
Riebeling
*a,
Christian
Riebeling
 a,
Andy M.
Booth
a,
Andy M.
Booth
 b,
Joseph D.
Brain
c,
Josephine
Brunner
a,
Cristina
Cerrillo
d,
Otto
Creutzenberg
e,
Irina
Estrela-Lopis
f,
Thomas
Gebel
g,
Gunnar
Johanson
h,
Harald
Jungnickel
a,
Heiko
Kock
e,
Jutta
Tentschert
a,
Ahmed
Tlili
i,
Andreas
Schäffer
j,
Adriënne J. A. M.
Sips
k,
Robert A.
Yokel
b,
Joseph D.
Brain
c,
Josephine
Brunner
a,
Cristina
Cerrillo
d,
Otto
Creutzenberg
e,
Irina
Estrela-Lopis
f,
Thomas
Gebel
g,
Gunnar
Johanson
h,
Harald
Jungnickel
a,
Heiko
Kock
e,
Jutta
Tentschert
a,
Ahmed
Tlili
i,
Andreas
Schäffer
j,
Adriënne J. A. M.
Sips
k,
Robert A.
Yokel
 l and
Andreas
Luch
a
l and
Andreas
Luch
a
aDepartment of Chemical and Product Safety, German Federal Institute for Risk Assessment, Max-Dohrn-Strasse 8-10, 10589 Berlin, Germany. E-mail: peter.laux@bfr.bund.de
bSINTEF Ocean, Trondheim N-7465, Norway
cHarvard T. H. Chan School of Public Health, Boston, MA, USA
dIK4-Tekniker, Tribology Unit, Iñaki Goenaga 5, 20600 Eibar, Spain
eDepartment of Inhalation Toxicology, Fraunhofer-Institute for Toxicology and Experimental Medicine (ITEM), Nikolai Fuchs Strasse 1, 30625 Hannover, Germany
fInstitute of Medical Physics & Biophysics, Leipzig University, Härtelstraße 16, 04107 Leipzig, Germany
gGerman Federal Institute for Occupational Safety and Health (BAuA), Friedrich-Henkel-Weg 1-25, 44149 Dortmund, Germany
hInstitute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden
iDepartment of Environmental Toxicology, Eawag, Swiss Federal Institute of Aquatic Science and Technology, Dübendorf, Switzerland
jInstitute for Environmental Research, RWTH Aachen University, Aachen, Germany
kNational Institute for Public Health & the Environment (RIVM), Bilthoven, The Netherlands
lPharmaceutical Sciences, University of Kentucky, Lexington, Kentucky, USA
First published on 28th November 2017
Abstract
The current lack of commonly used protocols for dispersion, characterization, and aquatic toxicity testing of nanomaterials (NMs) has resulted in inconsistent results, which make meaningful comparisons difficult. The need for standardized sample preparation procedures that allow the reproducible generation of relevant test conditions remains a key challenge for studies of the environmental fate and aquatic toxicity of NMs. Together with the further development of optimized and cost-effective analytical techniques for physicochemical characterization that depend on reproducible sample preparation, such methods have the potential to overcome the current uncertainties with regard to NM dispersion properties, effective dose, and particle dissolution. In this review, recent data available on the challenges are summarized, especially those associated with preparing and quantifying NM dispersions, determining NM uptake and accumulation in aquatic organisms, and the transformation of organic and inorganic NM in aquatic species. Additional limitations and challenges that are specific to certain types of NMs are highlighted. The release of highly persistent carbon nanotubes (CNTs) from nanocomposites is determined to be a potential source of environmental contamination. Furthermore, the role of NM dissolution and the contribution of ions versus particles to NM toxicity are discussed. A phenomenon of particular relevance for the environment is photoactivation of NMs. This is elucidated with regard to its consequences in complex aquatic ecosystems. Widespread implementation of standardized protocols alongside the consideration of phenomena associated with different life cycle stages of industrial products is crucial to the future establishment of NM environmental risk assessment.
Environmental significanceThe environmental fate assessment of nanomaterials lacks standardized analytical methods and scenarios for nanomaterial release. Production of nanomaterials and derived products increases, while potential adverse effects remain unpredictable. Development of suitable analytical methods and consideration of nanomaterials in multicomponent compositions are indispensable prerequisites for the environmental risk assessment of nanomaterials. Before new materials of uncertain effects in aquatic systems are produced in large scale, analytical methods for detection and analysis should be present. The recognition of scenarios that may lead to the release of potentially harmful materials into the aquatic environment contributes to higher risk awareness. Suitable analytical methods and the inclusion of relevant industrial products and their life cycle stages can help to prevent negative effects of nanotechnology on aquatic life. |
Introduction
Nanomaterials (NMs) are produced worldwide on a large scale and their applications are steadily increasing.1–3 However, there remains considerable concern regarding their release into the environment, fate, behavior, and subsequent potential for eliciting effects in organisms. The potential accumulation of NMs by aquatic organisms has previously been suggested to lead to transfer throughout food chains.4–6 While human toxicity and ecotoxicity of NMs have many aspects in common, the study of NM environmental fate and effects presents a number of additional challenges.Crucially, a lack of suitable standardized methods for NM dispersion, preparation, and characterization has contributed to significant divergences in published data on their ecotoxicity.7–9 Parameters such as pH, ionic strength, the presence of biological material such as proteins, and the occurrence of humic acids in environmental media that can adsorb to the NM surface, can have a strong influence on NM agglomeration, persistence, and particle release from nano-enabled products.10,11 An increasing number of studies investigated the influence of multiple environmental conditions on the physicochemical properties of NMs, NM environmental behavior, and their subsequent potential for exposure and ecotoxicity to a range of organisms from different environmental matrices. For CeO2 NMs, it was shown that humic acids prevent agglomeration at low CaCl2 concentrations and facilitate agglomeration at high concentrations.12 In the case of multi-walled carbon nanotubes (MWCNTs), agglomeration, physical interaction with cells, and shading were found to contribute to their toxicity toward algae.13,14 In a study with the benthic diatom Nitzschia palea, a strong increase in MWCNT toxicity by natural organic matter (NOM) was recorded and discussed in relation to the affinity of MWCNTs to biofilms and the potential contribution of MWCNTs to a shading effect.15 The detection, quantification, and characterization of NM physicochemical properties (e.g., agglomeration, dissolution, shape, chemical reactivity, and adsorbed molecules) in the various environment matrices and relevant exposure scenarios present many challenges and in many cases require new or improved methods and technologies. The current limitations with available sample preparation methods and analytical tools used to address environmental fate and aquatic toxicity of NMs are described in this article. Furthermore, data on NM accumulation, dissolution, and release from nano-enabled products are reviewed with regard to uptake and accumulation in the food chain and potential adverse effects in the aquatic environment. Additional limitations and challenges that are specific to certain types and groups of NM are highlighted. For example, recent findings on NM photoactivation, a phenomenon with specific relevance for aquatic organisms, are summarized.
Dispersion and characterization of nanomaterials in aqueous media
One of the biggest challenges with interpreting and comparing the large volume of published aquatic ecotoxicity data is the wide range of dispersion techniques and conditions employed.16 Most studies use energy, in the form of shaking, stirring, or sonication, to generate aqueous NM dispersions. However, organic solvents such as tetrahydrofuran, pyridine, methanol, ethanol, propanol, and dimethyl sulfoxide have been used to disperse nanomaterials (e.g., C60), although careful control of the solvent concentration is necessary to avoid negative impacts on organisms employed in nanotoxicity studies.17,18 This has led to inconsistent or even contradictory findings in different studies using the same NM and ecotoxicity assay.19 Reproducibility of NM dispersion in exposure media employed in aquatic ecotoxicity tests, and appropriate methods to characterize NMs in such dispersions, are crucial for obtaining accurate results that can be interpreted meaningfully.20–23 A recent study used the same titanium dioxide (TiO2) NMs (38 ± 10 nm) to investigate the effectiveness of four dispersion protocols that had been developed for past research projects.19 Variations among the four different protocols included the use of pre-wetting, the type of sonicator, sonication duration and power, cooling, and particle concentration. The results revealed a large degree of variability of the mean TiO2 particle dispersion diameter among the four protocols with a relative standard deviation of 26%. The authors identified particle concentration as well as sonication conditions (power and duration) as the main parameters influencing the final dispersion characteristics. Although sonication conditions are an important parameter in determining dispersion quality, it is clearly important to consider other parameters, such as particle concentration, age of the dispersion, and subsampling as potential sources of variability.19There is currently a lack of internationally-recognized standard dispersion protocols for NMs, but available information on protocols for the preparation of stock suspensions has recently been summarized.24 The need for clear and comprehensive guidelines for preparation of NM dispersions has been highlighted, together with the importance of a well-controlled sonication protocol.24–29 Standardizing the energy delivered to the system during dispersion is a key step towards achieving reproducible aqueous NM dispersions.30,31 Furthermore, the availability of internationally-accepted reference materials and benchmark data for assessing NM dispersion reproducibility, both within and across laboratories, is required. The generation of individual benchmarked reference datasets for the huge range of aquatic conditions studied in environmental fate and effects assessment is not feasible, yet it is important such studies are conducted using relevant environmental parameters. It is therefore suggested that initial standardization strategies focus on establishing reproducible stock dispersions in deionized water, which can be further diluted when specific media types are required in ecotoxicity tests or that mimic natural water bodies. This approach could overcome the issue of conducting tests on unnaturally dispersed NMs, which is likely to overestimate the exposure occurring in the natural environment.
Independent of their size, NMs of the same mass and chemical composition can have a completely different toxicity per unit mass.32 For toxicity testing of NMs, the metric for dose quantification needs to be carefully chosen. Particle volume, mass, surface area, and number have been used for this purpose.33 Each metric may provide a useful perspective. Delmaar et al., have outlined a simplified dose metric related to particle diameter for spherical SiO2 and Ag NMs.32 It appears unlikely that there is a single dose metric that is appropriate for all NMs and test systems.32,34 In aqueous suspensions, atomic force microscopy, scanning or transmission electron microscopy, and ultrafiltration are often applied for NM characterization.35 Field-flow fractionation and single particle ICP-MS are increasingly being used for NM size estimation in environmental samples.36,37
Carbon nanotubes
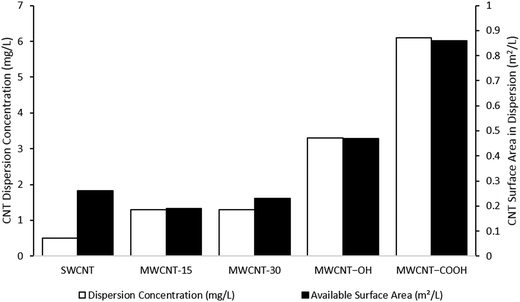
This review emphasizes carbon nanotubes (CNTs) for a detailed assessment of NM dispersion and characterization in aquatic media. CNTs present many of the same challenges observed with metal and metal oxide NMs, but also some additional factors unique to this family of materials. They represent the most commonly studied carbon NM, although environmental studies with fullerene and graphene families have also been reported.38–40 The preparation and characterization of CNT dispersions is challenging and has proven difficult to apply accurately and reproducibly in environmental assessment. The inherent physicochemical properties of CNTs mean that their dispersion, quantification, and characterization in aqueous environmental media and ecotoxicity tests require alternative approaches to those routinely employed for many metal and metal oxide NMs. Their hydrophobic nature and relatively large particle size means that CNTs disperse extremely poorly in water, although surface chemistry (e.g., –OH and –COOH functionalities) can reduce hydrophobicity, thereby increasing dispersion concentration (Fig. 1) and stability.41–47 CNT dispersibility and stability are further reduced with increasing ionic strength of natural waters and ecotoxicity media.38,43,48–50 NOM can significantly increase CNT dispersion concentration and stability in aqueous media.38,48,51–53 As such, some forms of NOM are increasingly employed in ecotoxicity studies with CNTs53–55 as well as inorganic NMs.56,57 Finally, CNT dispersions prepared using sonication will generate higher, more stable concentrations than shaking or stirring,51 although it is likely to result in breakage of the tubes and generation of artifacts depending on the sonication time and energy.18,58Many previous studies on CNT ecotoxicity employed specific and diverse sonication methods for dispersion.4,59,60 Differences in dispersion methods can lead to significant deviations in the final dispersion concentration, the agglomeration/aggregation state of the CNTs, and the degree of breakage.58,61 The issue of CNT breakage is rarely considered in reported CNT ecotoxicity studies, even though such damage has been shown to alter CNT behavior within the context of (eco)toxicological testing. For example, MWCNTs dispersed by sonication have been shown to be more toxic to the fresh water flea Ceriodaphnia dubia and the copepod Tigriopus japonicus than CNT dispersions prepared by stirring or shaking.62,63 In turn, these parameters affect the final exposure conditions of CNTs, rendering interpretation and comparison of ecotoxicity data generated challenging.
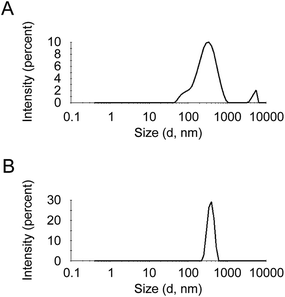
A recent study using a range of non-functionalized MWCNTs investigated the influence of different dispersion preparation techniques on both the comparability of the dispersions and the determination of CNT concentration by UV/vis absorbance.64 A sonication process for calibration dispersions using an ultrasonic probe for delivery of acoustic energy was optimized. Verification was conducted by preparing dispersions using an ultrasonic bath, and a procedure to select an appropriate wavelength for each type of MWCNT was also proposed. Results of the study clearly demonstrated that UV/vis absorbance is highly dependent on the dispersion method implemented. Dynamic light scattering was used to determine MWCNT agglomerate size, and revealed that the ultrasonic probe produced dispersions with lower MWCNT agglomeration levels (Fig. 2A) than the ultrasonic bath (Fig. 2B). This study has contributed to the development of standardization in the environmental assessment of CNTs. The European project NANoREG65 has also addressed the issue of dispersion reproducibility by developing a standard operating procedure (SOP) for NM dispersion preparation and characterization in environmental fate and ecotoxicity studies.
The separation and characterization of CNT dispersions in both laboratory ecotoxicity tests and in complex environmental/biological matrices represent additional challenges compared to other NMs. Relevant parameters for characterization in standard ecotoxicity tests include dispersion concentration (especially over time in an exposure), specific surface area (SSA), and the degree of aggregation/agglomeration (i.e., changes in average particle size distribution). Elemental-based quantification techniques (e.g., inductively coupled plasma mass spectrometry) routinely used for inorganic NMs are unsuitable for quantification of CNT dispersion concentrations, and thus alternative approaches are necessary.66 UV/vis spectroscopy represents the most common method for CNT quantification in aqueous samples. Despite advantages with respect to time and cost efficiency, this approach has limitations such as potential shading issues, interference from complex environmental matrices, and challenges establishing external calibration curves.41,47,67–70 The latter must be based on CNT dispersions with accurately known concentrations.51 Furthermore, variable wavelengths for absorbance measurements have been reported in the literature indicating that an optimized method has yet to be identified. Thermal analysis techniques (e.g., thermogravimetry, chemothermal oxidation, or thermal optical transmittance) can also be used to quantify CNTs in simple aqueous dispersions,51,71–74 but are limited in specificity to particular CNTs or for more complex environmental matrices.67,71 The high aspect ratio of CNTs means dynamic light scattering techniques are unsuitable for generating accurate particle sizes in dispersion.42,46,75 Scanning and transmission electron microscopy (SEM; TEM) imaging represent better options for determining more relevant particle size data and particle size distributions. However, TEM/SEM imaging of CNTs requires manipulation of the sample prior to analysis, which can significantly change CNT dispersion properties (e.g., aggregation). Vitrification and cryo-analysis have previously been suggested as methods to help overcome this issue,76 but both approaches also have the potential to significantly disturb the dispersion. CNTs dispersed in complex aqueous environmental samples may undergo a significant change in SSA due to aggregation and adsorption of NOM.44,77,78 However, standard methods for SSA measurement of dry NMs (e.g., BET) cannot be used for direct SSA measurements of CNTs in dispersion, meaning determination of this parameter currently remains unachievable. Thus, determination of SSA currently remains elusive.
Release of particles from nanocomposites and coatings
Polymers are frequently enabled with NMs in order to obtain properties such as increased mechanical and barrier strength, biocidal activity, or to repel water and dirt. While materials in which particles, platelets, or fibers dispersed in a polymer matrix are called nano-composites, surface coating represents a further common functionalization technology. Inorganic particles such as nano-Ag and nano-TiO2 are common components of polymeric materials of textiles, food contact materials, and other products of daily use. CNTs are dispersed in polymer matrices in low amounts (usually <5 weight%) to transfer some of their beneficial properties to the plastic matrix, mainly mechanical strength,79e.g., for motor helmets or tennis rackets, as well as electrical conductivity, e.g., for flexible electrodes, antistatic coatings, or piezoresistive sensors.80 It was assumed that ca. 0.2% of the 300 million tons of plastic material produced worldwide each year represent composites with embedded NMs.81 One of the main questions is whether and under which conditions NMs from such products might reach the aquatic environment. Release of NMs from nanocomposites and coatings can occur during the use phase, e.g., by wear or washing, by drilling or sanding, as well as by environmental weathering of composites after disposal, e.g., by solar irradiation and leaching processes.82–86 A study on the release of silver from nano-enabled textiles into artificial sweat demonstrated that coated products release a higher amount compared to nanocomposites, where particles are integrated in the polymer.85 Although release of nano-Ag was observed, this was not related to the primary particle size and chemistry of the silver compounds used for textile functionalization. The finding that NMs may not only originate from direct release of elemental silver but also from reduction of silver ions was confirmed by experiments on textile washing in which metallic Ag NMs were detected in experiments in which AgNO3 was introduced.86An analysis of NM release due to mechanical treatment was recently conducted with 11 acrylate coatings and 11 polypropylene composites.87 The samples were equipped with 10 pigments: TiO2, carbon black, and 8 nanoscale pigments. High numbers of released NMs were observed, however, no correlation to the primary pigment particle size was found. Standardized scenarios for measurement of NM release to air during occupational and daily life still remain to be developed. A protocol was recently established that reproducibly simulates the drilling of polyamide-6 nanocomposites containing 30 weight% of glass fiber and different concentrations of nano-SiO2 or organically modified montmorillonite.88
Degradation of CNT-containing composites induced by photooxidation under artificial sunlight has already been described.89–91 CNTs were uncovered during degradation of the embedding matrix and formed a network on the weathered surface resulting in potential release from the composite (Fig. 3).
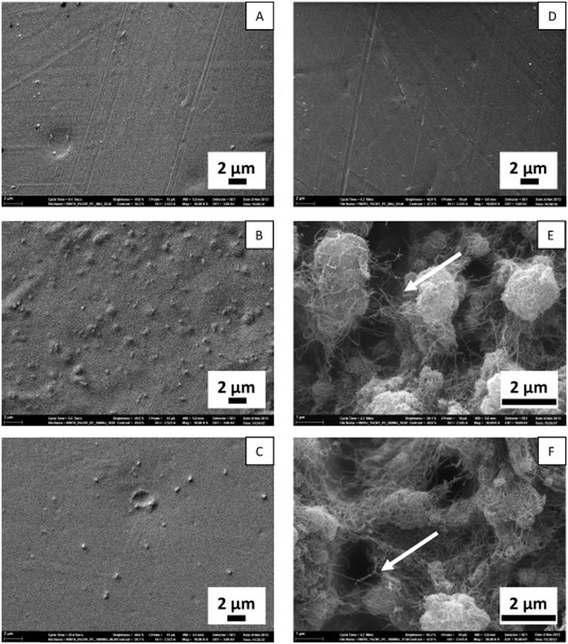 | ||
| Fig. 3 Scanning electron microscopic pictures of samples of carbon nanotubes (CNT) containing polycarbonate (PC) composites and controls with no CNT material; A: PC without CNT not irradiated; B: PC without CNT after 500 MJ m−2 irradiation with artificial sunlight, C: PC without CNT after 1000 MJ m−2 irradiation; D: PC with 1 weight% CNT not irradiated, E: PC with 1 weight% CNT after 500 MJ m−2 irradiation; F: PC with 1 weight% CNT after 1000 MJ m−2 irradiation. The arrow points to a single CNT.92 | ||
However, exact quantification of the released amounts upon environmental weathering is not possible without using a labelling approach. Rhiem et al.92 used 14C-labelling of the carbon skeleton of uncoated CNTs to establish a mass balance. They prepared a black CNT-polycarbonate composite sample (on average 138 mg per sample) containing 1 weight% of multi-walled 14C-CNT. After irradiation with simulated sunlight (lamp 220 W m−2, cut-off at 300 nm; total irradiation 1000 MJ m−2) and gentle tapping of the sample to remove loose material, further environmental scenarios were consecutively simulated: (a) 21 days shaking in water with six water exchanges during this period, (b) temperature stress (one week 70 °C, a temperature that black plastic material can achieve under natural sunlight), (c) freeze–thawing for one week (−22 °C, once per day thawed at 20 °C), (d) one week shaking in humic acid in water (25 ppm, pH 11.3), one week shaking in artificial acid rain (pH 2.6), and (e) one week shaking in artificial disposal site effluent prepared according to Rhiem et al. and Kalbe et al.92,93 The results in Table 1 were obtained with respect to released material (A: sample irradiated (conditions see above), B: sample not irradiated).
| Tapping | Shaking (water) | 70 °C | Freeze–thaw | Shaking (humic acid) | Shaking (acid rain) | Shaking (disposal effluent) | Tissue wiping | Sum | |
|---|---|---|---|---|---|---|---|---|---|
| A (μg) | 0.01 ± 0.01 | 2.6 ± 1.6 | 0.07 ± 0.004 | 0.77 ± 0.57 | 1.87 ± 0.76 | 2.50 ± 0.85 | 2.75 ± 2.57 | 4.12 ± 1.53 | 14.70 ± 1.86 |
| B (μg) | — | 0.1 ± 0.1 | 0.004 ± 0.004 | 0.002 ± 0.002 | 0.03 ± 0.03 | 0.04 ± 0.01 | 0.19 ± 0.27 | 0.02 ± 0.02 | 0.40 ± 0.27 |
Referring to the initial amount of 1 weight% CNT per composite sample, ca. 1.0 ± 0.2% of the CNTs were released from irradiated samples – equivalent to about 64 mg CNT equivalents per square meter composite surface – and only 0.03 ± 0.02% from non-irradiated control samples after all environmentally-relevant treatments. SEM analysis revealed that single CNTs, CNT agglomerates, and CNTs embedded in small matrix particles were released under these scenarios. In line with this release rate,94 approximately 100 mg CNT per m2 per year would be released at the surface of polyoxymethylene composites containing 5 weight% CNT.
CNTs released from the composites will have a very long half-life in the environment. In experiments with horseradish peroxidase, an enzyme known to catalyze the oxidation of aromatic compounds, a half-life of 80 years was determined by the slow mineralization of 14C-labelled CNT and quantification of formed 14C–CO2.95
Uptake, accumulation, and transformation of nanomaterials in the environment
Some recent studies on functionalized and non-functionalized MWCNT accumulation have employed single-celled organisms such as algae,39 bacteria, and protozoa.116 In the case of bacteria and protozoa, an approach employing separation of 14C-labeled functionalized MWCNTs by density gradient centrifugation was described.117 The bacterium Pseudomonas aeruginosa was found to adsorb the functionalized MWCNTs at concentrations of 0.18 ± 0.04 and 21.9 ± 4.2 μg per mg dry mass at respective nominal concentrations of 0.01 and 1 mg L−1. In the same study, an accumulation of up to 0.9 ± 0.3 μg of functionalized MWCNT per mg dry mass was recorded in the protozoan Tetrahymena thermophila following trophic transfer via MWCNT-encrusted Pseudomonas aeruginosa cells, while up to 3 ± 1 μg of functionalized MWCNT per mg dry mass was detected following direct uptake. Thus, protozoa were identified as a potential vector for the transfer of functionalized MWCNTs to the next trophic level.116 The alga Desmodesmus subspicatus was found to contain mean concentrations of 1.3 ± 0.5, 2 ± 2, and 5 ± 2 μg non-functionalized MWCNTs per mg dry weight following exposure to a suspension of 1 mg L−1 of MWCNTs for 24, 48, and 72 h respectively.81
Daphnia magna has been shown to internalize functionalized MWCNTs. Following a 48 h exposure to a suspension of 0.4 μg per ml, 63 ± 15 μg of the test substance was recorded per mg dry mass.98 Previously, a value of approximately 29 μg mg−1 dry mass was reported, following a 48 h exposure of D. magna to a 30 μg L−1 fullerene suspension.118 Interestingly, no facilitation of functionalized MWCNT excretion by NOM was observed, which has been described to facilitate suspension of CNTs.98 The mean level of non-functionalized MWCNTs in whole fish, following 48 or 168 h exposure to 1 mg L−1 test medium, was determined to be 73 ± 93 ng per mg dry weight, indicating a low bioaccumulation.119 Factors controlling CNT accumulation and retention are poorly understood, but the role of NOM appears to be important. For example, π–π interaction and hydrogen bonding have been suggested as the predominant interactions between CNTs and negatively-charged biocolloids.120 These forces were further found to regulate the interaction of CNTs with humic acids, natural biopolymers, and model solid-phase polymers in a systematic comparison by Zhao et al.,121 who described aromaticity and surface polarity as the most positive factors for CNT retention. A recent review by Hu et al.39 emphasized the need to consider the complete range of techniques for extraction, isolation, and characterization of CNTs in order to follow their transformation in natural environments and organisms. However, in the case of CNTs, few data on structural degradation in the environment have been reported. A strain of the bacterial species Trabusiella guamensis isolated from soil was shown to cause surface oxidation and structural changes in MWCNTs.122 Furthermore, a lignin peroxidase isolated from the mushroom Sparassis latifolia was shown to biodegrade SWCNTs.123
Current methodologies for isolating CNTs and their transformed forms from complex matrices are still limited, whilst techniques for accurately characterizing and quantifying CNTs also remain underdeveloped.23,98,117,124–126 Furthermore, the presence of low CNT concentrations in environmental matrices and the difficulty in distinguishing natural sources of carbon from those corresponding to CNTs compound the challenges of separation and analysis. The challenge of determining CNT uptake and accumulation in organisms and environmental samples highlights the necessity of using radiolabeling in laboratory studies.127
With respect to NM accumulation in fish, TiO2 particles were mainly identified in the kidney, liver, gills, and to a lesser extent in muscle of the marine species Trachinotus carolinus following intraperitoneal injection of 1.5 and 3.0 μg g−1 bodyweight.134 Animals were exposed for 24, 48, or 72 h; the size of the employed TiO2-NM was estimated by TEM as being 11–40 nm. As no size distribution was reported for the injected suspension, which was described as containing primary NMs and larger agglomerates/aggregates, no conclusion on a relationship between size and distribution is possible from this study.134 Facilitated uptake of larger-sized NMs is indicated in a study by Chen et al.,135 who observed enhanced accumulation of agglomerating nano-Fe3O4 in medaka fish (Oryzias latipes) when compared to the stable colloid Fe0. Fe2O3 NMs were further reported to adhere to the surface of zebrafish (Danio rerio) embryos during toxicity testing.136 In line with these results suggesting a facilitated uptake of larger particle sizes, an apparent sorption of nano-Ag of a primary particle size of 50 nm into exposed carp embryos was described for 400 nm, but not for 200 nm, agglomerates.137 In the same study, a relationship between NM agglomeration and toxicity has been described for common carp embryos. While there was no dose–response relationship observed in nano-Ag supplemented media with Ag concentrations of 5, 10, and 25 μM comprising aggregates up to a size of 200 nm, there was a clear dose–response observed for concentrations of 10, 25, and 50 μM when agglomerates up to a maximum size of 400 nm were present.
An in vitro barrier model was recently suggested to elucidate NM fate in the intestinal epithelial cells of fish. For this purpose, intestinal cells of rainbow trout (Oncorhynchus mykiss) were grown as monolayers on permeable membranes, establishing an upper (apical) and a lower (basolateral) compartment. The created polarized epithelium was shown to efficiently prevent the translocation of polystyrene-NMs between the apical and basolateral compartments.138
Bivalves are considered to be key organisms in the fate and transport of NMs in aquatic habitats because of their ability to filter and concentrate water-suspended particles. The influence of nano-CeO2 association to phytoplankton on the internalization of the compound by the marine mussel Mytilus galloprovincialis was investigated by Conway et al.128 While direct exposure to 3 mg L−1 CeO2 caused a mean dry tissue cerium concentration of 33 ± 9 μg g−1, a value of 28 ± 5 μg g−1 was measured following exposure to the same concentration sorbed to phytoplankton. Clearance rates and pseudofeces production were shown to increase with increasing exposure concentrations. In a further study, M. galloprovincialis was shown to accumulate 79 ± 13 μg Cu per g dry weight following exposure to 3 mg L−1 CuO NMs for four weeks. Biodeposits excreted by the animals were found to contain 110 mg Cu per g. This finding demonstrates that there is a potential for copper magnification in sediments and a possible contribution of mussels to trophic transfer of NMs to predators such as crabs and fish.139
The results achieved so far on NM accumulation in the aquatic environment demonstrate the need to further characterize NM interactions with organic substances such as humic acids and chitin. Both are suspected to influence trophic transfer and thus potential adverse effects. Moreover, dissolution is a key parameter for NM accumulation that is likely influenced by environmental compartments.
The role of nanoparticle dissolution in their ecotoxicity
Toxicity of inorganic NMs in organisms and complex ecological systems is poorly understood despite the widespread production, use, and release of these substances into the environment.140 Most profound effects are to be expected in ecosystems dominated by organisms highly sensitive to these compounds.140,141 Microorganisms in natural ecosystems are prime candidates because several NMs, such as nano-Ag or nano-ZnO, are deliberately designed and applied to exert antimicrobial effects. The causes of toxicity of such NMs to microorganisms are still largely unclear. Nevertheless, release of the ionic form of the metal from NMs has been suggested to play a key role.142,143 For instance, Navarro et al.142 have shown that the inhibitory effect of nano-Ag on the photosynthesis of algae was abolished when an Ag+ chelator was added to the culture medium. Similarly, results from a study by Xiu et al.144 indicated a lack of negative effects on bacteria when anaerobic conditions prevented the oxidative dissolution of nano-Ag. Consequently, the importance of the released amount of ions into the test medium has aroused great interest. A study by Rohder et al.145 showed that toxicity of nano-CeO2 to a green freshwater alga could be attributed to Ce3+ ions released in the NM suspension. Furthermore, the results of this study indicated that the measured toxicity increased with the amount of bioavailable Ce3+ ions in the media. In contrast, Rodea-Palomares et al.146 suggested that toxicity of nano-CeO2 to algal and bacterial cells was not caused by the released ions in the test media but by the attachment or sorption of the NMs to the outer surface of the microorganisms. Despite the importance of using single species microorganisms in understanding mechanisms of toxicity and ensuring comparability due to rigorous standardization, evaluation of the risks posed by soluble inorganic NMs should also consider the complex biodiversity, functions, and response dynamics in ecosystems.Few studies have investigated the role of dissolution in the ecotoxicity of NMs at the community level.147 Gil-Allué et al.148 and Tlili et al.149 selected two microbial communities that play key roles in fresh waters through primary production and organic matter decomposition, respectively, to provide convenient model systems to assess nano-Ag effects on complex ecological interactions and ecosystem processes. The first community of heterotrophic fungi and bacteria colonizes decomposing leaf litter; the second is an autotrophic community commonly referred to as periphyton, which consists of algae and bacteria attached to submerged surfaces. Together, these two systems capture much of the essence of stream ecosystem functioning. The studies showed that acute exposures to citrate coated nano-Ag (size 25 ± 13 nm; zeta potential −36.6 ± 3.2 mV) affected the majority of the functional endpoints tested. Most importantly, acute nano-Ag toxicity was mainly driven by dissolved Ag+ ions; however, this was not the case for all activities measured, such as bacterial and fungal growth.149 In the experiments with stream periphyton, the observed inhibition of leucine aminopeptidase, an extracellular enzyme involved in nutrient acquisition, by nano-Ag was attributed to a putative, physically-mediated particle effect independent of silver ions (Ag+).148 Indeed, addition of an Ag+ chelator failed to prevent the inhibitory effect of nano-Ag on the measured enzyme. Consequently, the hypothesis that toxicity of nano-Ag is caused by dissolved Ag+ could not fully explain the results obtained in short-term toxicity tests. Nano-Ag was described as exerting some particle-related effects on fungi, algae and bacteria, independent of the effect of Ag+, when complex microbial communities were considered. A study in zebrafish embryos and larvae on genes involved in detoxifying processes and oxidative stress responses revealed that Ag+ and nano-Ag induced similar gene responses and affected the same target tissues, pointing to an ion-mediated effect of nano-Ag.150
This notwithstanding, there is a debate whether particle-related effects, which can be the stimulation or inhibition of microbial activities, are mainly associated with changes in metal dissolution kinetics when particles are in contact with microorganisms.149 Indeed, NMs near microbial cells are susceptible to being oxidized by metabolic products such as H2O2.151 Such oxidation can lead to high local concentrations of ions, which are then rapidly taken up, and therefore cannot be measured in the test media. Firm conclusions cannot yet be drawn on the role of ion dissolution in the toxicity of NMs to microorganisms. However, these findings highlight the importance of investigating the physicochemical state of NMs (i.e., NMs vs. dissolved ions, primary particles vs. agglomerates) when ecotoxicity is assessed. They also underline the need to consider the complexity of ecosystems for studies on fate and toxicity of NMs in the environment and to focus on multiple functional endpoints for such investigations.
The impact of non-ionizing radiation on nanomaterial ecotoxicity
Ultraviolet radiation significantly influences the environmental toxicity of photoactive materials. Due to its redox ability and chemical stability, TiO2 is most frequently used as a photocatalyst.152–154 The impact of different particle sizes on TiO2 phototoxicity was compared in a study with D. magna. For all test materials the toxicity was significantly enhanced when organisms were simultaneously exposed to simulated sunlight irradiation in comparison to exposures under laboratory light. While intermediate-sized particles (NM-102, 20–25 nm) showed the highest potency followed by small particles (NM-101, 7–10 nm), larger particles (NM-100, 200–220 nm) were less toxic (Fig. 4).155 Median effective concentrations (EC50) for the three uncoated NM-types were 0.53, 1.28, and 3.88 mg L−1 at 48 h, respectively.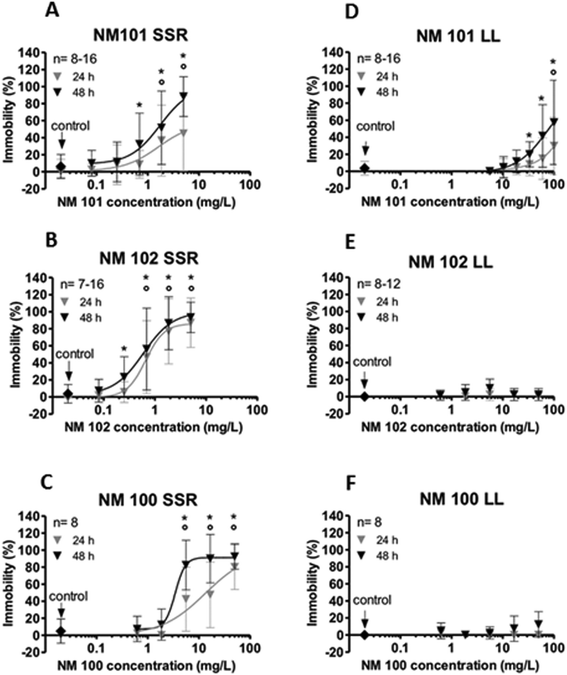 | ||
| Fig. 4 Ecotoxicological investigation of two titanium dioxide (TiO2) nanomaterials (NM-101, ø 7–10 nm, NM-102, ø 20–25 nm) and of a non-nano-sized TiO2 material (NM-100, ø 200–220 nm). The immobilization of daphnids as a function of TiO2 concentrations is shown under simulated sunlight irradiation (SSR; left; A–C) and under laboratory light (LL; right; D–F). Simulated sunlight irradiation was obtained from a mercury-vapor lamp which emitted a spectrum comparable to sunlight (280–800 nm, uva 2.36 mW cm−2 and uvb 0.15 mW cm−2). The spectrum of this light source is given in Wyrwoll et al. (2016).155 Laboratory light was emitted by a laboratory bulb (Osram Lumilux Cool White, HO 49W/840). Experiments were conducted in 10-fold diluted iso medium. Error bars represent standard deviations of the mean of the replicates from at least two independently conducted experiments. Circles (24 h of exposure) and asterisks (48 h of exposure) indicate significant differences from the control (p < 0.05). Reprinted with modifications from, A. J. Wyrwoll, P. Lautenschlager, A. Bach, B. Hellack, A. Dybowska, T. A. Kuhlbusch, H. Hollert, A. Schäffer, H. M. Maes, Size matters-the phototoxicity of TiO2 nanomaterials, Environ Pollut., 208, 859–867, Copyright 2016, with permission from Elsevier. | ||
The impact of different particle sizes of TiO2 was shown to depend on the generation of different reactive oxygen species (free and surface-adsorbed OH radicals) and the particle-D. magna interaction area, both influenced by particle size.155 The effect of photoactivation on toxicity of NMs has been described for several additional species. In the case of the nematode Caenorhabditis elegans, an increased toxicity of photoactivated nano-TiO2 associated with reactive oxygen species could be demonstrated with a median effect concentration decreasing from more than 100 mg L−1 to 53 mg L−1.156 Phototoxicity of nano-TiO2 was further confirmed for algae.157 Similar to TiO2, light-induced toxicity of nano-ZnO was described for algae,157 nematodes,158 and fish.159 In a further study using zebrafish embryos and a cell line originating from the caudal fin tissue of the bluegill sunfish (Lepomis macrochirus), TiO2 and Ag NMs were reported to express enhanced toxicity upon sunlight exposure, which was attributed to hydroxyl radical generation and Ag+-release, respectively.160
While sunlight-promoted particle toxicity is assumed in the case of TiO2, facilitated ion release is discussed as a potential reason for the increased effects of irradiated nano-ZnO.161 Since the toxicity of the tested NMs was significantly enhanced when organisms were simultaneously exposed to solar irradiation in comparison to exposures under laboratory light, this study gives further evidence that standard ecotoxicity tests such as D. magna immobilization underestimate the environmental hazard associated with photoactive NMs. For Ag NMs with a similar coating, a lower toxicity to D. magna was observed at high pH, in the presence of DOM and with increasing particle size, thereby supporting the major contribution of Ag species below 2 nm, including Ag+ ions, to toxicity.162
To date, mostly simple NM forms have been tested for phototoxicity. However, there are multiple approaches to improve the photocatalytic properties of commonly studied TiO2. As the molecule is only excited by UV light with a wavelength below 388 nm, sunlight absorption is limited to ca. 3%. Moreover, the electron–hole pair recombination reduces its photocalaytic activity.152,163,164 Multiple approaches, comprising noble metal deposition,165,166 semiconductor compounding,167 dye sensitizing,168 carbon composition,169,170 and doping171,172 were undertaken to extend the photocatalytic activity of TiO2 from UV to visible light range.
Doping attempts comprise rare earth metals,173 combined use of iron and nitrogen,174 as well as self-doping with Ti3+.175 A mineralization of 4-chlorophenol and the azo dye remazol was described for carbon-doped TiO2, while the base material was nearly inactive.164 Ag-doped TiO2 was shown to possess a 5-fold higher efficacy against bacteriophages in aqueous media when compared to the base material.176 However, the environmental impact of such procedures still requires investigation. Although Ag doping increases photocatalytic virus inactivation of TiO2 in the first instance by increased hydroxyl radical production, leaching of antimicrobial Ag+ was also concluded to account for antiviral activity.176 Ion-mediated toxicity is known as a main factor for the biocidal activity of Ag.177 Similarly, it was shown that the antibacterial activity of Cu-doped TiO2 is driven by Cu2+ release. While Fe-doping of CuO and ZnO NMs was described to have the potential of decreasing the release of toxic ions,178,179 the finding of rheotaxis dysfunction and DNA damage in zebrafish due to exposure to S-doped TiO2 underpins that toxic effects due to particle surface alterations, independent from ion release, cannot be ruled out.180 In addition to doping, the composition of TiO2 and carbon has received attention for remediation purposes.170 A TiO2 (P25)-graphene photocatalyst showed a narrowed band gap and an improved adsorption of the test substance methylene blue in comparison to straight TiO2 P25.169 Later on, a 2.3-fold increased photocatalytic generation of reactive oxygen species in visible light was recorded for the graphene-TiO2 nanocomposite compared to the base material TiO2 P25. Together with the enhanced photoreactivity of modified nano-TiO2 and its changed aquatic toxicity, its reactivity in the respective medium of application needs to be considered, as several environmental factors may affect agglomeration and quenching.163 In a study by Quik et al.181 utilizing water samples from the European rivers Rhine and Meuse, homoaggregation of CeO2 NMs was suggested to occur after sample spiking at initial high NM concentrations. Subsequent heteroaggregation or deposition of NMs on natural colloids resulting in NM removal according to first order kinetics was identified as the main mechanism leading to sedimentation.
The physicochemical properties of NMs depend on their functionalization. Coating-dependent degrees of degradation were recorded for three rutile TiO2 nanomaterials of a primary crystallite size of 30 nm. Following 10 days incubation in ultrapure water or in the dark, there was a stronger degradation observed for particles coated with SiO2 or SiO2 and polydimethylsiloxane (PDMS) in comparison to those possessing a capping of SiO2, Al2O3, and stearic acid.182 Aging of a TiO2-based nanocomposite used in sunscreen cosmetics, consisting of a TiO2 core and a coating of Al(OH)3 and PDMS, revealed a complete desorption of PDMS after 18 h of aging in ultrapure water, while Al remained at the surface of the altered composite.183 Coated TiO2 NMs were shown to undergo larger changes in the light compared to dark conditions.182,183
These factors need to be considered in future testing guidelines.
Outlook
Aquatic ecotoxicity assessment needs to employ standardized protocols for the reproducible and common preparation of aqueous NM dispersions. The European project NANoREG65 recently developed harmonized and benchmarked SOPs for probe sonication-based preparation of NM dispersions for use in in vitro and ecotoxicity studies. However, the adoption and widespread implementation of these standardized protocols within the scientific community and relevant regulatory authorities is a necessary next step for risk assessment. Furthermore, such standardized dispersion methods should be accompanied by a full physicochemical characterization of the resulting NM dispersion, when further diluted into specific test media and, importantly, at regular intervals throughout the duration of the selected test. This is of particular relevance for NMs that exhibit a high rate of dissolution or a tendency to agglomerate and/or settle. These properties significantly influence the actual exposure to test organisms. These parameters need to be determined to gain a more accurate assessment of potential toxicity in aquatic environments as is true for human toxicity testing.34,184The development of analytical techniques suitable for determination of NM uptake, accumulation, and transformation in complex environmental and biological matrices is increasingly necessary to gain a complete picture of NM behavior and fate, transfer through the food chain, and for interpreting toxicity data. Standardization efforts already started in projects such as VAMAS,185 NanoDefine,186 or ACEnano,187 need to be continued and transferred into routine testing. As the toxicity of NMs may be modulated by the varying availability of ions depending on the material being tested, it is therefore important to include several toxicological endpoints to fully assess their aquatic ecotoxicity. This is in addition to the need for conducting multiple toxicity tests to inform environmental guidelines for NMs. In principal, the existing OECD test guidelines are suitable. However, some nanospecific test modifications are necessary29 and were recently proposed for the growth inhibition with the green algae Raphidocelis subcapitata (TG 201),188 acute toxicity with the crustacean Daphnia magna (TG 202),189 development toxicity with the fish Danio rerio (TG 210),190 and reproduction of the sediment-living worm Lumbriculus variegatus (TG 225).191
As previously described, the stability of test dispersions remains a key issue for NM aquatic toxicity testing. No additional synthetic organic solvents or dispersants should be used. If natural dispersants such as NOM are added, the lowest concentration suitable to achieve a stable distribution should be applied.192 We must consider NMs with unique physicochemical properties, such as photoactivity in the case of TiO2 NMs. This property can have a significant impact on the aquatic toxicity of NMs. These important factors need to be considered and incorporated into current regulatory standard ecotoxicity tests conducted for use in environmental risk assessment. Newly emerging NMs with complex multicomponent compositions, increased functionalization, and surface activities will present additional challenges for characterization and environmental assessment. Furthermore, the degradation of nanocomposites and subsequent release of embedded NMs require attention as these may exert adverse effects on the environment and human health. As we understand more about the possible interactions of NMs with other organic and inorganic substances present in aquatic matrices, there is an additional need for analytical tools to support studies in this field.
Abbreviations
| CNT | Carbon nanotube |
| MWCNT | Multi-walled carbon nanotube |
| NM | Nanomaterial |
| NOM | Natural organic matter |
| PAA | Polyacrylate-coated |
| PDMS | Polydimethylsiloxane |
| SEM | Scanning electron microscopy |
| SSA | Specific surface area |
| SOP | Standard operating procedure |
| SWCNT | Single-walled carbon nanotube |
| TEM | Transmission electron microscopy |
| UV/vis | Ultraviolet-visible |
| P25 | Aeroxide TiO2 |
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors acknowledge support from the EU FP7 project ‘NANoREG’ (Grant Agreement number 310584). The authors wish to thank this project for financial support of their research and for periodic teleconferences and meetings. Acknowledgements (A. M. Booth): Supported by Polish-Norwegian Research Programme (Project Contract No. Pol-Nor/237761/98/2014) and the Research Council of Norway (Contract No. 239199/O70). Acknowledgements (C. Cerrillo): Gemma Mendoza, Olatz Areitioaurtena, Amaya Igartua, Gotzone Barandika, and the ZABALDUZ Program for financing her contract with UPV/EHU in collaboration with IK4-TEKNIKER. Acknowledgements (A. Tlili): Participants in this research include Renata Behra, Julien Cornut, Carmen Gil-Allué, Mark. O. Gessner and Jeremy Jabiol. The project was supported by the Swiss National Science Foundation (SNF, grant no. 200020_134750/1). Acknowledgements (A. Schäffer): Participants in this research included Hanna Maes, Anne Wyrwoll, Alexander Bach, Henner Hollert, Maximilian Gruber, Petra Lautenschläger, Anne Meister-Werner and Ralph Petto.References
- E. Heister, E. W. Brunner, G. R. Dieckmann, I. Jurewicz and A. B. Dalton, ACS Appl. Mater. Interfaces, 2013, 5, 1870–1891 CAS.
- B. K. Kaushik and M. K. Majumder, in Carbon Nanotube Based VLSI Interconnects: Analysis and Design, Springer India, New Delhi, 2015, pp. 17–37 Search PubMed.
- T. Yadav, A. A. Mungray and A. K. Mungray, Reviews of Environmental Contamination and Toxicology, 2014, vol. 230, pp. 83–110 Search PubMed.
- S. W. Bennett, A. Adeleye, Z. Ji and A. A. Keller, Water Res., 2013, 47, 4074–4085 CrossRef CAS PubMed.
- C. P. Firme 3rd and P. R. Bandaru, Nanomedicine, 2010, 6, 245–256 CrossRef PubMed.
- A. Baun, N. B. Hartmann, K. Grieger and K. O. Kusk, Ecotoxicology, 2008, 17, 387–395 CrossRef CAS PubMed.
- X. Zhao and R. Liu, Environ. Int., 2012, 40, 244–255 CrossRef CAS PubMed.
- A. Menard, D. Drobne and A. Jemec, Environ. Pollut., 2011, 159, 677–684 CrossRef CAS PubMed.
- J. Mwangi, N. Wang, C. Ingersoll, D. Hardesty, E. Brunson and H. Li, Environ. Toxicol. Chem., 2012, 31, 1823–1830 CrossRef CAS PubMed.
- L. V. Stebounova, E. Guio and V. H. Grassian, J. Nanopart. Res., 2011, 13, 233–244 CrossRef CAS.
- J. Beddow, B. Stolpe, P. A. Cole, J. R. Lead, M. Sapp, B. P. Lyons, I. Colbeck and C. Whitby, Environ. Microbiol., 2017, 19(2), 500–510 CrossRef CAS PubMed.
- K. Li and Y. Chen, J. Hazard. Mater., 2012, 209–210, 264–270 CrossRef CAS PubMed.
- Z. F. Long, J. Ji, K. Yang, D. H. Lin and F. C. Wu, Environ. Sci. Technol., 2012, 46, 8458–8466 CrossRef CAS PubMed.
- J. Farkas and A. M. Booth, Nanotoxicology, 2017, 11, 569–577 CrossRef CAS PubMed.
- L. Verneuil, J. Silvestre, F. Mouchet, E. Flahaut, J. C. Boutonnet, F. Bourdiol, T. Bortolamiol, D. Baque, L. Gauthier and E. Pinelli, Nanotoxicology, 2015, 9, 219–229 CrossRef CAS PubMed.
- R. Tantra, C. Oksel, K. N. Robinson, A. Sikora, X. Z. Wang and T. A. Wilkins, Particuology, 2015, 22, 30–38 CrossRef.
- S. M. Cook, W. G. Aker, B. F. Rasulev, H.-M. Hwang, J. Leszczynski, J. J. Jenkins and V. Shockley, J. Hazard. Mater., 2010, 176, 367–373 CrossRef CAS PubMed.
- T. B. Henry, F.-M. Menn, J. T. Fleming, J. Wilgus, R. N. Compton and G. S. Sayler, Environ. Health Perspect., 2007, 115, 1059–1065 CrossRef CAS PubMed.
- R. Tantra, A. Sikora, N. B. Hartmann, J. R. Sintes and K. N. Robinson, Particuology, 2015, 19, 35–44 CrossRef CAS.
- R. D. Handy, G. Cornelis, T. Fernandes, O. Tsyusko, A. Decho, T. Sabo-Attwood, C. Metcalfe, J. A. Steevens, S. J. Klaine, A. A. Koelmans and N. Horne, Environ. Toxicol. Chem., 2012, 31, 15–31 CrossRef CAS PubMed.
- F. Laborda, E. Bolea, G. Cepria, M. T. Gomez, M. S. Jimenez, J. Perez-Arantegui and J. R. Castillo, Anal. Chim. Acta, 2016, 904, 10–32 CrossRef CAS PubMed.
- M. Li and C. Huang, Carbon, 2011, 49, 1672–1679 CrossRef CAS.
- M. D. Montano, G. V. Lowry, F. von der Kammer, J. Blue and J. F. Ranville, Environ. Chem., 2014, 11, 351–366 CrossRef CAS.
- N. B. Hartmann, K. A. Jensen, A. Baun, K. Rasmussen, H. Rauscher, R. Tantra, D. Cupi, D. Gilliland, F. Pianella and J. M. R. Sintes, J. Toxicol. Environ. Health, Part B, 2015, 18, 299–326 CAS.
- R. Tantra, D. Gohil and P. Cumpson, Particuology, 2009, 7, 471–476 CrossRef CAS.
- R. Tantra and A. Shard, Nat. Nanotechnol., 2013, 8, 71–71 CrossRef CAS PubMed.
- J. S. Taurozzi, V. A. Hackley and M. R. Wiesner, Nanotoxicology, 2011, 5, 711–729 CrossRef CAS PubMed.
- J. S. Taurozzi, V. A. Hackley and M. R. Wiesner, NIST Special Publication 1200-2, National Institute of Standards and Technology, 2012 Search PubMed.
- K. Hund-Rinke, A. Baun, D. Cupi, T. F. Fernandes, R. Handy, J. H. Kinross, J. M. Navas, W. Peijnenburg, K. Schlich, B. J. Shaw and J. J. Scott-Fordsmand, Nanotoxicology, 2016, 10, 1442–1447 CrossRef CAS PubMed.
- J. S. Taurozzi, V. A. Hackley and M. R. Wiesner, Nanotoxicology, 2011, 5, 711–729 CrossRef CAS PubMed.
- J. S. Taurozzi, V. A. Hackley and M. R. Wiesner, NIST Spec. Publ., 2012, 1200, 4 Search PubMed.
- C. J. E. Delmaar, W. J. G. M. Peijnenburg, A. G. Oomen, J. W. Chen, W. H. de Jong, A. J. A. M. Sips, Z. Wang and M. V. D. Z. Park, Environ. Toxicol. Chem., 2015, 34, 1015–1022 CrossRef CAS PubMed.
- H. M. Hwang, P. C. Ray, H. Yu and X. He, RSC Green Chem. Ser., 2013, 190–212, 10.1039/9781849735469-00190.
- P. Laux, C. Riebeling, A. M. Booth, J. D. Brain, J. Brunner, C. Cerrillo, O. Creutzenberg, I. Estrela-Lopis, T. Gebel, G. Johanson, H. Jungnickel, H. Kock, J. Tentschert, A. Tlili, A. Schäffer, A. J. A. M. Sips, R. A. Yokel and A. Luch, NanoImpact, 2017, 6, 69–80 CrossRef PubMed.
- S. J. Klaine, P. J. J. Alvarez, G. E. Batley, T. F. Fernandes, R. D. Handy, D. Y. Lyon, S. Mahendra, M. J. McLaughlin and J. R. Lead, Environ. Toxicol. Chem., 2008, 27, 1825–1851 CrossRef CAS PubMed.
- D. C. Antonio, C. Cascio, Z. Jaksic, D. Jurasin, D. M. Lyons, A. J. A. Nogueira, F. Rossi and L. Calzolai, Mar. Environ. Res., 2015, 111, 162–169 CrossRef CAS PubMed.
- M. D. Montano, H. R. Badiei, S. Bazargan and J. F. Ranville, Environ. Sci.: Nano, 2014, 1, 338–346 RSC.
- M. H. Shen, Y. G. Yin, A. Booth and J. F. Liu, Water Res., 2015, 71, 11–20 CrossRef CAS PubMed.
- X. G. Hu, A. Q. Sun, L. Mu and Q. X. Zhou, TrAC, Trends Anal. Chem., 2016, 80, 416–428 CrossRef CAS.
- J. Zhao, Z. Y. Wang, J. C. White and B. S. Xing, Environ. Sci. Technol., 2014, 48, 9995–10009 CrossRef CAS PubMed.
- L. Jiang, L. Gao and J. Sun, J. Colloid Interface Sci., 2003, 260, 89–94 CrossRef CAS PubMed.
- B. Smith, K. Wepasnick, K. E. Schrote, A. R. Bertele, W. P. Ball, C. O'Melia and D. H. Fairbrother, Environ. Sci. Technol., 2009, 43, 819–825 CrossRef CAS PubMed.
- B. Smith, K. Wepasnick, K. E. Schrote, H. H. Cho, W. P. Ball and D. H. Fairbrother, Langmuir, 2009, 25, 9767–9776 CrossRef CAS PubMed.
- P. Jackson, N. R. Jacobsen, A. Baun, R. Birkedal, D. Kuhnel, K. A. Jensen, U. Vogel and H. Wallin, Chem. Cent. J., 2013, 7, 154 CrossRef PubMed.
- B. Krause, M. Mende, P. Potschke and G. Petzold, Carbon, 2010, 48, 2746–2754 CrossRef CAS.
- K. L. Chen, B. A. Smith, W. P. Ball and D. H. Fairbrother, Environ. Chem., 2010, 7, 10–27 CrossRef CAS.
- B. Glomstad, D. Altin, L. Sorensen, J. Liu, B. M. Jenssen and A. M. Booth, Environ. Sci. Technol., 2016, 50, 2660–2668 CrossRef CAS PubMed.
- N. B. Saleh, L. D. Pfefferle and M. Elimelech, Environ. Sci. Technol., 2008, 42, 7963–7969 CrossRef CAS PubMed.
- S. T. Yang, H. Wang, Y. Wang, Y. Wang, H. Nie and Y. Liu, Chemosphere, 2011, 82, 621–626 CrossRef CAS PubMed.
- A. N. Giordano, H. Chaturvedi and J. C. Poler, J. Phys. Chem. C, 2007, 111, 11583–11589 CAS.
- H. Hyung, J. D. Fortner, J. B. Hughes and J. H. Kim, Environ. Sci. Technol., 2007, 41, 179–184 CrossRef CAS PubMed.
- H. Hyung and J. H. Kim, Environ. Sci. Technol., 2008, 42, 4416–4421 CrossRef CAS PubMed.
- K. T. Kim, A. J. Edgington, S. J. Klaine, J. W. Cho and S. D. Kim, Environ. Sci. Technol., 2009, 43, 8979–8984 CrossRef CAS PubMed.
- S. Kang, M. S. Mauter and M. Elimelech, Environ. Sci. Technol., 2009, 43, 2648–2653 CrossRef CAS PubMed.
- A. J. Edgington, A. P. Roberts, L. M. Taylor, M. M. Alloy, J. Reppert, A. M. Rao, J. Mao and S. J. Klaine, Environ. Toxicol. Chem., 2010, 29, 2511–2518 CrossRef CAS PubMed.
- C. Cerrillo, G. Barandika, A. Igartua, O. Areitioaurtena and G. Mendoza, Sci. Total Environ., 2016, 543, 95–104 CrossRef CAS PubMed.
- M. Erhayem and M. Sohn, Sci. Total Environ., 2014, 468, 249–257 CrossRef PubMed.
- F. Hennrich, R. Krupke, K. Arnold, J. A. Rojas Stutz, S. Lebedkin, T. Koch, T. Schimmel and M. M. Kappes, J. Phys. Chem. B, 2007, 111, 1932–1937 CrossRef CAS PubMed.
- A. Di Crescenzo, D. Demurtas, A. Renzetti, G. Siani, P. De Maria, M. Meneghetti, M. Prato and A. Fontana, Soft Matter, 2009, 5, 62–66 RSC.
- I. Schwyzer, R. Kaegi, L. Sigg, A. Magrez and B. Nowack, Environ. Pollut., 2011, 159, 1641–1648 CrossRef CAS PubMed.
- A. J. Kennedy, J. C. Gunter, M. A. Chappell, J. D. Goss, M. S. Hull, R. A. Kirgan and J. A. Steevens, Environ. Toxicol. Chem., 2009, 28, 1930–1938 CrossRef CAS PubMed.
- M. H. Li and C. P. Huang, Carbon, 2011, 49, 1672–1679 CrossRef CAS.
- K. W. Kwok, K. M. Leung, E. Flahaut, J. Cheng and S. H. Cheng, Nanomedicine, 2010, 5, 951–961 CrossRef CAS PubMed.
- C. Cerrillo, G. Barandika, A. Igartua, O. Areitioaurtena, A. Marcaide and G. Mendoza, Environ. Toxicol. Chem., 2015, 34, 1854–1862 CrossRef CAS PubMed.
- NANoREG, A common European approach to the regulatory testing of Manufactured Nanomaterials, http://www.nanoreg.eu/, (accessed 13.01.2017) Search PubMed.
- M. Vippola, G. C. Falck, H. K. Lindberg, S. Suhonen, E. Vanhala, H. Norppa, K. Savolainen, A. Tossavainen and T. Tuomi, Hum. Exp. Toxicol., 2009, 28, 377–385 CAS.
- P. Bihari, M. Vippola, S. Schultes, M. Praetner, A. G. Khandoga, C. A. Reichel, C. Coester, T. Tuomi, M. Rehberg and F. Krombach, Part. Fibre Toxicol., 2008, 5, 14 CrossRef PubMed.
- N. W. Kam, M. O'Connell, J. A. Wisdom and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 11600–11605 CrossRef CAS PubMed.
- Z. F. Li, G. H. Luo, W. P. Zhou, F. Wei, R. Xiang and Y. P. Liu, Nanotechnology, 2006, 17, 3692–3698 CrossRef CAS.
- C. Y. Khripin, X. Tu, J. Howarter, J. Fagan and M. Zheng, Anal. Chem., 2012, 84, 8733–8739 CrossRef CAS PubMed.
- K. Doudrick, P. Herckes and P. Westerhoff, Environ. Sci. Technol., 2012, 46, 12246–12253 CrossRef CAS PubMed.
- L. S. K. Pang, J. D. Saxby and S. P. Chatfield, J. Phys. Chem., 1993, 97, 6941–6942 CrossRef CAS.
- D. L. Plata, C. M. Reddy and P. M. Gschwend, Environ. Sci. Technol., 2012, 46, 12254–12261 CrossRef CAS PubMed.
- A. Sobek and T. D. Bucheli, Environ. Pollut., 2009, 157, 1065–1071 CrossRef CAS PubMed.
- H. S. Lee and C. H. Yun, J. Phys. Chem. C, 2008, 112, 10653–10658 CAS.
- V. C. Moore, M. S. Strano, E. H. Haroz, R. H. Hauge, R. E. Smalley, J. Schmidt and Y. Talmon, Nano Lett., 2003, 3, 1379–1382 CrossRef CAS.
- X. Wang, J. Lu and B. Xing, Environ. Sci. Technol., 2008, 42, 3207–3212 CrossRef CAS PubMed.
- K. Yang, Q. Jing, W. Wu, L. Zhu and B. Xing, Environ. Sci. Technol., 2010, 44, 681–687 CrossRef CAS PubMed.
- J. D. Zhang, A. Panwar, D. Bello, T. Jozokos, J. A. Isaacs, C. Barry and J. Mead, Environ. Sci.: Nano, 2016, 3, 409–417 RSC.
- M. Castellino, M. Rovere, M. I. Shahzad and A. Tagliaferro, in Composites Part A: Applied Science and Manufacturing, Elsevier, 1st edn., 2016, vol. 87, pp. 237–242 Search PubMed.
- S. Rhiem, M. J. Riding, W. Baumgartner, F. L. Martin, K. T. Semple, K. C. Jones, A. Schäffer and H. M. Maes, Environ. Pollut., 2015, 196, 431–439 CrossRef CAS PubMed.
- L. Golanski, A. Guiot, M. Pras, M. Malarde and F. Tardif, J. Nanopart. Res., 2012, 14, 962 CrossRef.
- L. Schlagenhauf, B. T. Chu, J. Buha, F. Nuesch and J. Wang, Environ. Sci. Technol., 2012, 46, 7366–7372 CrossRef CAS PubMed.
- V. K. K. Upadhyayula, D. E. Meyer, M. A. Curran and M. A. Gonzalez, J. Cleaner Prod., 2012, 26, 37–47 CrossRef CAS.
- S. Wagener, N. Dommershausen, H. Jungnickel, P. Laux, D. Mitrano, B. Nowack, G. Schneider and A. Luch, Environ. Sci. Technol., 2016, 50, 5927–5934 CrossRef CAS PubMed.
- D. M. Mitrano, Y. A. R. Dasilva and B. Nowack, Environ. Sci. Technol., 2015, 49, 9665–9673 CrossRef CAS PubMed.
- D. Gohler, A. Nogowski, P. Fiala and M. Stintz, J. Phys.: Conf. Ser., 2013, 429, 012045 CrossRef.
- L. Gendre, V. Marchante, H. A. Abhyankar, K. Blackburn, C. Temple and J. L. Brighton, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2016, 51, 495–501 CrossRef CAS PubMed.
- S. Hirth, L. Cena, G. Cox, Z. Tomovic, T. Peters and W. Wohlleben, J. Nanopart. Res., 2013, 15, 1504 CrossRef PubMed.
- T. Nguyen, B. Pellegrin, C. Bernard, X. Gu, J. M. Gorham, P. Stutzman, D. Stanley, A. Shapiro, E. Byrd, R. Hettenhouser and J. Chin, J. Phys.: Conf. Ser., 2011, 304, 012060 CrossRef.
- W. Wohlleben, M. W. Meier, S. Vogel, R. Landsiedel, G. Cox, S. Hirth and Z. Tomovic, Nanoscale, 2013, 5, 369–380 RSC.
- S. Rhiem, A. K. Barthel, A. Meyer-Plath, M. P. Hennig, V. Wachtendorf, H. Sturm, A. Schäffer and H. M. Maes, Environ. Pollut., 2016, 215, 356–365 CrossRef CAS PubMed.
- U. Kalbe, W. W. Muller, W. Berger and J. Eckardt, Appl. Clay Sci., 2002, 21, 67–76 CrossRef CAS.
- W. Wohlleben, S. Brill, M. W. Meier, M. Mertler, G. Cox, S. Hirth, B. von Vacano, V. Strauss, S. Treumann, K. Wiench, L. Ma-Hock and R. Landsiedel, Small, 2011, 7, 2384–2395 CrossRef CAS PubMed.
- D. X. Flores-Cervantes, H. M. Maes, A. Schäffer, J. Hollender and H. P. Kohler, Environ. Sci. Technol., 2014, 48, 4826–4834 CrossRef CAS PubMed.
- J. Garcia-Alonso, F. R. Khan, S. K. Misra, M. Turmaine, B. D. Smith, P. S. Rainbow, S. N. Luoma and E. Valsami-Jones, Environ. Sci. Technol., 2011, 45, 4630–4636 CrossRef CAS PubMed.
- T. L. Botha, K. Boodhia and V. Wepener, Aquat. Toxicol., 2016, 170, 104–111 CrossRef CAS PubMed.
- E. J. Petersen, J. Akkanen, J. V. K. Kukkonen and W. J. Weber, Environ. Sci. Technol., 2009, 43, 2969–2975 CrossRef CAS PubMed.
- P. Rosenkranz, Q. Chaudhry, V. Stone and T. F. Fernandes, Environ. Toxicol. Chem., 2009, 28, 2142–2149 CrossRef CAS PubMed.
- F. Zindler, B. Glomstad, D. Altin, J. F. Liu, B. M. Jenssen and A. M. Booth, Environ. Sci. Technol., 2016, 50, 12446–12454 CrossRef CAS PubMed.
- H. Jungnickel, P. Laux and A. Luch, Toxics, 2016, 4, 5 CrossRef PubMed.
- M. Holzweber, A. G. Shard, H. Jungnickel, A. Luch and W. E. S. Unger, Surf. Interface Anal., 2014, 46, 936–939 CrossRef CAS PubMed.
- L. Y. Tan, B. Huang, S. Xu, Z. B. Wei, L. Y. Yang and A. J. Miao, Environ. Sci. Technol., 2016, 50, 7799–7807 CrossRef CAS PubMed.
- S. R. Tangaa, H. Selck, M. Winther-Nielsen and F. R. Khan, Environ. Sci.: Nano, 2016, 3, 966–981 RSC.
- R. M. Goodhead, J. Moger, T. S. Galloway and C. R. Tyler, Nanotoxicology, 2015, 9, 928–939 CrossRef PubMed.
- S. W. Kim, J. I. Kwak and Y. J. An, Chemosphere, 2016, 144, 1763–1770 CrossRef CAS PubMed.
- M. Mortimer, A. Gogos, N. Bartolome, A. Kahru, T. D. Bucheli and V. I. Slaveykova, Environ. Sci. Technol., 2014, 48, 8760–8767 CrossRef CAS PubMed.
- T. M. Scown, R. M. Goodhead, B. D. Johnston, J. Moger, M. Baalousha, J. R. Lead, R. van Aerle, T. Iguchi and C. R. Tyler, Environ. Chem., 2010, 7, 36–49 CrossRef CAS.
- M. J. C. Van Der Ploeg, J. H. J. Van Den Berg, S. Bhattacharjee, L. H. J. De Haan, D. S. Ershov, R. G. Fokkink, H. Zuilhof, I. M. C. M. Rietjens and N. W. Van Den Brink, Nanotoxicology, 2014, 8, 28–37 CrossRef CAS PubMed.
- E. Petersen, J. Akkanen, J. Kukkonen and W. Weber, Environ. Sci. Technol., 2009, 43, 2969–2975 CrossRef CAS PubMed.
- S. Rhiem, M. J. Riding, W. Baumgartner, F. L. Martin, K. T. Semple, K. C. Jones, A. Schäffer and H. M. Maes, Environ. Pollut., 2015, 196, 431–439 CrossRef CAS PubMed.
- F. Zindler, B. Glomstad, D. Altin, J. Liu, B. M. Jenssen and A. M. Booth, Environ. Sci. Technol., 2016, 50, 12446–12454 CrossRef CAS PubMed.
- P. Jackson, N. Jacobsen, A. Baun, R. Birkedal, D. Kuhnel, K. Jensen, U. Vogel and H. Wallin, Chem. Cent. J., 2013, 7, 154 CrossRef PubMed.
- H. Selck, R. D. Handy, T. F. Fernandes, S. J. Klaine and E. J. Petersen, Environ. Toxicol. Chem., 2016, 35, 1055–1067 CrossRef CAS PubMed.
- P. Jackson, N. R. Jacobsen, A. Baun, R. Birkedal, D. Kuhnel, K. A. Jensen, U. Vogel and H. Wallin, Chem. Cent. J., 2013, 7, 154 CrossRef PubMed.
- M. Mortimer, E. J. Petersen, B. A. Buchholz, E. Orias and P. A. Holden, Environ. Sci. Technol., 2016, 50, 8876–8885 CrossRef CAS PubMed.
- M. Mortimer, E. J. Petersen, B. A. Buchholz and P. A. Holden, Nanomaterials, 2016, 6, 10 CrossRef PubMed.
- E. Oberdorster, S. Q. Zhu, T. M. Blickley, P. McClellan-Green and M. L. Haasch, Carbon, 2006, 44, 1112–1120 CrossRef.
- H. M. Maes, F. Stibany, S. Giefers, B. Daniels, B. Deutschmann, W. Baumgartner and A. Schäffer, Environ. Sci. Technol., 2014, 48, 12256–12264 CrossRef CAS PubMed.
- J. Zhao, F. F. Liu, Z. Y. Wang, X. S. Cao and B. S. Xing, Environ. Sci. Technol., 2015, 49, 2849–2857 CrossRef CAS PubMed.
- Q. Zhao, E. J. Petersen, G. Cornelis, X. L. Wang, X. Y. Guo, S. Tao and B. S. Xing, Carbon, 2016, 99, 229–237 CrossRef CAS PubMed.
- R. S. Chouhan, A. Qureshi, B. Yagci, M. A. Gulgun, V. Ozguz and J. H. Niazi, Chem. Eng. J., 2016, 298, 1–9 CrossRef CAS.
- G. Chandrasekaran, S. K. Choi, Y. C. Lee, G. J. Kim and H. J. Shin, J. Ind. Eng. Chem., 2014, 20, 3367–3374 CrossRef CAS.
- E. J. Petersen and T. B. Henry, Environ. Toxicol. Chem., 2012, 31, 60–72 CrossRef CAS PubMed.
- B. F. G. Pycke, T. C. Chao, P. Herckes, P. Westerhoff and R. U. Halden, Anal. Bioanal. Chem., 2012, 404, 2583–2595 CrossRef CAS PubMed.
- F. von der Kammer, P. L. Ferguson, P. A. Holden, A. Masion, K. R. Rogers, S. J. Klaine, A. A. Koelmans, N. Horne and J. M. Unrine, Environ. Toxicol. Chem., 2012, 31, 32–49 CrossRef CAS PubMed.
- E. J. Petersen, D. X. Flores-Cervantes, T. D. Bucheli, L. C. C. Elliott, J. A. Fagan, A. Gogos, S. Hanna, R. Kagi, E. Mansfield, A. R. M. Bustos, D. L. Plata, V. Reipa, P. Westerhoff and M. R. Winchester, Environ. Sci. Technol., 2016, 50, 4587–4605 CrossRef CAS PubMed.
- J. R. Conway, S. K. Hanna, H. S. Lenihan and A. A. Keller, Environ. Sci. Technol., 2014, 48, 1517–1524 CrossRef CAS PubMed.
- A. Booth, T. Storseth, D. Altin, A. Fornara, A. Ahniyaz, H. Jungnickel, P. Laux, A. Luch and L. Sorensen, Sci. Total Environ., 2015, 505, 596–605 CrossRef CAS PubMed.
- M. Auffan, D. Bertin, P. Chaurand, C. Pailles, C. Dominici, J. Rose, J. Y. Bottero and A. Thiery, Water Res., 2013, 47, 3921–3930 CrossRef CAS PubMed.
- C. Tan and W. X. Wang, Environ. Pollut., 2014, 186, 36–42 CrossRef CAS PubMed.
- L. Y. Tan, B. Huang, S. Xu, Z. B. Wei, L. Y. Yang and A. J. Miao, Environ. Sci. Technol., 2017, 51, 932–939 CrossRef CAS PubMed.
- R. Bacchetta, N. Santo, M. Marelli, G. Nosengo and P. Tremolada, Environ. Res., 2017, 152, 128–140 CrossRef CAS PubMed.
- C. P. Vignardi, F. M. Hasue, P. V. Sartorio, C. M. Cardoso, A. S. D. Machado, M. J. A. C. R. Passos, T. C. A. Santos, J. M. Nucci, T. L. R. Hewer, I. S. Watanabe, V. Gomes and N. V. Phan, Aquat. Toxicol., 2015, 158, 218–229 CrossRef CAS PubMed.
- P. J. Chen, W. L. Wu and K. C. W. Wu, Water Res., 2013, 47, 3899–3909 CrossRef CAS PubMed.
- X. S. Zhu, S. Y. Tian and Z. H. Cai, PLoS One, 2012, 7, e46286 CAS.
- J. Oprsal, L. Blaha, M. Pouzar, P. Knotek, M. Vlcek and K. Hrda, Environ. Sci. Pollut. Res., 2015, 22, 19124–19132 CrossRef CAS PubMed.
- M. Geppert, L. Sigg and K. Schirmer, Environ. Sci.: Nano, 2016, 3, 388–395 RSC.
- S. K. Hanna, R. J. Miller and H. S. Lenihan, Nanomaterials, 2014, 4, 535–547 CrossRef PubMed.
- M. Bundschuh, F. Seitz, R. R. Rosenfeldt and R. Schulz, Freshwater Biol., 2016, 61, 2185–2196 CrossRef.
- M. O. Gessner and A. Tlili, Freshwater Biol., 2016, 61, 1991–2001 CrossRef.
- E. Navarro, B. Wagner, N. Odzak, L. Sigg and R. Behra, Environ. Sci. Technol., 2015, 49, 8041–8047 CrossRef CAS PubMed.
- C. N. Lok, C. M. Ho, R. Chen, Q. Y. He, W. Y. Yu, H. Sun, P. K. H. Tam, J. F. Chiu and C. M. Che, J. Biol. Inorg. Chem., 2007, 12, 527–534 CrossRef CAS PubMed.
- Z. M. Xiu, Q. B. Zhang, H. L. Puppala, V. L. Colvin and P. J. J. Alvarez, Nano Lett., 2012, 12, 4271–4275 CrossRef CAS PubMed.
- L. A. Rohder, T. Brandt, L. Sigg and R. Behra, Aquat. Toxicol., 2014, 152, 121–130 CrossRef CAS PubMed.
- I. Rodea-Palomares, K. Boltes, F. Fernandez-Pinas, F. Leganes, E. Garcia-Calvo, J. Santiago and R. Rosal, Toxicol. Sci., 2011, 119, 135–145 CrossRef CAS PubMed.
- M. Bundschuh, F. Seitz, R. R. Rosenfeldt and R. Schulz, Freshwater Biol., 2016, 61, 2185–2196 CrossRef.
- C. Gil-Allué, K. Schirmer, A. Tlili, M. O. Gessner and R. Behra, Environ. Sci. Technol., 2015, 49, 1165–1172 CrossRef PubMed.
- A. Tlili, J. Cornut, R. Behra, C. Gil-Allue and M. O. Gessner, Nanotoxicology, 2016, 10, 728–735 CrossRef CAS PubMed.
- O. J. Osborne, K. Mukaigasa, H. Nakajima, B. Stolpe, I. Romer, U. Philips, I. Lynch, S. Mourabit, S. Hirose, J. R. Lead, M. Kobayashi, T. Kudoh and C. R. Tyler, Nanotoxicology, 2016, 10, 1276–1286 CrossRef CAS PubMed.
- L. Sigg and U. Lindauer, Environ. Pollut., 2015, 206, 582–587 CrossRef CAS PubMed.
- Y. Hu, X. Song, S. M. Jiang and C. H. Wei, Chem. Eng. J., 2015, 274, 102–112 CrossRef CAS.
- S. Yin, B. Liu, P. L. Zhang, T. Morikawa, K. Yamanaka and T. Sato, J. Phys. Chem. C, 2008, 112, 12425–12431 CAS.
- S. Yin, Q. W. Zhang, F. Saito and T. Sato, Chem. Lett., 2003, 32, 358–359 CrossRef CAS.
- A. J. Wyrwoll, P. Lautenschlager, A. Bach, B. Hellack, A. Dybowska, T. A. Kuhlbusch, H. Hollert, A. Schäffer and H. M. Maes, Environ. Pollut., 2016, 208, 859–867 CrossRef CAS PubMed.
- J. S. Angelstorf, W. Ahlf, F. von der Kammer and S. Heise, Environ. Toxicol. Chem., 2014, 33, 2288–2296 CrossRef CAS PubMed.
- W. M. Lee and Y. J. An, Chemosphere, 2013, 91, 536–544 CrossRef CAS PubMed.
- H. Ma, N. J. Kabengi, P. M. Bertsch, J. M. Unrine, T. C. Glenn and P. L. Williams, Environ. Pollut., 2011, 159, 1473–1480 CrossRef CAS PubMed.
- N. R. Brun, M. Lenz, B. Wehrli and K. Fent, Sci. Total Environ., 2014, 476, 657–666 CrossRef PubMed.
- S. George, H. Gardner, E. K. Seng, H. Chang, C. Y. Wang, C. H. Y. Fang, M. Richards, S. Valiyayeettil and W. K. Chan, Environ. Sci. Technol., 2014, 48, 6374–6382 CrossRef CAS PubMed.
- Y. J. Shin, S. H. Nam and Y. J. An, Environ. Toxicol. Chem., 2016, 35, 1195–1200 CrossRef CAS PubMed.
- F. Seitz, R. R. Rosenfeldt, K. Storm, G. Metreveli, G. E. Schaumann, R. Schulz and M. Bundschuh, Ecotoxicol. Environ. Saf., 2015, 111, 263–270 CrossRef CAS PubMed.
- S. B. Li, X. Pan, L. K. Wallis, Z. Y. Fan, Z. L. Chen and S. A. Diamond, Chemosphere, 2014, 112, 62–69 CrossRef CAS PubMed.
- S. Sakthivel and H. Kisch, Angew. Chem., Int. Ed., 2003, 42, 4908–4911 CrossRef CAS PubMed.
- Y. M. Lin, Y. H. Tseng, J. H. Huang, C. C. Chao, C. C. Chen and I. Wang, Environ. Sci. Technol., 2006, 40, 1616–1621 CrossRef CAS PubMed.
- K. Hashimoto, K. Sumida, S. Kitano, K. Yamamoto, N. Kondo, Y. Kera and H. Kominami, Catal. Today, 2009, 144, 37–41 CrossRef CAS.
- Y. Bessekhouad, D. Robert and J. Weber, J. Photochem. Photobiol., A, 2004, 163, 569–580 CrossRef CAS.
- Q. Sun and Y. M. Xu, J. Phys. Chem. C, 2009, 113, 12387–12394 CAS.
- H. Zhang, X. J. Lv, Y. M. Li, Y. Wang and J. H. Li, ACS Nano, 2010, 4, 380–386 CrossRef CAS PubMed.
- K. Woan, G. Pyrgiotakis and W. Sigmund, Adv. Mater., 2009, 21, 2233–2239 CrossRef CAS.
- R. Quesada-Cabrera, C. Sotelo-Vazqueza, M. Quesada-Gonzalez, E. P. Melian, N. Chadwick and I. P. Parkin, J. Photochem. Photobiol., A, 2017, 333, 49–55 CrossRef CAS.
- V. D. Binas, K. Sambani, T. Maggos, A. Katsanaki and G. Kiriakidis, Appl. Catal., B, 2012, 113, 79–86 CrossRef.
- N. U. Saqib, R. Adnan and I. Shah, Environ. Sci. Pollut. Res., 2016, 23, 15941–15951 CrossRef CAS PubMed.
- J. Zhang, X. J. Wang, Y. J. Bu, X. Wang, J. K. Song, P. Xia, R. R. Ma, B. Louangsouphom, S. Ma and J. F. Zhao, Chem. Eng. J., 2016, 306, 460–470 CrossRef CAS.
- M. C. Wen, S. S. Zhang, W. R. Dai, G. S. Li and D. Q. Zhang, Chin. J. Catal., 2015, 36, 2095–2102 CrossRef CAS.
- M. V. Liga, E. L. Bryant, V. L. Colvin and Q. L. Li, Water Res., 2011, 45, 535–544 CrossRef CAS PubMed.
- B. Schafer, J. V. Brocke, A. Epp, M. Gotz, F. Herzberg, C. Kneuer, Y. Sommer, J. Tentschert, M. Noll, I. Gunther, U. Banasiak, G. F. Bol, A. Lampen, A. Luch and A. Hensel, Arch. Toxicol., 2013, 87, 2249–2262 CrossRef PubMed.
- H. Naatz, S. J. Lin, R. B. Li, W. Jiang, Z. X. Ji, C. H. Chang, J. Koser, J. Thoming, T. Xia, A. E. Nel, L. Madler and S. Pokhrel, ACS Nano, 2017, 11, 501–515 CrossRef CAS PubMed.
- S. George, S. Pokhrel, T. Xia, B. Gilbert, Z. X. Ji, M. Schowalter, A. Rosenauer, R. Damoiseaux, K. A. Bradley, L. Madler and A. E. Nel, ACS Nano, 2010, 4, 15–29 CrossRef CAS PubMed.
- X. J. He, W. G. Aker and H. M. Hwang, Nanotoxicology, 2014, 8, 185–195 CrossRef CAS PubMed.
- J. T. K. Quik, M. C. Stuart, M. Wouterse, W. Peijnenburg, A. J. Hendriks and D. van de Meent, Environ. Toxicol. Chem., 2012, 31, 1019–1022 CrossRef CAS PubMed.
- H. T. Lu, H. F. Dong, W. H. Fan, J. X. Zuo and X. M. Li, J. Hazard. Mater., 2017, 325, 113–119 CrossRef CAS PubMed.
- J. Labille, J. H. Feng, C. Botta, D. Borschneck, M. Sammut, M. Cabie, M. Auffan, J. Rose and J. Y. Bottero, Environ. Pollut., 2010, 158, 3482–3489 CrossRef CAS PubMed.
- D. Lichtenstein, J. Ebmeyer, P. Knappe, S. Juling, L. Böhmert, S. Selve, B. Niemann, A. Braeuning, A. F. Thünemann and A. Lampen, Biol. Chem., 2015, 396, 1255–1264 CrossRef CAS PubMed.
- VAMAS, Versailles Project on Advanced Materials and Standards, http://www.vamas.org/, (accessed 29.06.2017) Search PubMed.
- NanoDefine, NanoDefine: An integrated analytical approach to implement the EC definition of nanomaterial, http://www.nanodefine.eu/, (accessed 29.06.2017) Search PubMed.
- ACEnano, Analytical and Characterization Excellence, http://www.acenano-project.eu/, (accessed 29.06.2017) Search PubMed.
- OECD, TG 201. Freshwater Alga and Cyanobacteria, Growth Inhibition Test. OECD Guidelines for the Testing of Chemicals, Section 2. Paris, OECD Publishing., http://www.oecdilibrary.org/docserver/download/9720101e.pdf?expires¼1439305673&id¼id&accname¼guest&checksum¼3D13BDDF0E048F8EB821BAC54A807EE4 Search PubMed.
- OECD, TG 202. Daphnia sp Acute Immobilization Test. OECD Guidelines for the Testing of Chemicals, Section 2. Paris, OECD Publishing., http://www.oecd-ilibrary.org/environment/test-no-202-daphnia-sp-acute-immobilisation-test_9789264069947-en Search PubMed.
- OECD, TG 210. Fish, Early-life Stage Toxicity Test. OECD Guidelines for the Testing of Chemicals, Section 2. Paris, OECD Publishing., http://www.oecd-ilibrary.org/environment/test-no-210-fish-early-life-stage-toxicity-test_9789264203785-en Search PubMed.
- OECD, TG 225. Sediment Water Lumbriculus Toxicity Test Using Spiked Sediment. OECD Guidelines for the Testing of Chemicals, Section 2. Paris, OECD Publishing., http://www.oecd-ilibrary.org/docserver/download/9722501e.pdf?expires¼1439305457&id¼id&accname¼guest&checksum¼723742ECB5D9439F263B7FF83230FF7C Search PubMed.
- E. J. Petersen, S. A. Diamond, A. J. Kennedy, G. G. Goss, K. Ho, J. Lead, S. K. Hanna, N. B. Hartmann, K. Hund-Rinke, B. Mader, N. Manier, P. Pandard, E. R. Salinas and P. Sayre, Environ. Sci. Technol., 2015, 49, 9532–9547 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |