Open Access Article
Open Access ArticleMining legacy across a wetland landscape: high mercury in Upper Peninsula (Michigan) rivers, lakes, and fish
W. Charles
Kerfoot
 *a,
Noel R.
Urban
*a,
Noel R.
Urban
 b,
Cory P.
McDonald
b,
Cory P.
McDonald
 b,
Huanxin
Zhang
c,
Ronald
Rossmann
b,
Huanxin
Zhang
c,
Ronald
Rossmann
 d,
Judith A.
Perlinger
d,
Judith A.
Perlinger
 b,
Tanvir
Khan
b,
Ashley
Hendricks
b,
Mugdha
Priyadarshini
b and
Morgan
Bolstad
b
b,
Tanvir
Khan
b,
Ashley
Hendricks
b,
Mugdha
Priyadarshini
b and
Morgan
Bolstad
b
aDepartment of Biological Sciences, Michigan Technological University, Houghton, MI 49931, USA. E-mail: wkerfoot@mtu.edu
bDepartment of Civil & Environmental Engineering, Michigan Technological University, Houghton, MI 49931, USA
cDepartment of Geological & Mining Engineering & Sciences, Michigan Technological University, Houghton, MI 49931, USA
dVisiting Scientist, U.S. EPA, Mid-Continent Ecology Division, Large Lakes Research Station, Grosse Ile, MI 48138, USA
First published on 29th March 2018
Abstract
A geographic enigma is that present-day atmospheric deposition of mercury in the Upper Peninsula of Michigan is low (48%) and that regional industrial emissions have declined substantially (ca. 81% reduction) relative to downstate. Mercury levels should be declining. However, state (MDEQ) surveys of rivers and lakes revealed elevated total mercury (THg) in Upper Peninsula waters and sediment relative to downstate. Moreover, Western Upper Peninsula (WUP) fish possess higher methyl mercury (MeHg) levels than Northern Lower Peninsula (NLP) fish. A contributing explanation for elevated THg loading is that a century ago the Upper Peninsula was a major industrial region, centered on mining. Many regional ores (silver, copper, zinc, massive sulfides) contain mercury in part per million concentrations. Copper smelters and iron furnace-taconite operations broadcast mercury almost continuously for 140 years, whereas mills discharged tailings and old mine shafts leaked contaminated water. We show that mercury emissions from copper and iron operations were substantial (60–650 kg per year) and dispersed over relatively large areas. Moreover, lake sediments in the vicinity of mining operations have higher THg concentrations. Sediment profiles from the Keweenaw Waterway show that THg accumulation increased 50- to 400-fold above modern-day atmospheric deposition levels during active mining and smelting operations, with lingering MeHg effects. High MeHg concentrations are geographically correlated with low pH and dissolved organic carbon (DOC), a consequence of biogeochemical cycling in wetlands, characteristic of the Upper Peninsula. DOC can mobilize metals and elevate MeHg concentrations. We argue that mercury loading from mining is historically superimposed upon strong regional wetland effects, producing a combined elevation of both THg and MeHg in the Western Upper Peninsula.
Environmental significanceThe manuscript addresses the enigma of low atmospheric mercury deposition and falling emissions in modern-day Upper Peninsula environments, yet elevated THg and MeHg in rivers, lakes, and fish. For the first time, we reconstruct 140 years of historical mercury emissions from copper and iron mining, showing how mercury was broadcast broadly around regional environments up to the present. We compare historical deposition rates with a combination of modeling and sediment core studies. Mining discharges (smelter emissions, tailing releases, mine shaft seepage) appear superimposed upon high wetland methylation. With forest recovery, wetlands are becoming even more abundant. Rather than mercury concentrations in piscivorous fish declining due to reduced atmospheric inputs, we observe 1–3% increases. We show historically how the substantial mining inputs are superimposed upon wetland rebound with time delays in MeHg production, helping explain some of the curious reversals. |
Introduction
Mercury contamination of the environment from human activity continues to be a global problem.1–3 In 2015, 36 statewide mercury advisories were issued in the United States for freshwater fish from lakes or rivers.4 The extent of the problem is pervasive, as the 2010 National Listing of Fish Advisories included 4598 advisories that covered around 7.16 million hectares of lake area and 2.09 million km of river stretches, equivalent to 42 percent of the nation's total lake area and 36 percent of the nation's total river network. Four major medical and public health groups, as well as 13 states, submitted lawsuits that claim the U.S. government is not doing enough to protect people from mercury pollution.4In the state of Michigan, there is an intriguing geographic enigma. Due to intense monitoring activities of the National Atmospheric Deposition Program (NADP) over the past decade, much is now known about background atmospheric deposition of mercury in the Great Lakes region. The estimated rate of regional atmospheric wet deposition of total mercury (THg) is about 4–8 μg m−2 per year across the Upper Peninsula of Michigan, Wisconsin, and northern Minnesota (Fig. 1a, Table 1).5–7 The Upper Peninsula values contrast with higher deposition downstate, closer to present-day industrial sources (10.1–12.0 μg m−2 per year).7 Over a 10 year period (1994–2003), THg wet deposition was 2.1× greater in the Lower Peninsula of Michigan (Dexter, MI, near Detroit) than at the Eagle Harbor monitoring station in the Keweenaw Peninsula.7,8 Gross atmospheric deposition remains harder to estimate, although modeling suggests ranges for gross deposition at around 5–20 μg m−2 per year for the Upper Peninsula (Fig. 1b; wet + dry). Again, values are higher downstate (20–30 μg m−2 per year).9,10 If lakes and biota are responding primarily to modern-day atmospheric deposition, mercury levels should be lower in the Upper Peninsula and higher in the Lower Peninsula.
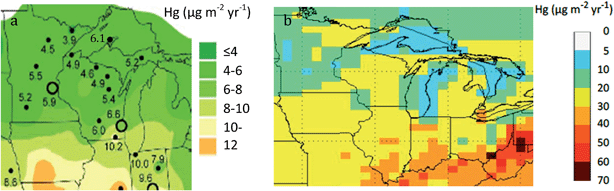 | ||
| Fig. 1 Modern-day atmospheric mercury deposition in the states of Minnesota, Wisconsin, and Michigan: (a) left panel shows wet deposition from National Atmospheric Deposition Program (NADP), with median values from monitoring sites (after Gay9); (b) right panel shows modeled estimates for gross deposition in the Great Lakes Region, following Zhang et al.10 | ||
| Location | N | Range | Mean ± 95%C.L. | Source |
|---|---|---|---|---|
| a Focusing corrected, 137-Cesium. | ||||
| Atmospheric NADP | ||||
| Lake Superior Basin | 8 | 4–5 | 4.9 ± 0.5 | 9 |
| Lake Superior region | 6 | 6–8 | 6.6 ± 0.5 | 11 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Lake Sediments | ||||
| Wisconsin Lakes | 7 | 5–9 | 6.7 ± 0.5 | 14 |
| WUP Lakes (“undisturbed”) | 16 | 5–24 | 11 ± 4 | 18 |
| N. Minnesota Lakes | 16 | 15–95 | 39 ± 23 | 16 |
| Lake Superior | 6 | 1–70 | 27 ± 26 | 17 |
| Lake Superior | 20 | 1–100 | 32 ± 11 | 85 |
| Lake Superior, Keweenaw “halo” | 19 | 8–100 | 39 ± 14a | 13 |
| Lake Superior, Keweenaw “halo” | 19 | 10–360 | 73 ± 48 | 13 |
| Keweenaw Waterway | 14 | 21–411 | 109 ± 69 | 13 |
Moreover, Lake Superior Basin mercury emissions have declined substantially over the past 30 years as primary sources have curtailed operations. In the 1990's, dominant emissions came from mining, although not all companies reported releases. A preliminary study of 1994 estimated four metric tonnes per year total emissions from mining operations.18 Dominant sources included three gold mines at Hemlo, Ontario (Golden Giant, ca. 998 kg per year; David Bell, 432 kg per year; Williams, 995 kg per year). High mercury incidence in Hemlo ores was well documented. Gold grains contained 6.5% mercury at Golden Giant, 11.6% at David Bell, and 5.7% at Williams. Golden Giant concentrate contained 6.1% mercury, with up to 29.5% mercury in sphalerite.19–21 Other significant regional emitters included: (1) the Algoma Sintering Plant (ca. 600 kg THg per year) at Wawa, Ontario; (2) White Pine smelter stack (640 kg per year) on the Keweenaw Peninsula, Michigan; and (3) accumulative Taconite Pellet Plant emissions (354 kg per year) from Minnesota.22–24 The Lake Superior Lakewide Management Plan (LaMP) estimated early 1990's basin mercury emissions (without Hemlo and Manitouwadge) originally at 1516 kg per year, with fuel combustion at 263 kg per year and incineration at 86 kg per year.25 Because of workplace contamination from mercury leaking back along induction furnace ducts, Hemlo operations decided to ship untreated concentrate out of the region after 1996 (to Johnson-Matthey, Salt Lake City).21 By 2015, only the Williams Mine remained operating at Hemlo, and both the Wawa Sinter Plant and White Pine Smelter closed down. Recently Minnesota total taconite emissions decreased to around 260 kg per year. Revised LaMP mercury emission estimates for 1990 are now 2.1 tonnes, declining to 0.4 tonnes by 2010, i.e. an estimated reduction of 81%.26 Mining is still recognized by the Lake Superior Binational Program (LSBP) as the single largest source of basin emissions, contributing around 63% of the recognized total.26
Reduction of regional mercury emission was expected to lead to declining mercury levels in fish. The Michigan Department of Environmental Quality (MDEQ) conducted a mercury water quality survey of 184 lakes and 84 streams/rivers in Michigan during 2001–2, comparing Upper Peninsula environments with the Lower Peninsula.27 As summarized by Gary Kohlhepp at the Romulus Workshop in 2006, the agency found “…mercury levels generally higher in the Upper Peninsula. Mercury…exceeded the Rule 57 Water Quality Value in 35% of Upper Peninsula lakes versus 8% of Lower Peninsula lakes.” Follow-up studies were carried out between 2005–2009. The sampling design included 250 randomly chosen sites, sampled at a rate of 50 sites each year over a 5 year period. Results from that survey were published only recently.28 Investigations confirmed that most parameters in the study (e.g. total phosphorus, chlorides, CaCO3 hardness) followed a pattern of decreased concentrations in the Upper Peninsula and northern Lower Peninsula with increased concentrations in the south and southeast Lower Peninsula. Yet two parameters, total mercury (THg) and dissolved organic carbon (DOC), followed a different, correlated pattern (Fig. 2). Some of the highest mercury concentrations were found in the Upper Peninsula portion of the Northern Lakes and Forests ecosystem. The unanticipated results from the 2001–2009 surveys prompted additional 2007–2013 investigations of lake sediments and fish, the results of which are reviewed here. In retrospect, some would have said that a strong correlation between waterborne Hg and DOC is well known for lakes in North America, attributed largely to organic complexation that increases the residence time of Hg in the water column (see Discussion). But at the time, the emergence of such strong regional patterns still spurred debate.
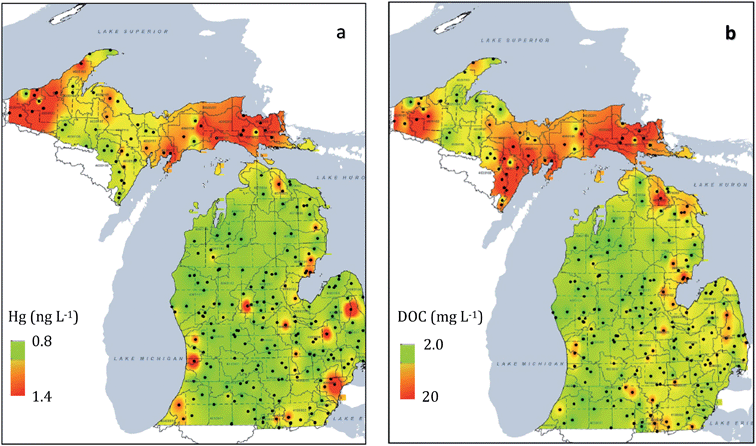 | ||
| Fig. 2 Results from 2005–2009 MDEQ surveys28 of Upper Peninsula and Lower Peninsula rivers and lakes, where the scale ranges from red (high) to green (low): (a) elevated mercury concentrations in Upper Peninsula rivers and lakes compared with the Lower Peninsula; (b) elevated patterns for DOC in Upper Peninsula rivers and lakes, compared with the Lower Peninsula. Scale derived from an inverse distance weighting interpolation technique28 applied to 250 sites. | ||
There are numerous hypotheses that could address the north-south Peninsula contrast, with resolution unclear at the moment. Some hypotheses vying for priority include: wetland prominence (which includes organic complexation along with activity of sulfate- and iron-reducing bacteria, principal methylators in anoxic zones, see Discussion),29–31 northern fish are older and grow more slowly,32 southern agricultural eutrophication dilutes MeHg food-web bioaccumulation,33 and MeHg bioaccumulation factors vary with latitude.34 Given that mining was so pervasive in the Lake Superior watershed, an additional regional variable centers on mercury in natural rock formations, historic mining releases (“legacy” effects),13,18,35 and 20–40 year lag times in watershed methylation.12 The regional challenge is to integrate knowledge about mining effects into a growing body of research about wetlands and biochemical cycling of mercury. The MDEQ concerns involve both regional loading of mercury (high THg in rivers, lakes, watersheds) and potentially associated elevated MeHg levels in fishes. Here we begin to address historical aspects of mercury loading due to mining in the Upper Peninsula and explore the association with MeHg levels in fishes. A major complication is that MeHg concentrations in fish depend not only on THg load but also on food web structure (which governs biomagnification) and biogeochemistry (which governs methylation).
What is the effect of mercury in naturally occurring rock formations, and what are the accumulative effects of a century and a half of mining releases scattered around the Upper Peninsula landscape? Cumulative impacts of mercury sources distributed to the environment are a challenge to assess geographically, but seem an increasingly important component in modern regional studies.36–38 One way to emphasize the pervasive influence of mining across the western Upper Peninsula is to plot the extensive distribution of mine shafts and smelters (Fig. 3; modified from Al Johnson, Michigan Tech Archives, and Kerfoot et al.39). To the west are the over 140 native copper mining sites and five smelters along the Keweenaw mid-rib, whereas to the south are the chalcosite copper and silver mines and one smelter (White Pine) of the Porcupine Copper–Silver District and, somewhat lower, the Gogebic Range Iron District. To the east is the Marquette Gold District, and further southeast and east are the hundreds of locations within the Baraga, Republic, Marquette, Gwinn, and Menominee Iron Ranges. The entire region was industrially active for over a century, economically rivaling downstate.40,41 Greater knowledge of contributing legacy sources and better insight into the roles of regional wetland methylation and ecosystem mercury cycling should help resolve key aspects of the north-south enigma. The connection to DOC concentrations in streams, rivers, and lakes suggests that wetlands are an integral component of the geographic patterns, although the exact nature of the interaction between wetlands and legacy mining requires additional study (see Discussion).
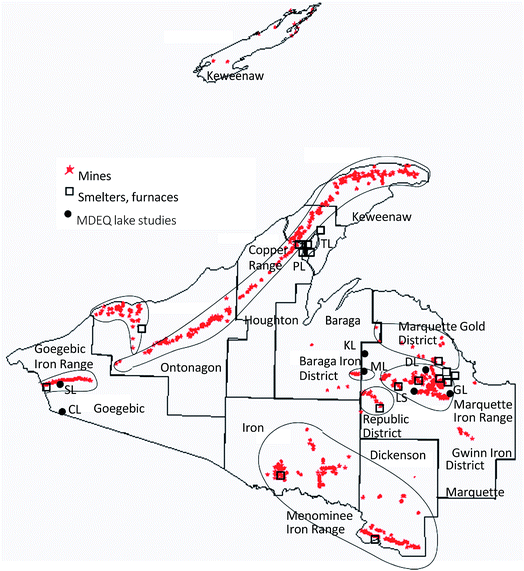 | ||
| Fig. 3 Historic mining regions and shaft locations across the Western Upper Peninsula of Michigan (modified from an Al Johnson plot, Michigan Tech Archives; and Kerfoot et al.39). Large squares superimposed near shaft locations mark sites of copper smelters, taconite plant operations, and early pig-iron furnaces. Solid dots with initials mark locations of specific THg lake sediment samples referred to in “Lake comparisons” section. | ||
Here we discuss mercury in copper and silver ores and calculate emissions and deposition in the vicinity of copper and iron mining operations. Natural bedrock outcrop effects and releases in gold mining districts are treated elsewhere. We address an old but important question: how does atmospheric deposition stack up against other factors affecting fish contamination? Historical smelter emissions are reconstructed from regional sources, and sediment mercury concentrations in lakes and rivers near mines are compared with similar measurements from more isolated locations. We also document the higher concentrations of MeHg in Western Upper Peninsula fish. In general, the regional geographic patterns suggest significant mining release superimposed upon a broad pattern of deposition over recovering forest and wetlands. The historical mining-wetland interaction poses an intriguing regional ecosystem challenge, but one that appears amenable to eventual resolution.
Methods and data sources
Mercury concentrations in Upper Peninsula mine ores
Mercury, silver, gold, copper, lead, and zinc are all similar with respect to their formation and geological occurrence. In the elemental state (Hg0), mercury can form natural amalgams with gold, silver, or copper–silver mixtures. An amalgam form “arguerite”, or mercurian silver (Ag12Hg), was previously documented at the Silver Islet Mine near Ontario's Sibley Peninsula, on Thunder Bay.42 Silver is characteristic of Lake Superior native copper lodes, producing the historically valued “Lake Copper” alloy.43 Mercury is also found in sulfide ores such as White Pine's chalcocite, Eagle Mine's copper–nickel Volcanogenic Massive Sulfide (VMS), and the proposed Back Forty's zinc–copper–lead VMS.13,44,45 Mercury generally occurs in low concentrations (<1 μg g−1) in iron ores, occurring both in association with sulfide (pyrite) and otherwise.17To identify the primary source of mercury associated with U.P. copper and silver mining, ore samples were previously collected from several mine sites.12 Subsamples were digested in a Milestone Thos 900 microwave digester, and analyzed for total mercury by the cold vapor technique using a Perkin-Elmer Model 5000 and a Perkin-Elmer MHS-10 mercury–hydride system. For every set of 10 samples, a minimum of two sets of standards were analyzed, in addition to two procedural blanks and one duplicate sample. Natural matrix certified reference materials included: Metals of soil/sediment #4 (Ultra Scientific; SRM 2704a) and Buffalo River sediment (National Bureau of Standards, NIST 1990). Mercury recovery from these reference materials was 96.7 ± 9.0% (n = 35).
Prior analyses confirmed trace mercury in native copper, and increasing concentrations in half-breeds and native silver samples from the Portage Lake Volcanic deposits.12,13,18,35,46 Other published references to mercury in Keweenaw copper and silver ores include Newhouse47 and Votava and Bornhorst.48 Here we include samples from the White Pine Mine ores (Nonesuch Shale deposits) and discussion of iron ore emissions. Moreover, recent archaeological research has determined the elemental composition of Keweenaw Native Copper/Silver ores and White Pine Mine copper and silver ores for comparisons with native copper artifacts.49 We review these cross-comparisons.
Mercury emissions from metal processing, tailings releases on land and in water
Given the mean mercury concentrations in copper and silver ores, and the amount of copper and silver processed, we estimate emission of mercury from Keweenaw native copper smelters and the White Pine mine smelter in addition to iron mine taconite pellet processing. Historic smelter sources are plotted geographically in Fig. 3. Emissions from the White Pine smelter were also monitored periodically,22,50,51 providing independent checks.We looked at sediment cores from the smelting “epicenter” of Keweenaw mining, the location of five smelters and numerous stamp mills in the Keweenaw Waterway (Portage & Torch Lakes) of the Torch Lake Superfund Site. Of the two northern lakes (Portage, Torch) near native copper mines, Portage Lake was less environmentally impacted from mill discharges for several reasons: the smaller amount of tailings released, the larger lake and watershed drainage area, and the higher natural sedimentation rate. Official Company Reports from the MTU Archives gave us specific information on yearly copper production and tailings release from smelters and mills. A total of 34 million metric tonnes of copper tailings were sluiced into Portage Lake, whereas 179 million metric tonnes were discharged into Torch Lake.12,39 Portage Lake is 4.6× larger (surface area = 44.4 km2) than Torch Lake (9.7 km2), shallower (maximum depth = 16 m, mean depth = 9 m), and polymictic.52 The Portage Lake watershed has a much larger catchment area than Torch Lake (26× larger; 5200 vs. 200 km2) and receives more uncontaminated sediment-laden runoff from several large rivers and streams, including the extensive Sturgeon River Sloughs and Pilgrim River drainage. Because of these inputs, post-mining sedimentation rates in Portage Lake are much higher (60–120 mg cm−2 per year)53 than in Torch Lake (20–30 mg cm−2 per year).54,55
Concentrations of Hg in iron ore are relatively low, but the ore tonnage processed is quite large, creating circumstances similar to coal burning. Historic iron mines are geographically spread across the landscape (Fig. 3). Calculating mercury releases from early and later regional iron mine operations (forges, blast furnaces, shaft operations) is challenging. Fortunately, early Upper Peninsula forges and blast furnaces are treated in Reed50 along with the amount of iron produced. We applied Pirrone et al.'s3 emission factors for pig iron and steel production (grams mercury per million grams iron processed = 0.04 g Mg−1) to estimate cumulative emissions from the early pig iron furnace operations. Later vertical shaft iron operations often shipped ore directly to lower Great Lakes smelters, minimizing regional emissions.56 Additional local sources of mercury that are much more difficult to estimate come from associated sulfides (e.g. sphalerite), ferric iron assay procedures, and blasting caps (mercuric fulminate).57
Iron mining shifted to taconite pellet production in the 1950's. Amounts of taconite pellets processed by individual mining operations are also summarized by Reed56 and updated (Cliffs, Annual Reports). Two operating Marquette Taconite pellet plants have discharged continuously, since 1963 (Empire Mine) and 1973 (Tilden Mine). Stack emissions appear to be the dominant pathway for mercury release from taconite processing, as Hg(II) in ore concentrate is converted to Hg(0) during the firing of pellets.23 Emissions were estimated in several ways. In Minnesota, emissions for taconite processing range from 1 to 17 kg Hg per million long tons of pellets.23 Regressions for calculation of emissions factors (Minnesota Taconite Operations) can be found in Jaing et al.23 and Berndt.24 Mean Minnesota Emission Factors were applied to Michigan Empire/Tilden operations to estimate probable yearly mercury emission. Although mercury emissions are not publically released by Cleveland Cliffs in Michigan, USEPA and MDEQ58 periodically checked stack emissions, providing independent checks.
Mercury deposition and AERMOD plume modeling
Rates of wet deposition of mercury were assembled from the Mercury Deposition Network (MDN) of the National Atmospheric Deposition Network (NADP). The MDN site in Michigan's Upper Peninsula, MI48, has been in operation since 2003; the Eagle Harbor site operated from 1994 through 2007, but recently closed down; rates at two stations in northeastern Wisconsin (WI95, WI09) are also used to evaluate geographic gradients (Fig. 1a). Litterfall deposition of Hg was monitored at the same sites since 2007 by Risch.59Local deposition of Hg from Cu smelting facilities was estimated in two ways. Based on Hg accumulation rates in multiple lake sediment cores around the large Cu smelter in Flin Flon, Wiklund et al.60 estimated that 11% of Hg emissions were deposited within a 50 km radius. However, at 825 ft, the smelter stack at Flin Flon for the last 36 years of its operation (1975–2010) was much taller than the original (1930) 100- and 225-ft stacks (Naylor61) that were comparable to the 100–200 ft stacks at the local smelters including Calumet & Hecla (200 ft), Quincy (75 ft), Michigan Smelter (150 ft) or even White Pine (504 ft). It is likely that a larger percentage of emissions would be deposited locally from smaller stacks. Until better estimates become available, we use an upper limit of 25% of emissions deposited within a 50 km radius. The range of 11–25% deposition within a 50 km radius was applied to the emissions from the copper smelting through 1968. While the White Pine facility continued smelting until 1995, it had a taller stack (504 ft), and was located outside the 50 km radius centered on the smelters along the Keweenaw Waterway. Modeling of White Pine deposition awaits more credible information on ore source processing during the last 10 years of operation. The Hg emissions from the Keweenaw smelters multiplied by the range of possible deposition efficiencies (11–25%) discussed above yielded a range of estimates of local deposition. This was then compared with an estimate of Hg deposition from long-range transport derived from sediment cores. We averaged the sediment Hg accumulation rate profiles from four seepage lakes in Wisconsin and northern Minnesota reported by Engstrom et al.62,63
AERMOD plume deposition modeling methods: emission data
Copper was smelted at the C&H Smelter from 1885 to 1948.18,64 New furnaces were installed in 1914 that increased the amount of copper that could be smelted (Conant65). To estimate total Hg emission rate from the smokestack at the C&H Smelter, values of the following parameters were obtained as follows: amount of copper in the concentrate, 32.9%, the amount of concentrate smelted, 1 × 105 lbs h−1, and Hg concentration in the concentrate, 2.4 × 10−6 g Hg g−1. Thus, the emission rate (g Hg s−1) was calculated as: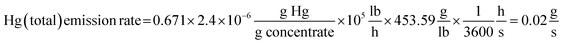 | (1) |
Because at the C&H Smelter, copper smelting operations were carried out at nighttime only (Conant65), we used a factor of 0.5 to reduce the emission rate to 0.01 g Hg s−1 for dispersion modeling. Following Rice et al.,66 the mercury species in the flue gas were assumed to consist of 50% elemental mercury (Hg0), 48% divalent oxidized gaseous mercury (Hg2+), and 2% particle-bound mercury (HgP).
To model the spatial distribution of mercury emitted and deposited from the C&H Smelter, the U.S. EPA's air quality dispersion modeling system (Peters et al.67) American Meteorological Society-Environmental Protection Agency Regulatory Model (AERMOD) (Perry et al.68) was applied. In this work, a commercial interface of the AERMOD modeling system, AERMOD View (version 9.4.0; Lakes Environmental™, Waterloo, Ontario, Canada) was used. The model consists of three components: AERMET View (preprocessor for meteorological data), AERMAP (terrain preprocessor), and AERMOD View (the dispersion model).
In this study, hourly averaged preprocessed surface meteorological data were collected at the Houghton County Memorial Airport (CMX) from the Michigan Department of Environment Quality (MDEQ) AERMOD data support document (MDEQ69) and the upper layer data were collected at the nearest upper air meteorological station (GRB) in Green Bay, Wisconsin for years 2012–2016. The terrain data (i.e., Digital Elevation Maps) from the U.S. Geological Survey with ∼90 m spatial resolution were used to define the modeling domain. Receptor networks within the modeling domain of 50 km radius from the point source were defined using uniform Cartesian grids with spacing of 5000 m. The reference point for the modeling domain was 47°10′37′′ N and 88°25′26′′ W. Land use categories for the modeling domain consisted of water bodies, forest, and suburban areas & forest. The characteristics data for the C&H Smelter used in this study are summarized in Table 2. For each year (2012–2016), the model simulations were conducted to determine total deposition rates (averaged annually) of three Hg species (i.e., Hg0, Hg2+, and HgP) within the modeling domain. Here we report results from a representative meteorological year. After Calumet-Hecla, calculations were extended to the entire group of five Keweenaw Waterway smelters.
| Parameter | Value | Reference |
|---|---|---|
| a Assumed to be slightly less than the melting temperature of copper of 2700 °F. b Approximated following Briggs (1969)128 assuming the stack was designed to avoid stack-tip downwash. | ||
| Hg (total) emission rate (g s−1) | 0.01 | See eqn (1) and related information |
| Release height (m) | 61.11 | 129 |
| Gas exit temperature (°F) | 2000 (assumeda) | (F. Quivik, personal communication) |
| Stack inside diameter (m) | 3.05 | 129 |
| Gas exit velocity (m s−1) | 1.5 × max. Surface wind speedb | 128 |
| Building height (m) | 21.94 | 130 |
| Building length (m) | 32.30 | 130 |
| Building width (m) | 32.00 | 130 |
Mercury in lake sediment studies and surface waters (lakes, rivers)
To illustrate enhanced mercury fluxes in the immediate vicinity of mining operations, we collected sediment cores with a 5 cm diameter K–B style gravity corer (WildCo) from Torch and Portage Lakes in the Keweenaw Waterway. Multiple cores were collected at two sites (10- and 20-m water depths) in the eastern half of Torch Lake and two cores were collected at a single site (14 m water depth) in (Portage Lake).12 Multiple cores of 60–80 cm length were previously retrieved from other sites within the Keweenaw Waterway, brought to the lab and X-rayed at Portage Health Hospital to aid in the correlation of depths and ages. Sediments were then extruded and sliced into 1 cm depth increments. A portion of the core slices was dried and ground, and then analyzed for 210Pb and copper. Another portion of the slices was preserved wet and shipped on dry ice to the USEPA Lab at Grosse Ile for measurement of mercury species.Analyses for 210Pb (sediment dating) and Cu were done at MTU. Total 210Pb was measured (as the daughter isotope, 210Po) by alpha spectrometry (EG&Ortec Octete Plus) following extraction and plating by the methods of Eakins and Morrison70 as modified by Engstrom et al.71 Isotope extraction and plating efficiencies were measured by spiking all samples with known activities of 209Po. Supported 210Pb and 137Cs were measured on all samples by gamma spectrometry (low background Germanium well detectors, Ortec DSpec model spectrometer); supported 210Pb was calculated as the average of eight 214Bi and seven 214Pb peaks. Profiles in Portage and Torch Lake showed continuous, uninterrupted deposition. Dating of the Portage Lake core was preceded by additional 210Pb, 137Cs, and varve dating studies at additional sites.12,13,18,72 Dating of the full profiles in Portage and Torch Lakes is discussed elsewhere.12,53–55,72
Sediments were digested in the microwave (CEM MDS-2100) using EPA method 3051A prior to measurement of total copper. Copper was measured using a Perkin Elmer model 3100 spectrophotometer. Digestion efficiencies were verified using NIST standard reference material Buffalo River Sediments (SRM 2704), and instrument calibration was checked using the Plasma-Pure standard from Leeman Labs, Inc. Digestion efficiencies averaged 104%, and the calibration standard was, on average, measured as 101% of the certified value.
As mentioned earlier, mercury analysis was done at the USEPA Grosse Ile Lab. Wet sediment samples for mercury analyses were sub-sampled. Those for total mercury analysis were freeze-dried and stored dry, whereas those for methyl mercury were weighed into pre-weighed vials and stored frozen. Water contents determined at the time of freeze-drying were used to convert methyl mercury wet weight results to dry weight results. Total mercury was analyzed by using a LECO AMA-254 mercury analyzer (LECO73). Within the instrument, dry samples were thermally combusted to release mercury. All mercury was converted to Hg0 within a catalyst chamber. The Hg0 was collected on a gold trap. After collection, the gold trap was heated, releasing the mercury for detection with an atomic absorption spectrophotometer.
Methyl mercury was analyzed using a PS Analytical Hg 7000 mercury speciation system. Major components of the system are an Agilent 6890 GC system and a PSA 10.750 detector. The method used was a slight modification of the Cai et al.74 method which was based on the original work by Jones et al.75 Using this method, samples were extracted with acidic potassium bromide/copper sulfate solution. The brominated mercury species were dissolved into methylene chloride for separation. The mercury species were then back extracted into sodium thiosulfate solution, extracted with acidic potassium bromide/copper sulfate solution, and dissolved into methylene chloride. After passing through an anhydrous sodium sulfate column, the methylene chloride containing the mercury species was then introduced into the gas chromatograph to separate the various species. As each species was driven off the column, it was converted to Hg0 in a pyrolyzer and was quantified with an atomic fluorescence detector.
Mercury profiles in sediment cores were determined from a variety of geographic sites in Upper Peninsula lake sediments. In addition to previous work,12,13,18 we included sediment mercury reported by Knauer et al.,76 Drevnick et al.,77 and Parsons et al.78 We summarize here mercury concentrations in surface sediments and mercury accumulation rates in 210Pb-dated cores.
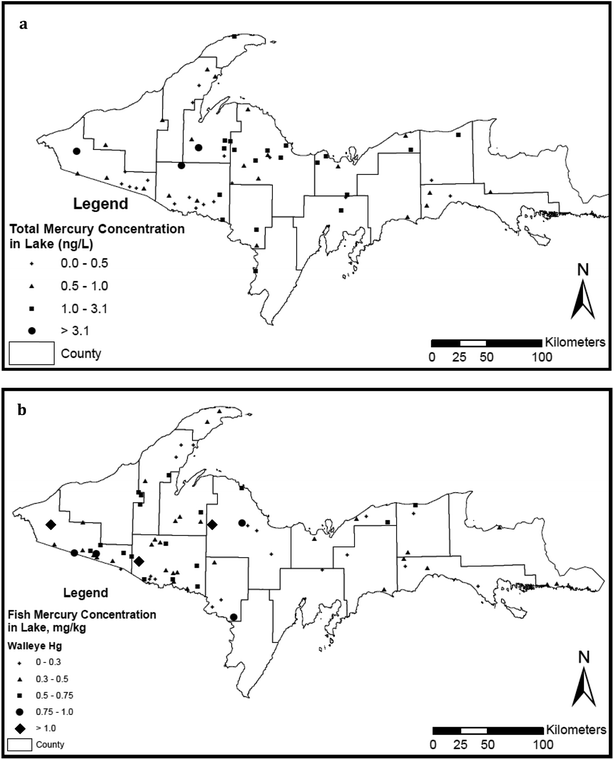
The State of Michigan has surveyed mercury concentrations in surface waters on several occasions (Fig. 4a). We compiled data here on lakes from state reports (MDEQ;73 Degraeve20; Knauer76) and the U.S. EPA National Lake Assessment Project (2007). The State reports on rivers came from MDEQ.28,58,76,79–82 Both the Michigan (MISWIMM) and EPA (STORET) databases were searched for any mercury measurements in Upper Peninsula lakes. We identified a total of 62 lakes with mercury measurements made in the time interval 2000–2017, and additional 38 measurements from rivers and streams. Rivers were assigned to “disturbed” versus “undisturbed” categories based on the presence or absence of tailings or seepage from nearby mining sites. The intent of the MDEQ 2011 lake study68 was “to gather mercury information on selected lakes in the Upper Peninsula of Michigan that have fish with very elevated concentrations of mercury”. One of our primary purposes here is to increase public awareness of the MDEQ data patterns of metal concentrations in waters and sediments and the relatively high values of MeHg in fish across the Upper Peninsula (Fig. 4b). More detailed discussion of physical and chemical correlations within the 62-lake study are treated in the Discussion and can be found in a separately submitted manuscript.83
Regional fish comparisons and local long-term analyses
The Western Upper Peninsula and Northern Lower Peninsula fall within MDEQ's “Northern Lakes and Forests” Ecoregion. Walleye (Sander vitreus), bass (smallmouth, Micropterus dolomieu; rock bass, Ambloplites rupestris), and northern pike (Esox lucius) were sampled from scattered Western Upper Peninsula (WUP) and Northern Lower Peninsula (NLP) lakes during 2007–10 by MDEQ for mercury content. To assess patterns, about ten fish were collected from each of 20–40 regional lakes (Fig. 4b) using a combination of fyke netting and electrofishing. Fish samples were kept on ice in the field and returned to the laboratory where they were frozen until analyzed. Total lengths were measured, fish filleted and analyzed as an edible portion, determining mercury concentrations per wet wt. Mercury in fish tissue was analyzed at the Michigan Department of Community Health (MDCH) laboratory in Lansing, Michigan, by thermal decomposition, amalgamation, and atomic adsorption according to USEPA (2007) method 7473. Mercury concentration is expressed as μg g−1 wet wt. The instrument was calibrated using ISO certified standards from Inorganic Ventures. Two QC levels (DORM-4 Lobster tissue and TORT-2 fish tissue) of certified reference materials (CRM) from the National Research Council in Canada were utilized to verify the calibration curve and batch run each day of analysis. The reference ranges generated by MDCH for these CRM's met or exceeded the established ranges from the manufacturer. We stress here that this analysis is also valuable for cross-comparing regions of little metal mining (Northern Lower Peninsula) with regions of metal mining (Western Upper Peninsula). Data from the analysis were forwarded to MTU by Joe Bohr (MDEQ) in the fall of 2011. For each fish category (walleye, bass, northern pike), mercury values for individual fish were plotted against fish length. WUP and NLP regressions were fit to Hg values using SYSTAT. To separate significance of site contrasts (WUP vs. NLP) from length patterns, ANCOVA was run, using the General Linear Model on log-transformed Hg values.One difficulty with standard state regional surveys is that relatively few fish are sampled each year from a particular location. However, a few collections are large enough to utilize regressions to standardize size values and compare time trends. For example, in the 2013 sampling efforts, a total of 30 fillet samples were sampled from Torch Lake, Houghton County, in a single year. All samples were analyzed for mercury by the MDHHS Analytical Chemistry Laboratory. Analytical results were reviewed and entered into the FCMP database. The complete dataset is available electronically (by request) or through the FCMP Web site (http://www.deq.state.mi.us/fcmp). For time comparisons of individual lakes, mercury concentrations are calculated for a fish of “standardized length” in μg g−1 dry wt. MDEQ Standardization procedures are published. Because mercury concentrations increase with fish size, values are length-normalized for graphical comparisons. For individual lakes, a concentration versus length regression line is fit to large samples, giving a standard length versus concentration regression. For comparison with smaller samples and over time, the contaminant concentrations were adjusted to occur in a fish of standard length for the species. Following previous state calculations, standard lengths for the comparisons in Torch Lake were set at 24 inches (61 cm) for northern pike, 16 inches (41 cm) for smallmouth bass, and 19 inches (48 cm) for walleye. The formula applied here for length-normalization was CLN = CA − S × (L − St), where CLN = length-normalized concentration, CA = actual concentration, S = slope of the concentration versus length regression, L = fish length, and St = standard length for the species (see Bohr84). Analysis of Variance and t-tests were utilized for regression analyses of time trend data (Minitab 15; Systat, Wilkinson86). Again, we thank Joe Bohr (MDEQ) for providing the time series comparisons with his standardization calculations. Discussion of the time series is also included in a state report (Bohr84).
Results
Modern-day atmospheric (NADP) mercury fluxes compared with isolated lake sediment values
NADP monitoring shows that wet and total mercury deposition in the Upper Peninsula is less than, about half, the deposition found in the Lower Peninsula (Fig. 1). Moreover, wet deposition of SO4 in the Upper Peninsula (4–8 kg ha−1) is also now less than in the Lower Peninsula (12–15 kg ha−1; MDEQ28). Wet deposition of mercury in Michigan's Upper Peninsula (2007–2014) averages about 6.7 ± 1.3 μg m−2 per year with an additional 6.2 ± 1.1 μg m−2 per year supplied by dry deposition.59Data from lake sediment cores, when handled with appropriate care, can indicate historical mercury deposition. Rates of mercury accumulation in sediment cores can be higher than NADP rates both because of Hg runoff from the catchment and sediment focusing within the lake.14 Estimates of annual THg flux from isolated inland lakes (Table 1) vary among states, ranging from slightly higher than the regional 4–8 μg m−2 per year NADP wet deposition values to much higher. Sediment Hg accumulation rates range from 5–9 μg m−2 per year in Wisconsin,15 to 2–24 μg m−2 per year in the Upper Peninsula of Michigan,8,9 to 15–95 μg m−2 per year in Minnesota.16 For these comparisons, lake coring sites were purposely chosen away from active mining areas (Minnesota, Michigan). When fluxes in Lake Superior (Table 1) are examined,13,18,85,87,88 central deep-water values (27–32 μg m−2 per year) for mercury deposition are higher than modern NADP wet-only values, yet only slightly higher than total mercury deposition in some inland lakes. However, off the tip of the Keweenaw Peninsula (in the “halo”, an elevated copper inventory region that extends kilometers into Lake Superior, related to copper mining13,18) or especially in the Keweenaw Waterway, formerly part of the Torch Lake Superfund site, surface fluxes increase several-fold above NADP and small isolated lake values to 73–109 μg m−2 per year. There are also strong correlations among Cu, Hg, and Ag concentrations in the coastal “halo” region and inland sediments (reflecting mining discharges), plus buried peaks that coincide with the time of native copper mining.13,18,88 These correlations and buried peaks provide strong evidence for tailing releases and smelter emissions dispersing out kilometers from coastal margins. A closer look at mining operations (below) clarifies both the regional sources of mercury and the amount of emissions.
Mercury in metal ores of the Upper Peninsula
Samples of native copper and silver from 29 abandoned Keweenaw mines were analyzed for mercury, and found to contain mean values of 3.9 μg g−1 (ppm; SD = ±2.1) in native copper; 154(±198) ppm in half-breeds (copper & silver mixtures), and 394(±187) ppm in native silver (Table 3). Exceptionally high values were also found in the associated gangue zinc mineral sphalerite [mean = 190(±55) ppm, N = 6]. Native copper contains silver in a ratio of around 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, whereas mercury is strongly correlated with silver concentrations. For example, Votava and Bornhorst48 characterized ore from the Caledonia Mine, finding that Hg and Ag had a rank correlation coefficient of 0.966 when data were plotted on a log–log scale (Fig. 5a). Additional measurements of mercury in native copper samples come from archeological comparisons, i.e. attempts to cross-compare native ores with copper artifacts (Table 3). Concentrations of mercury in archeological comparisons include: 6.5 ± 8.3 μg g−1;89 3.6 ± 6.7 μg g−1 and 2.3 ± 1.3 μg g−1.90 The archeological values (mean = 4.1 ppm) are comparable with earlier determinations. The essential point here is that Hg occurs naturally in native copper, native silver, and zinc mineral samples from the Keweenaw Portage Lake Volcanic deposits, apparently as a solid solution substitution in the mineral lattice.18,91
1000, whereas mercury is strongly correlated with silver concentrations. For example, Votava and Bornhorst48 characterized ore from the Caledonia Mine, finding that Hg and Ag had a rank correlation coefficient of 0.966 when data were plotted on a log–log scale (Fig. 5a). Additional measurements of mercury in native copper samples come from archeological comparisons, i.e. attempts to cross-compare native ores with copper artifacts (Table 3). Concentrations of mercury in archeological comparisons include: 6.5 ± 8.3 μg g−1;89 3.6 ± 6.7 μg g−1 and 2.3 ± 1.3 μg g−1.90 The archeological values (mean = 4.1 ppm) are comparable with earlier determinations. The essential point here is that Hg occurs naturally in native copper, native silver, and zinc mineral samples from the Keweenaw Portage Lake Volcanic deposits, apparently as a solid solution substitution in the mineral lattice.18,91
| Sites | Ore | Samples N | Mean (SD) μg g−1, ppm | Range μg g−1, ppm | Source |
|---|---|---|---|---|---|
| 29 Keweenaw native copper mines | Native copper | 60 | 3.9(2.1) | 0.1–47.1 | 12 |
| Half-breed (Cu,Ag) | 10 | 154(198) | 0.7–981 | 12 | |
| Native Silver | 30 | 394(187) | 34–2548 | 12 | |
| Sphalerite (ZnS) | 6 | 190(55) | 12 | ||
| 2 Keweenaw native copper mines | Native copper | 20 | 6.5(8.3) | 0.45–27.85 | 89 |
| 3 Houghton County mines | Native copper | 16 | 3.6(6.7) | NR | 90 |
| 3 Keweenaw County mines | Native copper | 16 | 2.3(1.3) | NR | 90 |
| White pine mine | Native copper | 5 | 2.4(1.1) | 12 | |
| Native Silver | 3 | 330(275) | 12 | ||
| Bornite (Cu5FeS4) | 3 | 2.5(1.4) | 13 | ||
| Chalcocite (Cu2S) | 9 | 3.2(1.9) | 13 | ||
| Native copper | 8 | 1.4(0.2) | NR | 90 | |
| White pine mine | Native copper-vein | 15 | 9.6(7.9) | 1.0–32 | 81 |
| Native copper-sheet | 49 | 13.9(26.3) | 1.1–55 | 92 | |
| Native copper-disseminated | 27 | 8.1(10.3) | 1.5–27 | 92 |
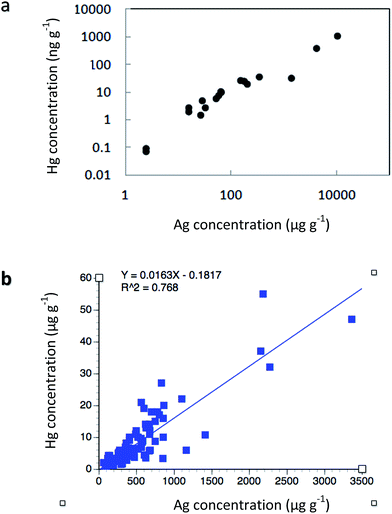 | ||
| Fig. 5 Relationship of mercury to silver concentrations in ore samples: (a) Caledonia Mine, rank correlation coefficient = 0.966 on log–log plot (after Votava and Bornhorst41); (b) White Pine Mine, correlation coefficient on arithmetic plot, r = 0.876. | ||
To the south, copper and silver deposits at the White Pine Mine are associated with another lode, the Nonesuch Shale. White Pine operations reached copper production peaks well after northern native copper and silver mines began to play out. Again, there is a strong relationship between silver and mercury concentrations in White Pine Mine ores (Fig. 5b; r2 = 0.768; regression equation = Y = 0.016X; p < 0.000). Initial analysis of native copper and silver ore from the White Pine Mine detected 2.4 ± 1.1 μg g−1 mercury in native copper, 330 ± 275 μg g−1 in native silver ores, 2.5 ± 1.4 μg g−1 in bornite (Cu5FeS4), and 3.2 ± 1.9 μg g−1 in chalcocite (Cu2S), respectively.13 A separate analysis of 85 native copper samples by Mauk and Hancock92 from White Pine utilized neutron activation methods to compare concentrations relative to copper artifacts. In vein, sheet, and disseminated copper samples, they found mercury concentrations of 9.6 ± 7.9 μg g−1, 13.9 ± 26.3 μg g−1, and 8.1 ± 10.3 μg g−1. In White Pine deposits, silver was present at a ratio of ca. 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 parts copper, slightly higher than in the previous more northern native copper ores (1
1000 parts copper, slightly higher than in the previous more northern native copper ores (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 ratio). The variance of mean mercury concentrations was higher in White Pine samples, probably reflecting the more heterogeneous mineral composition.
1000 ratio). The variance of mean mercury concentrations was higher in White Pine samples, probably reflecting the more heterogeneous mineral composition.
To date, we have found no measurements of Hg content for Upper Peninsula iron ores. Values reported for Minnesota iron deposits range from 0.001 to 0.032 μg g−1.24 The large masses of iron ore processed compensate for low Hg content. For example, the 1.3 billion metric tons of ore shipped from the Upper Peninsula during the interval 1850–1990 contained an estimated 26 tons of Hg that were released by steel plants elsewhere into the atmosphere.
Mercury emissions from copper and iron mining
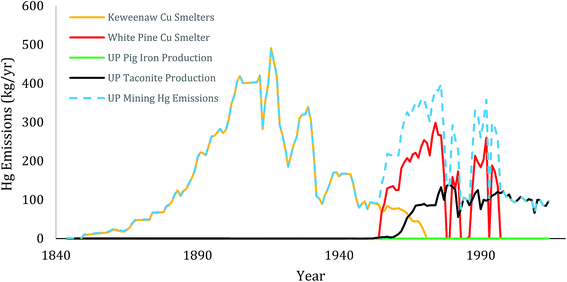
Early in the Keweenaw Peninsula history, the primary source of mercury was from copper and silver ores. Between 1850–1929, the Keweenaw district was the second largest producer of copper in the world, a key part of the industrial revolution.93,94 During that interval, 140 mines worked the central deposits of the Peninsula (Portage Lake Volcanics, Copper Harbor Conglomerates) and over 40 mills processed ores (Fig. 3). The concentration of copper in the parent rock averaged between 0.5–6.1% of total mass, and the ratio of Hg to Cu averaged around 4 ppm (see above, Table 3). That ratio is very similar to recently reported mean values for Cu mines worldwide.3,91 Mills discharged vast amounts of tailings, the so-called “stamp sands” and shipped concentrates to smelters.56 In the Keweenaw Waterway, Portage Lake had eleven copper stamp mills (7 early gravity-driven stamp mills, 4 later large steam-driven mills) between 1855 and 1947, as well as four smelters along its shoreline.39 Six large stamp mills and a smelter operated on the shores of Torch Lake between 1868–1968. Coarse “stamp sand” tailings were deposited in the immediate vicinity of stamp mills (tailings beach piles), whereas the “slime” (i.e., clay-sized) fraction dispersed out into deeper regions of the lakes.In the Upper Peninsula, smelting broadcast mercury over the entire Keweenaw landscape. Together, emissions from northern native copper and silver mining (Keweenaw Waterway) and the southern native copper and silver, chalcocite, chalcopyrite and bornite ores (White Pine) continued with only brief interruptions for 140 years. From concentrations of mercury in ores (Table 3), we derived approximate estimates of total amounts emitted (Fig. 6).
Prevailing winds came from the northwest and blew towards the east to southeast. The northern four native copper and silver smelters on Portage Lake included the Portage Lake Smelting Works, later renamed the Detroit and Lake Superior Smelter (1860–1905), the Dollar Bay Smelting Works (1898–1919); the Quincy Smelter (1898–1931), and the Michigan Smelter (1903–1945). The Torch Lake Calumet and Hecla Smelter (1860–1968) processed Portage Lake Volcanic Series and Calumet conglomerate.
Amygdaloid and conglomerate ores
The Keweenaw smelters produced about 4.8 million tonnes of copper and about 5000 tonnes of silver. Taking the mean Hg concentration in native copper (ca. 4.0 ppm) plus the mean concentration in native silver (394 ppm; Table 3), the Keweenaw Waterway smelters emitted around 20–24 tonnes of mercury, with yearly collective emissions reaching around 400–500 kg per year during the peak years between 1910–1930 (Fig. 6; Table 4). Another estimated additional 17 tonnes of mercury were released in tailings (stamp sand) from stamp mills.13| Sources | Total | Yearly | SO2 emissions |
|---|---|---|---|
| a Shipped downstate. | |||
| Waterway Smelters (1860–1968) | 20–24 tonnes | Peak 400–600 kg per year (1880–1930) | NR |
| White pine Smelter (1955–1995) | 7.6 tonnes | Peak 635 kg per year (1990) | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 tonnes SO2 per year (1970's) 100 tonnes SO2 per year (1970's) |
| Pig iron operations 1860–1880s | 69 kg | — | NR |
| Marquette iron range | 13.4 tonnesa | NR | |
| Tilden/Empire mines, Marquette | 2.1 tonnes (1963–1989) | 27–36 kg per year (Tilden) | 1110 tonnes SO2 per year (Tilden) |
The EPA program AERMOD was used to model emissions from individual Keweenaw Waterway smelters. Fig. 7a shows predicted average annual total (wet + dry) deposition of gaseous Hg(II) (gaseous oxidized mercury, GOM) from the Calumet-Hecla Smelter near Torch Lake for the years 1914–1948. About 85% of total deposition was GOM, 15% was Hg0, and only 0.1% was as particulate Hg. Maximum local annual deposition was 38.3 μg m−2, and mercury dispersed over more than 50 km around the Keweenaw landscape. Recall that Calumet-Hecla was only one of five smelters operating in the Keweenaw Waterway, and had a 200 ft tall stack. Estimated mercury deposition from the five northern Keweenaw Waterway smelters doubled long-range deposition values in the region during the peak of native copper mining (Fig. 7b).
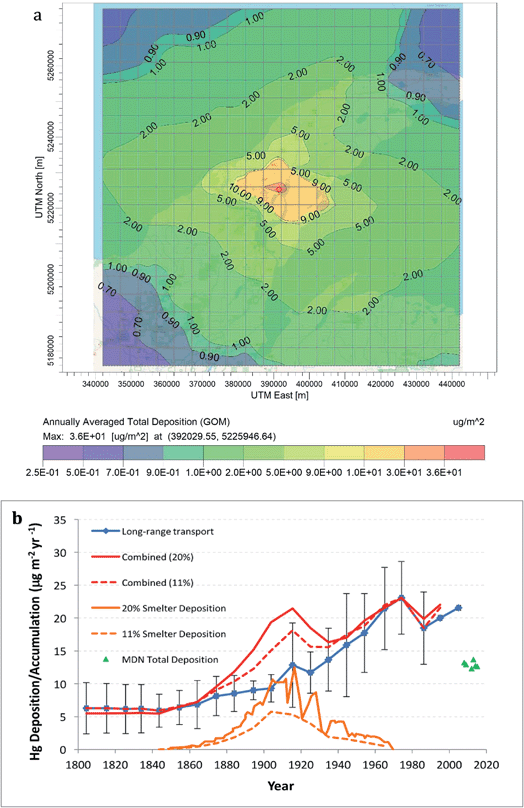 | ||
| Fig. 7 Deposition of Hg from individual and collective smelters: (a) AERMOD modeling of Hg deposition around Calumet-Hecla Smelter. Shading shows relatively high deposition around smelter, yet distant dispersal around the Peninsula. (b) Smelter deposition from the 5 Keweenaw Waterway smelter emissions superimposed upon long-range deposition (Engstrom's values from 4 remote Wisconsin and Minnesota lakes62,63). Green triangles show modern-day MDN total mercury deposition. | ||
The more southern White Pine Smelter processed primarily chalcocite (copper sulfide and silver) and native copper ores from the Nonesuch Shale between 1955–1995. Over its active life, White Pine operations produced around 1.8 million tonnes of copper and 1276 tonnes of silver (Table 4). The White Pine Mine also generated around 5.6 million tons of tailings per year (U.S.EPA 1992). Recall that mean concentrations of THg in component ores averaged: native copper (1.4–13.9 μg g−1; Table 3), native silver (330 μg g−1), chalcosite (3.2 μg g−1) and bornite (2.5 μg g−1). Given an average 80% chalcosite: 20% native copper mixture in ores, weighted mean ratios of mercury to copper averaged around 4.0 μg g−1 (i.e. 3.98 μg g−1), similar to the earlier Portage Lake smelter ratios. Using 4.0 as the mean value, the White Pine smelter would have emitted around 7.2 tonnes of mercury from copper and 0.4 tonnes from silver out of its 500′ high main stack (Fig. 6). However, between 1982 and closure in 1995, additional ores from largely unreported sources were custom-smelted along with Nonesuch Shale ores. In 1990, MDNR directly measured THg emissions at White Pine smelter as 635 kg per year mercury. If the 1990 estimate is representative of emissions during the final 1982–1995 interval, 8.3 tonnes of THg were emitted over the last 13 years of operation. Combining the first 27 with the last 13 years of operation gives a total of around 13.1 tonnes of THg emitted from the White Pine Smelter between 1955 and 1995 (Table 4). In Fig. 6, we plot only the estimated emissions from local White Pine ore between 1982–1995, until we can incorporate the undisclosed additional contract operations. Unfortunately, also, there is no corresponding estimate on how much mercury was released in the 5.6 million tonnes of tailings discharged over the 40 year interval, although tailings were deposited into a nearby tailings pond, with a river overflow into Lake Superior. The White Pine Mine also released a large amount of SO2, around 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 tonnes per year in the 1970's (Table 4).
100 tonnes per year in the 1970's (Table 4).
Mercury releases also came from extensive iron, gold and silver mining districts in the Upper Peninsula (Fig. 3). Below the Keweenaw Peninsula was the Gogebic Range Iron District. Further east and southeast were the hundreds of locations within the Marquette and Menominee Iron Ranges. During the 1860's to 1880's around 29–30 separate pig iron furnaces operated in the Iron Districts (Fig. 3), with SO2 discharges most pronounced in the western Menominee Range, but found at almost all sites. However, the ore volumes processed were relatively low. Estimates for early pig iron operations in the Upper Peninsula were 1.9 million short tons.56 Using mercury emission factor estimates for global pig iron and steel production (0.04 g THg per million grams iron; Pirrone et al.3), the early pig iron operations emitted only around 69 kg of THg (Table 4). Later, the Marquette Iron Range produced a total of 328.6 million long tons of iron before switching to pellet formation. Using Pirrone et al.'s3 emission factor of 0.04 on these totals yields substantial mercury emission estimates of 13.4 metric tonnes THg. However, most of this ore production was shipped southward out of the Upper Peninsula for final smelter processing and thus did not contribute to northern U.P. totals.56
Later manufacturing shifted to taconite pellet plants in the 1950's. Iron mining stack emissions are the dominant pathway for mercury release from modern-day taconite processing, as Hg(II) in ore concentrate is converted to Hg(0) during the firing of pellets.23 In Minnesota, emissions have been directly monitored for years. For comparable taconite operations in Minnesota, emission factors ranged from 1 to 17 kg Hg per million long tons of pellets processed. Between 2003–7, Minnesota operations emitted a total of 342–388 kg Hg per year, or between 23–104 kg Hg per year from individual plant operations. The two operating Marquette, Michigan, taconite pellet operations have discharged continuously since 1963 (Empire Mine) and 1974 (Tilden Mine), with accompanying elevated SO2 discharges. Applying average Minnesota pellet regressions (8 kg Hg per million long tons of pellets) to the total Marquette Iron Range pellet production (259.1 M long tons) gives an estimate of 2.1 tonnes THg emitted up to 1989 by pellet activities (Fig. 6; Table 4). If the annual production is 7.4 million long tons of pellets per year at the Tilden plant, using the average Minnesota regression value predicts around 59 kg of mercury emitted annually. Checks of Tilden Plant mercury emissions in 2002 recorded yearly values of 27–36 kg (Table 4).80 Total emissions from taconite processing in Upper Michigan in 2002 were estimated to be 40 kg per year.81 Between 2010 and 2013, the Tilden Plant also acknowledged emitting around 1110 tonnes of SO2 per year (Table 4).80 Two other ancillary contributing sources of mercury in iron mining operations were the mercury used in iron ore assays82 and mercury fulminate used in blasting caps.57
Lake sediment mercury profiles near mining operations confirm elevated mercury net accumulation
Emission studies suggest relatively large amounts of mercury released during the period of active copper mining. Although mercury loading in nearby Keweenaw Waterway lakes (Portage Lake, Torch Lake) involved both smelter emissions and tailings releases, we could check net accumulation fluxes through time using sediment core studies. We used 210Pb profiles to date the strata from Portage and Torch Lake and to calculate net accumulation fluxes for copper (Cu) and total mercury (THg). In the Portage Lake core (Fig. 8), measurements of flux helped correct for dilution and concentration effects associated with variable sedimentation and allowed comparison with NADP fluxes. Fluxes for copper and total mercury are correlated (r = 0.844, N = 23, 14 m deep Portage site). Fluxes increase after 1868, and achieve major peaks during the mining era: two peaks of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240 and 8130 mg m−2 per year for Cu and two peaks of 7080 and 5120 μg m−2 per year for THg, with THg averaging around 1500 μg m−2 per year. Thus, during the mining era, mercury fluxes at this site averaged 50–250 fold greater than present day gross atmospheric deposition (12–30 μg m−2 per year) and reached peaks 240- to 1400-fold greater. After mill operations ceased in 1947, fluxes for both Cu and THg declined rapidly, but still remained elevated above background (THg, 21–411 μg m−2 per year).
240 and 8130 mg m−2 per year for Cu and two peaks of 7080 and 5120 μg m−2 per year for THg, with THg averaging around 1500 μg m−2 per year. Thus, during the mining era, mercury fluxes at this site averaged 50–250 fold greater than present day gross atmospheric deposition (12–30 μg m−2 per year) and reached peaks 240- to 1400-fold greater. After mill operations ceased in 1947, fluxes for both Cu and THg declined rapidly, but still remained elevated above background (THg, 21–411 μg m−2 per year).
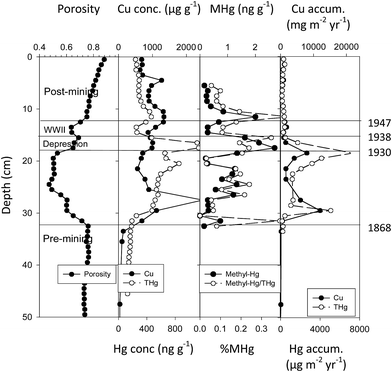 | ||
| Fig. 8 Sediment profiles at central 14 m depth site in Portage Lake. Measurements include porosity, total copper concentration (μg g−1), total Hg (ng g−1) and methyl mercury (ng g−1) concentrations, ratios of methyl to total Hg (% MHg), and fluxes for Cu (mg m−2 per year) and Hg (μg m−2 per year). The horizontal dashed lines mark dated historical periods (labels on left). Major fluxes for Cu peak between 1868–1947 and correspond to tailings (‘slime clay’) discharges from stamp mills into the lake. Superimposed total Hg fluxes show a similar pattern, following build-up of local smelting capability (1880–1938). MeHg concentrations before mining are low (NR), increase during mining (1868–1947), peak during the Depression (1930–1938) and immediately after mill closure (1947), then decline. Cu and Hg measures are dry wt. (After Kerfoot et al.12). | ||
In the Portage Lake core, fluxes for MeHg and THg (ng cm−2 per year) were also highly correlated (N = 33; r = 0.775; p = 1.16 × 10−7). Concentration profiles for THg and MeHg are also correlated (r = 0.774). Correlations would be higher, except that MeHg appears time-delayed relative to Cu and THg fluxes (Fig. 8). MeHg concentration peaks occur near the end of the high Cu and THg fluxes and remain high in the immediate post-mining strata. Although there are fluctuations, MeHg concentrations rise slowly throughout the mining era, reach peak concentrations during Depression-era sediments, show a post-WWII sub-peak, and then decline substantially in post-mining strata. In Portage Lake sediments, methyl mercury was present throughout the mining era. Moreover, the concentration of mercury present as methyl mercury generally varied between 1 to 3 mg g−1 THg (0.1–0.3%). A regression of methyl mercury on total mercury was highly significant (Portage Lake; y = 0.002X − 0.203; r = 0.775; N = 44, d.f. = 1, F = 62.8, p = 7.11 × 10−10). The MeHg on THg regression slope was 0.00198 with a SE ± 0.00024, i.e. MeHg averaged about 2.0 mg g−1 THg or about 0.2% (Fig. 8).
Additional information on THg versus Cu fluxes comes from twelve core sites distributed around the Keweenaw Waterway.12,13 Peak THg concentrations during the mining era range from 130 to 1060 ng g−1 (mean = 676 ng g−1 dry wt., SD = 325) and THg fluxes range from 139 to 5027 μg m−2 per year (mean = 1586 μg m−2 per year; SD = 1416). The mean Waterway values again emphasize elevated loading during the mining era (50 to 260-fold modern-day NADP values). The primary source of THg was probably smelter discharge, whereas the primary source of Cu was probably stamp mill discharge (stamp sands).13 However, we emphasize that the elevated mercury net accumulation came from both smelting and tailing inputs.
Torch Lake is north of Portage Lake (Fig. 3) and varies from Portage Lake in several aspects: (1) it received a much greater amount of mine tailings (178.5 million metric tonnes versus 34.3 million metric tonnes for Portage Lake, a 5.2× difference), (2) deposition was into a smaller area (9.7 km2versus 44.4 km2, i.e. 4.6× smaller), (3) extraction practices varied historically (mill discharge plus dredging of older tailings piles and subsequent chemical floatation treatment64), (4) a later date of cessation from discharges (approximately 20 years later, 1968 in Torch Lake; compared to 1947 in Portage Lake), and (5) a lower rate of post-mining sediment accumulation. Again, determination of detailed sedimentation rates from 210Pb dating allowed calculation of copper and mercury fluxes through time. However, due to the large amount of tailings discharged into Torch Lake, the meter-length sediment cores did not penetrate through the deep slime clay (tailing) layers, so the bottom record begins during the early mining period. Again, there is a high correlation between fluxes for Cu and THg (r = 0.834, N = 23). Because of greater mill discharges, fluxes for Cu are larger in Torch Lake than in Portage Lake, varying between 3–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 mg m−2 per year during the early mining period and increasing to 9–56
800 mg m−2 per year during the early mining period and increasing to 9–56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 mg m−2 per year during the late mining period (Fig. 9). Total mercury fluxes (THg) are also higher than in Portage Lake, varying between 280–3070 μg m−2 per year in the early mining period and reaching maxima of 10
800 mg m−2 per year during the late mining period (Fig. 9). Total mercury fluxes (THg) are also higher than in Portage Lake, varying between 280–3070 μg m−2 per year in the early mining period and reaching maxima of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 130–21
130–21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 μg m−2 per year during the late mining period. During the mining era in Torch Lake, fluxes for THg reached peaks 10- to 710-fold greater than present day atmospheric deposition. That is, as in Portage Lake, there was a major increase in THg loading, from both smelting and tailing inputs, closely correlated with copper mining.
300 μg m−2 per year during the late mining period. During the mining era in Torch Lake, fluxes for THg reached peaks 10- to 710-fold greater than present day atmospheric deposition. That is, as in Portage Lake, there was a major increase in THg loading, from both smelting and tailing inputs, closely correlated with copper mining.
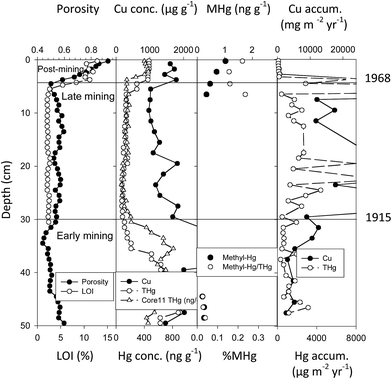 | ||
| Fig. 9 Sediment profiles at 20 m depth site in Torch Lake. Profiles again include porosity, total copper concentration (μg g−1), total mercury (ng g−1) and methyl mercury (ng g−1), ratios of methyl to total Hg (%MHg), and fluxes for Cu (mg m−2 per year) and Hg (μg m−2 per year). Horizontal lines mark historical periods of early mining (before 1945), late mining (until 1968), and post-mining (after 1968). Copper, total mercury, and methyl mercury concentrations were depressed in late mining strata due to double processing of tailings. Despite double processing, fluxes for Cu and Hg were greatly elevated during the late mining era. Note that concentrations of Cu, Hg, and MeHg increase in post-mining strata (After Kerfoot et al.12). | ||
However, in Torch Lake, circumstances were more complicated than in Portage Lake, because changing mining practices influenced metal concentrations in sediments. The profiles of total Cu and THg in Torch Lake sediments show elevated concentrations during the early mining period, yet relatively low concentrations in late mining era strata. Wilfley tables, combined with the second-stage grinding implemented in 1910, reduced copper and associated mercury losses in tailings by 70%.56 In addition, leaching and enhanced flotation, implemented in 1914, were able to reduce metal losses an additional 20–30%.95 These technological advances prompted the largest company (Calumet-Hecla) to dredge early coastal tailings piles and to re-extract copper, releasing the “double-processed tailings” back into Torch Lake. Enough additional copper was re-extracted to provide a third of Calumet-Hecla yearly profits.64,95 The metal-depleted tailings resulted in lower Cu and THg concentrations in late-mining era sediments relative to concentrations in the earlier period of conventional mining. In post-mining sediments, fluxes of both Cu and THg dramatically decline.
In contrast to Portage Lake, there is a substantial increase of THg and Cu concentrations in post-mining strata, suggesting a much greater “lingering effect”. In addition to lateral transport of metals from covered shoreline piles, we have postulated an upward diffusion of metal-laden pore waters from the extensive buried slime clay strata, depositing metals as they come into contact with carbon-rich post-mining strata.55,72 In the Torch Lake core, MeHg and THg concentrations are again highly correlated (regression y = 0.0017x + 0.0187; r2 = 0.775; F = 96.2; p = 1.47 × 10−10). Although early mine strata contain elevated concentrations of total mercury (502–882 ng g−1, i.e. ppb), the high concentrations of both THg (451–466 ng g−1) and MeHg (0.7–1.1 ng g−1) found in post-mining organic-rich sediment layers are reminiscent of the peaks observed during the Depression Era and post-WWII strata in Portage Lake sediments. Methyl mercury concentrations are present at the base of the core (early mining-era strata), decline during the late mining period, then increase to much higher concentrations (0.5–1.0 ng g−1) in recent, post-mining sediments. During the late mining era, dilution from double-treated tailings again probably contributes to the mid-profile decline. MeHg/THg ratios are relatively low in early mining strata (0.02–0.03%), and increase during late-mining and post-mining strata (0.2–0.3%). The sediment MeHg profiles suggest active methylation in the neighborhood, coincident with wetlands bordering Torch Lake.
Comparison of THg concentrations in Upper Peninsula sediments from remote versus mining-impacted lakes
The complete set of surveyed lakes (Fig. 3, 4a, 10; Table 5) for THg in water and sediments show scattered values. Fig. 10 plots “undisturbed” versus “mining-disturbed” surface (top 10 cm; N = 10 subsamples) sediment concentrations of THg in Western Upper Peninsula lakes. With the exception of Lake Superior, all the lakes show elevated concentrations above typical undisturbed levels of 175 ppb THg (horizontal line). Even relatively isolated lakes show elevated concentrations of THg in sediments. However, lakes with evidence of mining disturbance, those in close proximity to mining regions, and those down-wind from smelters, show significantly increased enrichment above remote lakes. If we compare “mining-impacted” and “non-impacted, remote” lakes scattered around the Western Upper Peninsula and selected by MDEQ,76 there are widely different THg and MeHg sediment concentrations (Fig. 10; Table 5). Our application of “Mining-impacted” in Table 5 involves a conservative criterion, as it includes only lakes with tailings present (e.g. Portage, Torch, Sunday, Lake Michigamme, Deer Lake, Lac LaBelle), or those immediately downwind from smelters (Roland, Mud Lake). The “non-impacted, remote” lakes have no shoreline disturbance and are distant from smelter or river sources (e.g. Keewaydin, Chaney, Big Lake, McDonald Lakes). A nested ANOVA of “mining-impacted” and “non-impacted” sediment THg values from Fig. 10, shows a strong, significant difference (d.f. 1,86, F = 21.4, p = 1.29 × 10−5). Applying a single-classification ANOVA to Table 5 THg values, “mining-impacted” and “non-impacted” lakes also indicates significant differences (N = 16, F = 6.79, p = 0.019).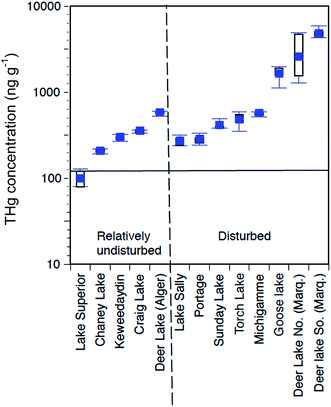 | ||
| Fig. 10 Total mercury (THg) concentrations in Western Upper Peninsula (WUP) top (<10 cm) lake sediments. Lakes are classified as mining-disturbed or undisturbed, depending on the presence of tailings in sediments. These lakes were selected in the MDEQ (2011) survey, with locations plotted in Fig. 3. Concentrations are in ppb THg dry wt., plotted on a log scale, expressed as box and whisker diagrams (mean, solid square; median, quartile, and range, horizontal lines). The dark horizontal line at 175 ppb dry wt. represents values normally associated with remote, undisturbed lakes, suggesting that almost all the regional lakes selected by MDEQ, with the exception of Lake Superior, contain moderately to highly elevated sediment concentrations of mercury. | ||
| Lake | THg (μg kg−1, ppb) | MeHg (μg kg−1, ppb) | Lat/Long |
|---|---|---|---|
| a Tailings in lake. b Proximity to smelter. | |||
| Mud Lake (NLA 0734)b | 484 | 0.53 | 47°7′55.67′′N/88°18′46.01′′W |
| McDonald Lake (NLA 1206) | 217 | 0.93 | 46°2′27.13′′N/86°48′19.51′′W |
| Lake Gogebic (NLA 1358) | 261 | 0.66 | 46°32′50.75′′N/89°36′49.03′′W |
| Forestville Basin (NLA 1654) | 214 | 1.51 | 46°34′23.74′′N/87°27′24.01′′W |
| Bailey Lake (NLA 1758) | 97 | 0.93 | 47°27′32.22′′N/88°5′18.06′′W |
| Big Lake (NLA 1742) | 359 | 0.5 | 46°36′51.30′′N/88°34′47.82′′W |
| Deer Lake(N), Marquette Co.a | 1500–5000(2860) | 2.1–8.0(5.8 ± 1.5) | 46°30′58.75′′N/87°40′1.78′′W |
| Michigamme, Marquette Co.a | 510–590(544 ± 26) | 0.6–2.0(1.8 ± 2.2) | 46°35′25.08′′N/87°56′5.78′′W |
| Lake Roland, Houghton Co.b | 300–500(400 ± 150) | — | 46°53′28.37′′N/88°51′14.14′′W |
| Rice Lake, Houghton co. | 100–300(100 ± 80) | — | 47°9′42.35′′N/88°16′39.29′′W |
| Goose Lake, Marquette Co.a | 1000–2000(1700 ± 300) | 2.0–4.7(2.8 ± 1.0) | 46°27′36.06′′N/87°30′9.72′′W |
| Lake Sally, Marquette Co.a | 220–330(260 ± 100) | 0.9–1.2(1.0 ± 0.1) | 46°28′14.89′′N/87°39′30.6′′W |
| Lac LaBelle, Keweenaw Coa | 200–500(200 ± 100) | — | 47°23′1.95′′N/88°0′59.44′′W |
| Portage Lakea | 300–500(400 ± 100) | 0.1–2.1(0.6 ± 0.7) | 47°3′14.79′′N/88°28′23.25′′W |
| Torch Lakea | 300–1100(900 ± 200) | 0.4–1.0(0.7 ± 0.3) | 47°11′12.99′′N/88°24′33.86′′W |
| Lake Keewaydin | 280–320(282 ± 100) | 0.3–1.0(0.6 ± 0.3) | 46°35′50.55′′N/88°7′26.21′′W |
| Chaney Lake | 180–220(203 ± 106) | 0.1–0.8(0.4 ± 0.2) | 46°18′13.73′′N/89°55′15.10′′W |
| Sunday Lakea | 360–500(417 ± 47) | — | 46°28′50.22′′N/89°28′50.22′′W |
High mercury values for Western Upper Peninsula fish
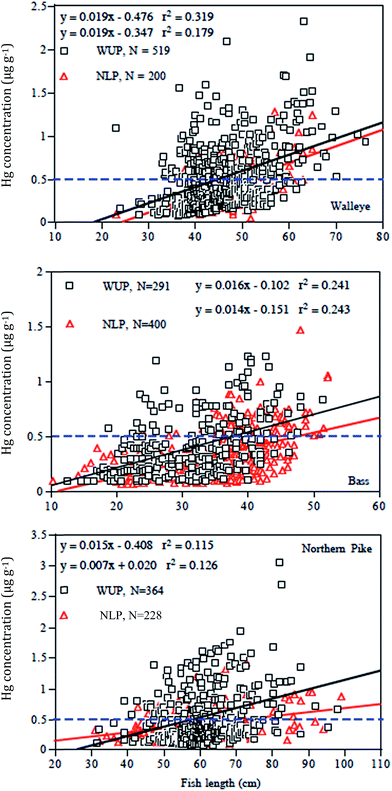
Despite the low present-day atmospheric deposition of THg in Western Upper Peninsula watersheds relative to Lower Peninsula sites (Fig. 1), and statements that emissions from industry have dropped around 80%, surveys of Upper and Lower Peninsula lakes indicate that concentrations of mercury are higher in Western Upper Peninsula (WUP) fish than in Northern Lower Peninsula (NLP) fish (Fig. 11; see Methods). In the figure, mercury concentrations (ppm) in large predatory fish (bass, walleye, northern pike) from WUP and from NLP are plotted against fish total length. Increasing mercury concentrations with fish length are expected, a carry-over from regressions in individual lakes. Dark squares represent WUP fish, whereas red diamonds represent NLP fish. Linear regressions fit to the data are similarly colored (black, WUP; red NLP) with statistics for regressions summarized in Table 6. The dashed horizontal line marks the “choices to avoid” for individual fish consumption.96 Notice that substantial numbers of fish fall above the EPA probable effects level (0.3 ppm) and that many fall above the EPA non-consumption level (0.97 ppm).| Fish | N | L | Equation | F value | Significance (p) | Slope SE | Intercept SE |
|---|---|---|---|---|---|---|---|
| Walleye (WUP) | 519 | 42 | Y = 0.019X − 0.35 | 112.8 | 5.69 × 10−24 | 0.002 | 0.08 |
| Walleye (NLP) | 200 | 20 | Y = 0.019X − 0.48 | 92.9 | 2.82 × 10−18 | 0.002 | 0.09 |
| Bass (WUP) | 291 | 31 | Y = 0.016X − 0.10 | 91.6 | 4.91 × 10−19 | 0.002 | 0.05 |
| Bass (NLP) | 400 | 37 | Y = 0.014X − 0.15 | 127.3 | 8.14 × 10−26 | 0.001 | 0.04 |
| Northern pike (WUP) | 364 | 37 | Y = 0.015X − 0.41 | 46.9 | 3.27 × 10−11 | 0.002 | 0.14 |
| Northern pike (NLP) | 228 | 33 | Y = 0.007X + 0.02 | 32.6 | 3.54 × 10−8 | 0.001 | 0.07 |
Despite considerable scatter in individual fish, in all cases the regressions for WUP walleye, bass, and northern pike fall above corresponding regressions for NLP fish. For example, the comparison for walleye contains Hg values from 719 fish, 519 fish from WUP (42 lakes, N = 519; mean ± SD = 12.3 ± 7.2 fish per lake; Y = 0.019X − 0.347, r2 = 0.179) and 200 fish from NLP (13 lakes, N = 200; 10.0 ± 4.9 fish per lake; Y = 0.019X − 0.476, r2 = 0.319). Slopes are similar, yet the regression is higher for WUP walleye relative to NLP walleye, regardless of fish length. WUP fish have a higher regression intercept value relative to that of NLP fish (WUP = −0.347 ± 0.166 95% C.L.; NLP = −0.476 ± 0.186). Specific locations for Western Upper Peninsula fish are given in Fig. 4b.
In the three fish comparisons, mercury concentration versus length regressions may have similar slopes but different intercepts (walleye), or they may have significantly different slopes (bass), or significant slope differences and close to significant intercept values (northern pike). All regressions have significant correlations with fish length (positive slope), a carry-over from anticipated highly significant relationships within individual lakes (Table 6). However, because the data set involves a mixed collection of fish from several lakes, regression r2 values are lower than expected if sampling came from single lakes (i.e. r2 range 0.115–0.319). Table 6 lists standard errors for regression slopes and intercepts. In the case of northern pike, the WUP slope has a value of 0.015, a standard error (S.E.) of 0.002, and 95%C.L. of 0.011–0.019 compared with an NLP slope of 0.007, a S.E. of 0.001, and 95%C.L. of 0.005–0.009. Likewise, the intercept for WUP has a value of −0.41, a S.E. of 0.14, and 95% C.L. of −0.68 to −0.13, whereas the intercept for NLP has a value of +0.02, a S.E. of 0.07, and 95% C.L. of +0.12 to −0.16. That is, the slopes are significantly different and the intercepts nearly so.
Untransformed mercury fish data for WUP versus NLP showed significant differences for all three species. However, to separate the contributing relationships of regional source and fish size, we conducted an Analysis of Covariance (ANCOVA) on log-transformed Hg values (log-transformation to better normalize data). For example, ANCOVA results indicated that walleye from the WUP have significantly higher values than walleye from the NLP (d.f. 1, 718; F = 14.3, p < 0.001). ANCOVA also indicated highly significant effects of body length in walleye (d.f. 1, 718; F = 199.8, p < 0.001) on mercury content, a dominating relationship expected from typical within-lake mercury versus fish length relationships. Highly significant regional (WUP vs. NLP) differences were also confirmed for bass and northern pike (bass, d.f. 1, 689; F = 13.8, p < 0.001; northern pike, d.f. 1, 590; F = 14.7, p < 0.001).
An example of EPA criteria applied to decent sample size collections from the Torch Lake AOC walleye is shown in Fig. 12a. As mentioned previously, mercury levels (ppm, wet wt.) increase with fish length (Y = 0.172X − 2.74; r2 = 0.782) and the correlations are tighter when dealing with individual lakes. Almost all fish from the 2013 data set are near to or above the EPA 0.3 ppm Human Health Criterion and 8 (40%) fall above the EPA no consumption level (0.97). A temporal comparison (Fig. 12b–d), using standardized length fish scores (regression-based single size; see Methods) indicates about a 1% increase in mercury concentration for walleye from Torch Lake (Fig. 12b, p = 0.06, not significant), 2% for Northern Pike (Fig. 12c, p = 0.02, significant), and 2% for smallmouth bass (Fig. 12d, p = 0.003, highly significant) over time.84 We emphasize that the northern inland lake fish concentrations are higher than those found for the same fish species in Lake Superior (Fig. 12b, walleye, see LBDN) or downstate.
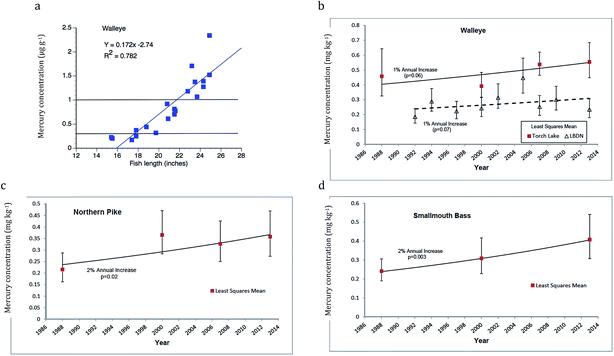 | ||
| Fig. 12 Mercury in Torch Lake fishes: (a) Recent length-related mercury concentrations (ppm), showing application of EPA 0.3 and 1.0 ppm standards for probable effects and no consumption. Regression equation and R2 value given above figure. Long-term trends in Torch Lake for standard length (b) walleye, (c) northern pike, and (d) smallmouth bass. (b) Compares trends in L'anse Bay, Lake Superior. Fish mercury concentration is expressed in mg kg−1 = ppm wet wt.; means with 95% C.L.; trends show increases (1–2%) with time (Data from Bohr84). | ||
Discussion
Mercury concentrations in ores, smelter emissions and mill tailing releases
Over a hundred years ago, mercury was imported into gold and silver mining regions of Lake Superior and used in the amalgamation process.35 Yet mercury also occurs as an impurity in copper, silver, zinc, lead and nickel ores, as well as in gold ores.18,91 Early compilations for zinc97 and copper and silver,13,18,46,91 have transformed into application of global emission factors.3,98 Global emission studies list Cu smelting as emitting 5.0–6.0 g Mg−1 (i.e. 5.0–6.0 g THg for 1 million g copper smelted), pig iron and steel production at 0.04 g Mg−1, coal combustion from power plants at 0.04–0.3 g Mg−1, and gold production at 0.5 g g−1.3 Our values for Keweenaw native copper and White Pine (both 4.0 g Mg−1) are very comparable to Pirrone et al.'s3 USGS global values and compare favorably with other global averages (4.6 g Mg−1 (ref. 91)). Kocman et al.98 argue that, relative to global atmospheric emissions, non-ferrous metal (Cu, Pb, Zn) contributions are second only to artisanal and commercial gold mining. However, the considerable variation among mercury concentrations in individual ore deposits, and differences in refining methods, require that general emission factors be applied with caution to individual operations.91Scattered tailings piles and drainage from mining shafts (Fig. 3) continue to leach metals into streams and rivers, spreading copper and mercury over watersheds. Additional dispersal came until recently from historic smelter emissions. Using EPA AERMOD modeling, we illustrated how emissions from the Calumet-Hecla Smelter at its peak operation were spread tens of kilometers over the Keweenaw Peninsula landscape. Sediment core records from nearby Portage and Torch Lakes, Lac La Belle to the north, and Lake Roland sediments east of White Pine, show elevated metal and total mercury peaks in profiles that coincide with the time of smelter and tailings releases.18,35,53,99 The White Pine Smelter closed immediately following a court settlement aimed at reducing mercury, sulfate, lead, and cadmium emissions.100 The settlement acknowledged elevated mercury levels in Native American populations located up to 80 km east of the smelter. Recently reported MeHg concentrations from Portage Lake ranged between 0.2–3 μg kg−1 during the mining era.12 Similar surface values are found in recent surveys of MeHg near historic mining sites (Fig. 3; MDEQ 2011: Lake Michigamme, MeHg 0.6–2 μg kg−1; Goose Lake 2.0–4.7 μg kg−1; Deer Lake 2–8 μg kg−1).76 Ancillary SO2 emissions (Table 4) from White Pine and iron mining also may have promoted Hg methylation in widely distributed wetlands, a process investigated by Gilmouret al.101 and Watras et al.102
The compilation of mercury emissions from copper smelter operations combined with estimates of deposition based on local AERMOD modeling suggest that local ore processing contributed importantly to historic atmospheric mercury deposition (Fig. 7). Direct measurements of fluxes in Torch Lake and Portage Lake sediment cores confirmed major mining releases (smelter and tailings) during the active mining period. Releases locally increased THg deposition rates around 50- to 250-fold and led to a pulse of methylation.12 The existence of methyl mercury deep within the mining-era sediments of both Portage and Torch Lakes provided unambiguous proof that some of the mercury in local mining discharges was subject to methylation. The majority of MeHg was deposited during the mining era in both Portage Lake (91%) and Torch Lake (76%) core records. Good preservation of MeHg and other organic remains in Portage and Torch Lake might have been favored by high copper concentrations, which reduced microbial diversity to only a few resistant strains.103 However, there was a subsequent time lag of about 20–40 years in MeHg production, compared with the earlier THg deposition, stretching into recent periods in Torch Lake. Some might argue that the MeHg was generated recently in situ by modern bacteria. However, the high Cu concentrations might also restrict active methylating bacteria in lower core strata, and that could be investigated by microbe analysis. Time delays in MeHg concentration profiles are expected, since elemental mercury emitted from smelter discharges is not only deposited in the immediate Waterway vicinity, but spread around surrounding watersheds and incorporated into forests. The total accumulating in surficial Waterway sediments might include local methylation plus return from the watershed.62,104,105 Some workers have tried to estimate the time lag in loading to lakes, and point out that the effect is magnified in lakes with large watersheds, similar to the Keweenaw Waterway landscape.106,107
Lingering effects of mining
Total mercury deposition in 91 sediment cores from Great Lakes inland lakes, supposedly “removed from disturbed watersheds”, was recently reviewed by Drevnick et al.77 Mercury accumulation in pre-industrial sediments averaged around 12 μg m−2 per year, 1970 strata 35 μg m−2 per year, post-industrial peak (1985) strata around 54 μg m−2 per year, and recent levels around 43 μg m−2 per year, suggesting general regional decline. However, many of the coring sites used in this compilation are scattered between large down-state cities (Chicago, Detroit, Cleveland, Buffalo), and are more comparable to mid-continental NADP and gross depositional values. The Engstrom62,63 sites used in Fig. 7b are northern and removed from “disturbed watersheds”, and show less elevated values.Despite 45 years and 66 years, respectively, since native copper mining closed around the northern Keweenaw Peninsula (Keweenaw Waterway), total Hg concentrations in recent Torch Lake (240–650 ng g−1) and Portage Lake (250–800 ng g−1) sediments clearly exceed background concentrations (13–48 ng g−1) measured in deep sediments of Lake Superior and Portage Lake, and are at the upper end of concentration ranges reported for other lake sediments in the region. When fluxes of THg from modern sediments of Portage (50–500 μg m−2 per year) and Torch Lakes (60–300 μg m−2 per year) are compared with accumulation rates in surface sediments of non-mining impacted lakes, the Keweenaw Waterway stands out today as having rates 2–60 times higher.12 Measured sediment focusing factors in both Portage and Torch Lake range between 0.2–1.7, too low to account for the observed elevated accumulation rates.53,72 That is, there seems to be a persistent lingering effect of mining in the Keweenaw region that overrides complete recovery, decades after cessation of active mining.
As far as stream and river connections, it is clear that legacy mining continues to release mercury into surface waters in the copper mining region of the Keweenaw Peninsula. Concentrations of mercury as high as 310 ng L−1 were measured in the outflow from the Kingston mine, and concentrations in the outflow from the Osceola mine were 130 ng L−1.27 Concentrations of mercury in mining impacted streams (n = 17, Degraeve et al.27; MDEQ79) are significantly higher (p < 0.05) than in non-impacted streams (n = 38) in Michigan's Upper Peninsula (Degraeve et al.27; Knauer et al.76; MDEQ28). Flow monitoring at the Osceola mine discharge indicates that the annual release of mercury from the mine is on the order of 400 g per year. If all of this mercury was transported to Torch Lake, it would amount to a loading of 44 μg m−2 per year, much higher than the loading from atmospheric deposition (∼15 μg m−2 per year). Given the very high number of exposed vertical shafts (Fig. 3), horizontal adits, and tailings piles scattered across the Western Upper Peninsula, there is probably widespread influence on drainage systems. The higher total mercury in stream, river, and lake sediments found by MDEQ across the Western Upper Peninsula makes sense as largely a consequence of legacy mining effects and bedrock influence. However, the number and location of mine shaft discharges into rivers have not been systematically assessed, so the total magnitude of mercury loading from shaft seepage and the magnitude of geographic effects cannot yet be determined.
Widespread wetland effects and mercury in fish
Our studies underscore that the potential risk to human health created by high mercury levels in inland Great Lakes fish is serious. The newly recommended meal frequency categories developed by the Great Lakes Fish Advisory Workshop (2007), using the U.S.EPA human health criterion of 0.30 ppm, underscore concerns about north-south geographic patterns. Based on updated U.S.EPA threshold levels, piscivorous fish (smallmouth and largemouth bass, walleye, northern pike, and muskellunge) from many northern inland lakes pose a serious consumption risk, many large fish falling above the EPA “no consumption” category.108,109 Long-term mercury trends in fish and herring gull in the entire Great Lakes Region show a general decline, largely attributed to the previous cited general reductions in regional mercury emissions.110,111. However, the north-south Michigan Peninsula inland lake patterns appear to run contrary to expectations. Concentrations have not fallen and seem to be increasing in several monitored lakes.84 The global presence of Hg contamination is largely attributable to a 3–5 fold increase in atmospheric Hg concentrations over the past 150 years, with regional and local releases from mining and industrial operations contributing to the loading. Of course, the copper and iron mining activities of the Upper Peninsula were an integral part of the early industrial revolution releases in North America.We have emphasized historic THg loading to the Upper Peninsula associated with legacy mining. However, fish MeHg levels depend not only on THg load but also on food web structure (which governs biomagnification) and biogeochemistry (which governs methylation). In nature. methylation is a function both of microbial activity and the bioavailability of inorganic Hg to methylating microbes. Dissolved total mercury and DOC correlations found in the Eastern Upper Peninsula by MDEQ (Fig. 3, 4a) suggest that biomagnification of mercury up food webs and MeHg mobilization are associated broadly across the Upper Peninsula with biogeochemical cycling in wetlands. Early studies by Grieb et al.112 in the Upper Peninsula, using a stratified random sampling design weighted for low pH (to assess acidification effects) found MeHg concentrations in yellow perch negatively correlated with pH and acid neutralizing capacity (ANC), suggesting the importance of wetlands. We stress that the abundant wetlands in the Upper Peninsula and northern Wisconsin are well recognized now to enhance MeHg production (e.g. Watras' studies in Wisconsin waters,15,102,113 Hurley and collaborators studies in the Seney Tract of the Eastern U.P.29–31). In general, both field studies and experimental data have demonstrated correlations between dissolved organic carbon (DOC) concentrations and MeHg production.
Our local comparisons of fish MeHg concentrations and lake physical and chemical properties in 62 scattered lakes (Fig. 4b) are mentioned elsewhere;83 but we briefly review correlations with chemical and landscape variables here. A major factor making Upper Peninsula lakes vulnerable to high bioaccumulation of MeHg is primarily the abundance of wetlands. In the Upper Peninsula, 29% of the land area is occupied by wetlands. Wetlands are important sites of Hg methylation, subsequently transporting MeHg to lakes.114,115 Wetlands also export DOC to lakes. The DOC can bind with and transport inorganic Hg to lakes. Among the lakes, the median DOC was 7.7 mg L−1. The study of MeHg in walleye found highly significant positive geographic correlations locally with % wetland (r = 0.718) and watershed area:lake area (r = 0.636), and significant negative correlations with secchi disk transparency (r = −0.659) and maximum depth (r = −0.482). These comparisons recall results from previous surveys in the Upper Peninsula, northern Wisconsin and inland Great Lakes.112,113 The first positive correlation emphasizes proximity to wetland environments, whereas the second underscores the well-known relationship between basin catchment area and sediment mercury concentrations.14 The negative secchi disk and depth correlations suggest that elevated concentrations are more likely in smaller lakes where epilimnetic temperatures are greater and where enhanced DOC reduces water column transparency and enhances anaerobic activity. A glance at Fig. 4b shows that peak fish values are scattered about the landscape, but seem more clustered around western and middle mining regions of the Western Upper Peninsula than in the eastern (wetland only) region. Statistical tests show concentrations of Hg in walleye were higher in western than in eastern Upper Peninsula lakes (t-test, p = 0.037), although admittedly more eastern lakes need to be sampled.
In MDEQ studies, the DOC-MeHg connection should not be surprising. For biomagnification up food webs, the bioavailability of Hg is related to the fraction of total Hg present as methyl mercury. Availability reflects numerous processes, including relative rates of methylation and demethylation and relative mobilities of MeHg and THg.116,117 Recent assessments of mercury geochemistry suggest that in most natural waters, Hg(II) exists as a mixture of dissolved, colloidal, and particulate phases. Furthermore, the dissolved (and possibly colloidal) forms of mercury are associated with natural organic matter (NOM, DOC), particularly binding with sulfhydryl functional groups on the organic matter. In other settings located near a source of sulfide, this Hg(II) can be complexed by inorganic sulfides.101,118 The combination of elevated mercury and SO2 discharges (White Pine Smelter; iron furnaces, taconite plants) could be synergistic to methylation in regional wetlands. From Watras's studies in Wisconsin lakes, there is an important theoretical relationship between mercury methylation rate and two key substrates, inorganic mercury and sulfate (Fig. 13). The interaction of Hg(II) and SO4 on methylation rates in anoxic waters and sediments can be described by the equation:
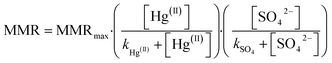 | (2) |
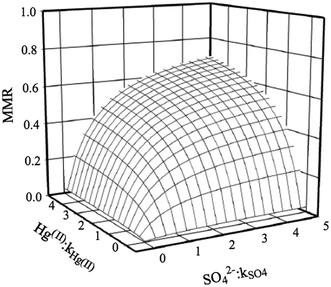 | ||
| Fig. 13 Theoretical relationship between mercury methylation rate (MMR) and two key substrates, inorganic mercury and sulfate (source: McDonald et al.,120 after Watras119). | ||
Lake to lake variability suggests that additional factors are also contributing to Upper Peninsula patterns. In northern Wisconsin, a seepage lake with little catchment showed a rapid decline in water and fish Hg over the same time that a drainage lake with considerable wetland in the catchment showed a decline in total Hg but little change in MeHg.121 Another factor is the recently discovered strong negative interaction between selenium from iron mining (Empire Mine, Tilden Mine) and mercury levels in fish.122 Selenium competes with mercury and reduces MeHg concentrations in fish. Unfortunately, partly because MDEQ water/sediment surveys and fish surveys were not carried out in the same lakes, nor checked with selenium levels, the relationship between high water and sediment mercury values and MeHg concentrations in fish could not be better disentangled.
Historic forest/wetland recovery and production of MeHg
The geographic correlation of mercury with DOC and wetlands suggests that wetlands are key ingredients to the north-south pattern, over-riding present-day atmospheric deposition. However, landscape composition is not static, forest and wetland recovery may also be contributing to regional MeHg production beyond simple joint effects. Key time intervals include: (1) Euro-American settlement, circa 1850; (2) the period of maximum clearing for agriculture following widespread forest logging, ca. 1935; and (3) ca. 1993 farm abandonment and forest recovery. More detailed regional reconstructions include “witness tree” records compared with USDA Forest Service Inventory and Analysis (FIA) Program data.123,124 In particular, the USGS LUHNA Program utilized a combination of “witness tree”records (General Land Office Survey), pollen core diagrams, and Forest Service Inventory information to reconstruct historical landcover changes in the Great Lakes Region over the past 1000 years.124 Looking at changes over the past 150 years, since European colonization, the LUHNA Program initially found two dramatic changes: conversion of forest to farmland and transformation through logging of other forest types to aspen-birch forest. The total amount of forested area initially declined over 40%, largely through conversion of northern mesic forest and oak forest to farmland in southern regions. An additional 21% of the presettlement forests, mostly pine in the northern regions, was converted to early successional forests of aspen and birch following logging. Only 39% of presettlement forests did not change major type since settlement. Forest types that declined initially included pine forest (−78%), boreal forest and conifer swamp (−62%), and northern mesic forest (−61%). The only forest type that increased was aspen-birch forest (+83%). Farmland practices drained wetlands, swamps, and wet mesic environments.From about 1840 to 1900, most of the Michigan forests were cut down for farms and to produce lumber for buildings, ships, and mines. Michigan was the nation's leading lumber producer between 1869 and 1900. Logs were floated down rivers and across lakes to sawmills. The first sawmill was built in 1832 at the mouth of the Menominee River in the Upper Peninsula. Sawn lumber was loaded onto ships for transport to down-state markets, or stacked on railroad cars. Mining created a large local demand for timber. Timber was used for mining shaft support and for charcoal in smelting pig iron. The furnaces of the Upper Peninsula burned around 30 acres of hardwood timber each day. Most wetlands were logged, but many were ditched and drained. Early copper and iron smelters used hardwood for fuel, whereas copper and iron mines used timbers for shaft support.40 Settlers would often burn the woods to help clear the land, starting terrific forest fires. By 1897, over 160 billion board feet was logged from Michigan forests. For comparison, today's forests hold about 70 billion board feet of saw-timber (Michigan Forest History, Michigan State Extension: Teacher's Guide http://mff.dsisd.net). Recently, forested and wetland environments are returning as large northern tracts are managed more for sustainability, regional watersheds are converted to state and federal forests, and wetland ditching is reduced. Forests and wetlands are under transition; simply put, there are now more wetlands and the numbers are increasing. We believe increases in wetlands may also be modifying mercury cycling and should be checked as an additional factor potentially contributing to recent local upward trends of MeHg in fishes.
There are additional geographic interactions that go beyond point loading (smelter emissions, tailings releases, mine seepage into streams), since DOC seepage through tailings also greatly increases metal concentrations.125 Whereas the exact geographic mix of sources (atmospheric deposition, bedrock mineral release, mining discharges) and details of watershed processing (methylation in marshes, lakes, river hyporheic systems, sequestration in forests and soils) require additional work,12 we suggest that the regional interaction between legacy mining effects and methylation environments contributes to enhanced MeHg concentrations in Western Upper Peninsula fish. Legacy mining inputs of both Hg and SO42− could enrich MeHg concentrations and perpetuate “lingering effects”. The prediction from ELA watershed experimental studies (Harris et al.126) that mercury emission reductions will yield rapid (years) reductions in fish methylmercury concentrations and associated risk does not appear to be playing out in the Upper Peninsula. Mercury levels are high in fish near mining sites decades after operations cease with evidence for either little change or actual increase (1–2%) through time.84 We suggest that “lingering effects” from Hg and SO42− mining inputs are evident throughout the region and are superimposed upon elevated regional wetland methylation, both contributing to a pattern that reverses latitudinal gradients expected from modern atmospheric deposition.
Conclusions
The north-south geographic enigma in Michigan appears real, associated with elevated mercury loading in the U.P. by legacy mining operations for over 140 years superimposed upon active wetland MeHg mobilization. Legacy loading effects from mining and Hg-rich bedrock help explain north-south differences in river and lake sediment THg levels. Elevated regional MeHg concentrations in fish also appear real, and the regional comparison suggests a 10–20% increase in the Western Upper Peninsula. A future test with more power would be a larger, random sampling of fish Hg levels from three sites: Western Upper Peninsula (mining influence + wetland), Eastern Upper Peninsula (wetland, no mining influence), and Northern Lower Peninsula (lower Peninsula, no mining influence). That 3-way comparison would evaluate MeHg increases due to both mining and wetland influence in the Upper Peninsula relative to the Lower Peninsula.In a sense, we are simply saying that one of the worst landscapes for mining mercury releases is into a wetland environment. There are at least five regions in the Lake Superior Basin where mining activities may have increased background mercury loading to the landscape and fish. Most have wetlands, and all are also experiencing degrees of forest recovery: Upper Peninsula of Michigan; NE Minnesota; Thunder Bay, Lake Nipigon, and Wawa regions of Ontario.35 Even though mining was not as severe in Wisconsin as in Minnesota and Michigan, iron mining was extensive along the northern portion of the state, so comparisons along the northern rim also might be worthwhile. If there are distinctive “fingerprints” from local copper and iron ores, application of mercury stable isotope studies127 in the Upper Peninsula and elsewhere should help untangle mining versus distant atmospheric source contributions to fish, ultimately resolving some of the patchwork patterns.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was funded in part by a Michigan Department of Environmental Quality (MDEQ) grant (Long-Term Monitoring Study, Torch Lake Superfund Site) with additional support from the NSF OCE 97-12872 (NOAA/NSF KITES) Project to WCK and NRU and from NSF ICER-1313755 to JAP and NRU. The authors acknowledge Lakes Environmental, which provided the AERMOD View software used here. The information in this document, especially MeHg and THg analyses, also has been funded (in part) by the U.S. Environmental Protection Agency (Ronald Rossmann). Regional fish mercury concentration data, comparing Western Upper Peninsula with Northern Lower Peninsula areas, were forwarded by Joe Bohr, MDEQ, as part of the Michigan DEQ fish contaminant monitoring program. Many of the ore samples analyzed for mercury content came from the A. E. Seaman Mineral Museum, Michigan Tech University, where we thank George W. Robinson for assistance. Mining company reports and communications in Michigan Tech Archives, Van Pelt and Opie Library, aided reconstruction of annual copper production from Keweenaw Waterway operations and the White Pine Mine. This is contribution number 51 of the Great Lakes Research Center at Michigan Tech.References
- L. Jarup, Hazards of heavy metal contamination, Br. Med. Bull., 2003, 68(1), 167–182 CrossRef PubMed.
- E. B. Swain, P. M. Jakus, G. Rice, F. Lupi, P. A. Maxxon, J. M. Pacyna, A. Penn, S. J. Spiegal and M. M. Veiga, Socioeconomic consequences of mercury use and pollution, Ambio, 2007, 36, 45–61 CrossRef PubMed.
- N. Pirrone, S. Cinnirella, X. Feng, R. B. Finkelman, H. R. Friedii, J. Leaner, R. Mason, A. B. Mukherjee, G. B. Stracher, D. G. Streets and K. Teimer, Global mercury emissions to the atmosphere from anthropogenic and natural sources, Atmos. Chem. Phys., 2010, 10, 5951–5964 CrossRef CAS.
- D. M. Steinway, K. A. Ewing, D. R. Case, K. J. Nardi and K. J. Brownell, Environmental Law Handbook, Government Institutes, 20 edn, 2009 Search PubMed.
- C. Seigneur, K. Vijayaraghavan, K. Lohman, P. Karamchandani and C. Scott, Global source attribution for mercury deposition in the United States, Environ. Sci. Technol., 2004, 38(2), 555–569 CrossRef CAS PubMed.
- M. Cohen, R. Artz, R. Draxler, P. Miller, L. Poissant and D. Niemi, et al., Modeling the atmospheric transport and deposition of mercury to the Great Lakes, Environ. Res., 2004, 95, 247–265 CrossRef CAS PubMed.
- M. R. Risch, D. A. Gay, K. K. Fowler, G. J. Keeler, S. M. Backus, P. Blanchard, J. A. Barres and J. T. Dvonch, Spatial patterns and temporal trends in mercury concentrations, precipitation depths, and mercury wet deposition in the North American Great Lakes Region, 2002–2008, Environ. Pollut., 2012, 161, 261–271 CrossRef CAS PubMed.
- G. L. Keeler and J. T. Dvonch, Atmospheric mercury: A decade of observations in the Great Lakes, in Dynamics of mercury pollution on regional and global scales: Atmospheric processes and human exposures around the world, ed. N. Pirrone and K. R. Mahaffey, Springer Publishers, Norwell, MA, 2005, pp. 611–636 Search PubMed.
- D. Gay, An overview of the Mercury Deposition Network in the US & Upper Midwest. Illinois State Water Survey, University of Illinois, Champaign, IL, 2009 Search PubMed.
- L. Zhang, et al., Assessment of modeled mercury dry deposition over the Great Lakes region, Environ. Pollut., 2012, 161, 272–283 CrossRef CAS PubMed.
- T. Butler, G. Likens, M. Cohen and F. Vermeylen, Final Report: Mercury in the Environment and Patterns of Mercury Deposition from the NADP/MDN Mercury Deposition Network. 2007, p. 87 Search PubMed.
- W. C. Kerfoot, N. R. Urban, C. P. McDonald, R. Rossmann and H. Zhang, Legacy mercury releases during copper mining near Lake Superior, J. Great Lakes Res., 2016, 42, 50–61 CrossRef.
- W. C. Kerfoot, S. L. Harting, J. Jeong, J. A. Robbins and R. Rossmann, Local, regional, and global implications of elemental mercury in metal (copper, silver, gold, and zinc) ores: Insights from Lake Superior sediments, J. Great Lakes Res., 2004, 30(suppl. 1), 162–184 CrossRef CAS.
- E. B. Swain, D. R. Engstrom, M. E. Bringham, T. A. Henning and P. L. Brezonik, Increasing rates of atmospheric mercury deposition in midcontinental North America, Science, 1992, 257, 784–787 CAS.
- C. J. Watras, N. S. Bloom, R. J. M. Hudson, S. Gherini, R. Munson, S. A. Claas, et al., Sources and fates of mercury and methylmercury in Wisconsin lakes, in Mercury Pollution, Integration and Synthesis, ed. C. J. Watras and J. W. Huckabee, Lewis Publ., Ann Arbor, Michigan, 1994, pp. 153–177 Search PubMed.
- D. R. Engstrom, S. J. Balogh and E. B. Swain, History of mercury inputs to Minnesota lakes: influences of watershed disturbance and localized atmospheric deposition, Limnol. Oceanogr., 2007, 52(6), 2467–2483 CrossRef CAS.
- A. L. Kemp, J. D. H. Williams, R. L. Thomas and M. L. Gregory, Impact of man's activities on the chemical composition of the sediments of lakes Superior and Huron, Water, Air, Soil Pollut., 1978, 10, 381–402 CrossRef CAS.
- W. C. Kerfoot, S. L. Harting, R. Rossmann and J. A. Robbins, Elemental mercury in copper, silver, and gold ores: an unexpected contribution to Lake Superior sediments with global implications. Geochemistry: Exploration, Environment, Analysis, 2002, 2, 185–202 CAS , Special Volume: Metal Mining and The Environment.
- D. C. Harris, The mineralogy and geochemistry of the Hemlo Gold deposit, Ontario, Economic Geology Report no. 38, Geological Survey of Canada, 1989, p. 88.
- W. G. Powell and D. R. M. Pattison, An exsolution origin for low-temperature sulfides at the Hemlo gold deposit, Ontario, Econ. Geol. Rep., 1997, 92, 569–577 CrossRef CAS.
- D. W. Hendriks and G. Chevalier, Recovery of gold using gravity concentration: the Hemlo experience, Eur. J. Miner. Process. Environ. Protect., 2001, 208–219 Search PubMed , Kneisen Group, Technical Reports and Abstracts.
- MDNR, Stack Test: Main stack of the Copper Range Company, Almega Corporation for Air Quality Division, MDNR, White Pine, Michigan, 1990 Search PubMed.
- H. Jiang, C. Y. Wu and T. Biewen, Metals emissions from taconite ore processing facilities in Minnesota, Minnesota Pollution Control Agency, 520 Lafayette Road North, St. Paul, MN 55155, 2000, p.11 Search PubMed.
- M. E. Berndt, Mercury and mining in Minnesota. Minerals Coordinating Committee Final Report, Minnesota Department of Natural Resources, St. Paul, MN, 2003, p. 58 Search PubMed.
- LSBP (Lake Superior Binational Program), Lake Superior LaMP 2002 Progress Report, 2002.
- LSBP (Lake Superior Binational Program), 2012 Lake Superior Lakewide Management Plan: 1990–2010 Critical chemicals milestones document, 2012, p. 104 Search PubMed.
- M. Degraeve and D. McCauley, Low Level Mercury Concentrations in Selected Michigan Surface Waters, Summer/Fall 2002, Great Lakes Environmental Center, Traverse City, MI, 2003, p. 9 Search PubMed.
- MDEQ/WRD, Michigan's water chemistry monitoring program, A report of statewide spatial patterns 2005–2009, and fixed station status and trends 1998–2008. Water Chemistry Monitoring Program, Surface water assessment Section, Water Resources Division, 2013, Report 13/005, p. 71 Search PubMed.
- V. L. St. Louis, J. W. M. Rudd, C. A. Kelly, K. G. Beaty, R. J. Flett and N. T. Roulet, Production and loss of methylmercury and loss of total mercury from boreal forest catchments containing different types of wetlands, Environ. Sci. Technol., 1996, 30, 2719–2729 CrossRef CAS.
- J. P. Hurley, J. M. Benoit, C. L. Babiarz, M. M. Shafer, A. W. Andren and J. R. Sullivan, et al., Influences of watershed characteristics on mercury levels in Wisconsin rivers, Environ. Sci. Technol., 1999, 29(7), 1867–1875 CrossRef PubMed.
- R. W. Stoor, J. P. Hurley, C. L. Babiarz and D. E. Armstrong, Subsurface sources of methyl mercury to Lake Superior from a wetland-forested watershed, Sci. Total Environ., 2006, 2006(368), 99–110 CrossRef PubMed.
- H. A. Simonin, J. J. Loukmas, L. C. Skinner and K. M. Roy, Lake variability: Key factors controlling mercury concentrations in New York State fish, Environ. Pollut., 2008, 154(1), 107–115 CrossRef CAS PubMed.
- C. L. Folt, C. Y. Chen and P. C. Pickhardt, Using plankton food web variables as indicators for the accumulation of toxic metals in fish, in Biomarkers of environmentally associated disease: Technologies, concepts, and perspectives, ed. S.H. Wilson and W.A. Suk, CRC Press/Lewis Publishers, Boca Raton, 2002, pp. 287–306 Search PubMed.
- K. R. Rolfhus, B. D. Hall, B. A. Monson, M. J. Paterson and J. D. Jeremiason, Assessment of mercury bioaccumulation within the pelagic food web of lakes in the western Great Lakes region, Ecotoxicology, 2011, 20, 1520–1529 CrossRef CAS PubMed.
- W. C. Kerfoot, J. Jeong and J. A. Robbins, Lake Superior mining and the proposed Mercury Zero-discharge Region in State of Lake Superior, ed. M. Munawar and I. F. Munawar, Ecovision World Monograph Series, 2009, pp. 153–216 Search PubMed.
- M. S. Gustin, M. F. Coolbaugh, M. A. Engle, B. C Fitzgerald, R. E. Keislar and S. E Lindberg, et al., Atmospheric mercury emissions from mine wastes and surrounding geologically enriched terrains, Environ. Geol., 2003, 43, 339–351 CAS.
- J. E. Gray, M. E. Hines, P. L. Higueras, I. Adatto and B. K. Lasorsa, Mercury speciation and microbial transformations in mine wastes, stream sediments, and surface waters at the Almaden mining district Spain, Environ. Sci. Technol., 2004, 38(16), 4285–4292 CrossRef CAS PubMed.
- G. Qiu, X. Feng, S. Wang and L. Shang, Mercury and methylmercury in riparian soil, sediments, mine-waste calcines, and moss from abandoned Hg mines in east Guizhou province, southwestern China, Appl. Geochem., 2005, 20(3), 627–638 CrossRef CAS.
- W. C. Kerfoot, G. Lauster and J. A. Robbins, Paleolimnological study of copper mining around Lake Superior: Artificial varves from Portage Lake provide a high resolution record, Limnol. Oceanogr., 1994, 39(3), 649–689 CrossRef CAS.
- L. D. Lankton, Hollowed ground: Copper mining and community building on Lake Superior, 1840’s-1990's, Detroit, MI, Wayne State University Press, 2010 Search PubMed.
- N. Langston, Sustaining Lake Superior, Yale University Press, 2017 Search PubMed.
- A. Wilson, Silver Islet, The Mineralogical Record, 1986, vol. 17, ch. 1, pp. 49–60 Search PubMed.
- H. B. Cronshaw, Silver Ores, John Murry, Albemaric Street, W. London, 1921 Search PubMed.
- Aquila Resources Inc., Geochemical Investigation Report, ID: 14A021. Treatment and containment plan for tailings and waste rock, Aquila Resources Inc., Stephenson, MI, 2015 Search PubMed.
- MDEQ, Air deposition impact analysis for the Aquila Back Forty Project, Air Quality Division. Lansing, MI, 2016, p. 6 Search PubMed.
- W. C. Kerfoot, S. L. Harting, R. Rossmann and J. A. Robbins, Mercury in metal ore deposits: an unrecognized widespread source to Lake Superior sediments, Abstract in 11th Annual International Conference on Heavy Metals in the Environment, ed. J. Nriagu, University of Michigan, School of Public Health, Ann Arbor, MI (CD-ROM), 2000 Search PubMed.
- W. H. Newhouse, Mercury in native silver, American Mineralogist, 1933, vol. 18, pp. 295–299 Search PubMed.
- J. E. Votava and T. J. Bornhorst, Towards a geochemical characterization of native copper ores of the Keweenaw. Proceedings, Institute on Lake Superior Geology, Ashland, Wisconsin, 2011, vol. 57 Search PubMed.
- A. L. Hawkins, J. A. Petrus, L. M. Anselmi and G. Crawford, Laser ablation-inductively coupled plasma-mass spectrometry analysis of copper-based artifacts from Southern Ontario and the chronology of the indirect contact period, J. Archaeol. Sci., 2016, 6, 332–341 CrossRef.
- USEPA-600, Design and Operating Parameters for Emission Control Studies: White Pine Copper Smelter, U.S. Environmental Protection, 1976, p. 39 Search PubMed.
- USEPA., Site Visit Report: Copper Range Company, White Pine Mine, Office of Solid Waste, 401 M. Street SW, Washington DC., 1992, p. 48 Search PubMed.
- J. H. Churchill and W. C. Kerfoot, The impact of surface heat flux and wind on thermal stratification in Portage Lake., Michigan., J. Great Lake. Res., 2007, 33, 143–155 CrossRef.
- W. C. Kerfoot and J. A. Robbins, Nearshore regions of Lake Superior: Multi-element signatures of mining discharges and a test of Pb-210 deposition under conditions of variable sediment mass flux, J. Great Lake. Res., 1999, 25(4), 697–720 CrossRef CAS.
- C. P. McDonald and N. R. Urban, Sediment isotope dating across a stratigraphic discontinuity in a mining-impacted lake, J. Environ. Radioact., 2007, 92, 80–95 CrossRef CAS PubMed.
- C. P. McDonald, N. R. Urban, J. H. Barkach and D. McCauley, Copper profiles in the sediments of a mining-impacted lake, J. Soils Sediments, 2010, 10, 343–348 CrossRef CAS.
- R. C. Reed, Economic geology and history of metallic minerals in the Northern Peninsula of Michigan, in Early sedimentary evolution of the Michigan Basin, ed. P. A. Catacosinos and P.A. Daniels Jr, USGS Special Paper 256, 1991, p. 248 Search PubMed.
- T. S. Reynolds, “The miners dread to use it”: A comparative study of the introduction of nitroglycerin in the Lake Superior Copper and Iron Ranges 1865–1880, J. Soils Sediments, 2011, 18, 66–86 Search PubMed.
- MDEQ, 2006. Air Quality Division's Atmospheric Mercury Monitoring activities. Monitoring in the Upper Peninsula, Lansing Area, and Detroit Area. 2006, July 2005, https://www.michigan.gov/documents/deq-aqd-toxics-July_2005_Merc_Mon_Rpt_158779_7.pdf Search PubMed.
- M. R. Risch, Mercury and Methylmercury Concentrations and Litterfall Mass in Autumn Litterfall Samples Collected at Selected National Atmospheric Deposition Program Sites in 2007–2009 and 2012–2015, U.S. Geological Survey data release, U.S. Geological Survey, 2017 Search PubMed.
- J. A. Wiklund, J. L. Kirk, D. C. G. Muir, M. Evans, F. Yang and J. Keating, et al., Anthropogenic mercury deposition in Flin Flon Manitoba and the Experimental Lakes Area Ontario (Canada): A multi-lake sediment core reconstruction, Sci. Total Environ., 2017, 586, 685–695 CrossRef CAS PubMed.
- J. Naylor, 40 years on, stack remains an icon of north, Flin Flon Reminder, Glacier Media Group, Flin Flon, Manitoba, Canada, 2014 Search PubMed.
- D. R. Engstrom, E. B. Swain, T. A. Henning, M. E. Brigham and P. L. Brezonik, Atmospheric mercury deposition to lakes and watersheds: A quantitative reconstruction from multiple sediment cores, Adv. Chem. Ser., 1993, 237, 33–66 CrossRef.
- D. R. Engstrom and E. B. Swain, Recent declines in atmospheric mercury deposition in the upper Midwest, Environ. Sci. Technol., 1997, 31, 960–967 CrossRef CAS.
- C. H. Benedict, Lake Superior milling practice, Michigan College of Mining and Technology Press, Houghton, MI, 1955 Search PubMed.
- H. D. Conant, Min. Congr. J., 1931, 17, 531–532 Search PubMed.
- G. Rice, R. Ambrose, O. Bullock and Swartout, Mercury study report to Congress. Fate and transport of mercury in the environment, Environmental Protection Agency, Research Triangle Park, NC (United States). Office of Air Quality Planning and Standards, 1997, vol. 3 Search PubMed.
- W. Peters, A. Venkatram, J. Weil, R. Wilson, S. Paine, S. Perry, R. Lee and A. Cimorelli, Comparison of Regulatory Design Concentrations AERMOD vs. ISCST3, CTDMPLUS, IST-PRIME, EPA-454/R-03–002, US Environmental Protection Agency Report, North Carolina. USA, 2003 Search PubMed.
- S. G. Perry, A. J. Cimorelli, R. J. Paine, R. W. Brode, J. C. Weil, A. Venkatram, R. B. Wilson, R. F. Lee and W. D. Peters, AERMOD: A dispersion model for industrial source applications. Part II: Model performance against 17 field study databases, J. Appl. Meteorol., 2005, 44, 694–708 CrossRef.
- MDEQ. Meteorological Data, 4561,7–135-3310_70487-66831-,00.html, accessed 25 Sept. 2017, available at, http://www.michigan.gov/deq/0.
- J. D. Eakins and R. T. Morrison, A new procedure for the determination of lead-210 in lake and marine sediments, Int. J. Appl. Radiat. Isot., 1978, 29, 531–536 CrossRef CAS.
- D. R. Engstrom, E. B. Swain and J. C. Kingston, A paleolimnological record of human disturbance from Harvey's Lake, Vermont: geochemistry, pigments and diatoms, Freshw. Biol., 1985, 15, 261–288 CrossRef CAS.
- C. P. McDonald, Historical sedimentation dynamics and a model for copper in Torch Lake, Houghton County, MI., [M.S.]Michigan Technological Univ., Houghton, MI, USA, 2005 Search PubMed.
- LECO Corporation, AMA254 Instruction Manual, LECO Corporation, St. Joseph, MI, 2003 Search PubMed.
- Y. Cai, R. Tang, R. Jaffe and R. Jones, Evaluation of some isolation methods for organomercury determination in soil and fish samples by capillary gas chromatography-atomic fluorescence spectrometry, Int. J. Environ. Anal. Chem., 1997, 68, 331–345 CrossRef CAS.
- R. Jones, M. Jacobson, R. Jaffe, J. West-Thomas, C. Arfstrom and A. Alli, Method development and sample processing of water, soil, and tissue for the analysis of total and organic mercury by cold vapor atomic fluorescence spectrometry, Water, Air, Soil Pollut., 1995, 80(1–4), 1285–1294 CrossRef CAS.
- D. Knauer, G. Pelkola, S. Casey, D. Krabbenhoft and J. F. Dewild, The distribution of total and methyl mercury in Michigan's Upper Peninsula Lakes, Michigan Dept. Environmental Quality, Lansing, MI, 2011, p. 76 Search PubMed.
- P. E. Drevnick, D. R. Engstrom, C. T. Driscoll, E. B. Swain, S. J. Balogh and N. C. Kamman, et al., Spatial and temporal patterns of mercury accumulation in lacustrine sediments across the Laurentian Great Lakes region, Environ. Pollut., 2012, 161, 252–260 CrossRef CAS PubMed.
- M. J. Parsons, D. T. Long, S. S. Yohn and J. P. I. Giesy, Spatial and temporal trends of mercury loadings to Michigan inland lakes, Environ. Sci. Technol., 2007, 2007(41), 5634–5640 CrossRef.
- MDEQ, Total Maximum Daily Load for Mercury for Hammell Creek, Michigan Dept. Environmental Quality, Houghton County, Lansing, Michigan. 2002, p. 7 Search PubMed.
- MDEQ, 2005 estimates of anthropogenic mercury air emissions in Michigan, Air Quality Division, Lansing, MI. 2011, p. 51 Search PubMed.
- MDEQ, Mercury Strategy Staff Report, Michigan Dept. Environmental Quality, Lansing, MI, 2008 Search PubMed.
- MDEQ, Stage 2 Remedial Action Plan for the Deer Lake Area of Concern, Office of the Great Lakes, MDEQ, Lansing, Michigan, 2011 Search PubMed.
- J. A. Perlinger, N. R. Urban, A. Giang, N. E. Selin, A. N. Hendricks, H. Zhang, A. Kumar, S. Wu, V. S. Gagnon, H. S. Gorman and E. S. Norman, Responses of deposition and bioaccumulation in the Great Lakes region to policy and other large-scale drivers of mercury emissions, Environ. Sci.: Processes Impacts, 2018, 20, 195–209 CAS.
- J. Bohr, Status of fish contaminant levels in the Torch Lake Area of Concern 2013. Michigan Department of Environmental Quality, Report 16/003, Water Resources Division, Lansing, p. 24, 2016 Search PubMed.
- R. Rossmann, Horizontal and vertical distributions of mercury in 1983 Lake Superior sediments with estimates of storage and mass flux, J. Great Lake. Res., 1999, 25(4), 683–696 CrossRef CAS.
- L. Wilkinson, SYSTAT: The system for statistics, SYSTAT, Inc, 2007 Search PubMed.
- K. R. Rolfhus, H. E. Sakamoto, L. B. Cleckner, R. W. Stoor, C. L. Babiarz and R. C. Back, et al., Distribution and fluxes of total and methylmercury in Lake Superior, Environ. Sci. Technol., 2003, 37(5), 865–872 CrossRef CAS PubMed.
- S. B. Gewurtz, L. Shen, P. A. Helm, J. Waltho, E. J. Reiner, S. Painter, I. D. Brindle and C. H. Marvin, Spatial distributions of legacy contaminants in sediments of Lakes Huron and Superior, J. Great Lake. Res., 2008, 34, 153–168 CrossRef CAS.
- M. A. Hill, The benefit of the gift: Exchange and social interaction in the Late Archaic Western Great Lakes. 2009, Washington State University Search PubMed.
- J. L. Whattam, Chemical provenance of pre- to post-contact period copper and copper-rich artifacts from archaeological sites in Nova Scotia, Canada: a laser ablation ICP-MS study, Honours B.S. thesis, Saint Mary’s University, Halifax, Nova Scotia., 2014, p. 85 Search PubMed.
- C. A. Cooke, P. H. Balcom, W. C. Kerfoot, M. B. Abbott and A. P. Wolfe, Pre-Columbian mercury pollution associated with the smelting of argentiferous ores in the Bolivian Andes, Ambio, 2011, 40, 18–25 CrossRef CAS PubMed.
- J. L. Mauk and R. G. V. Hancock, Trace element geochemistry of native copper from the White Pine Mine, Michigan (USA): Implications for sourcing artefacts, Archaeometry, 1998, 40(1), 97–107 CrossRef CAS.
- A. Murdoch, Boom Copper, Drier/Koepel, Calumet, 1943 Search PubMed.
- C. H. Benedict, Red Metal: The Calumet and Hecla Story, University of Michigan Press, Ann Arbor, Michigan, 1952 Search PubMed.
- D. J. Quirk, Copper from sand: A History of Copper Reclamation on Torch lake, Michigan Technological University, Houghton County, Michigan [M.S.], USA, 1999 Search PubMed.
- U.S.EPA., EPA-FDA Fish Advice: Technical Information, U.S. Environ. Protection Agency, Washington, D.C., 2017 Search PubMed.
- M. O. Schwartz, Mercury in zinc deposits: Economic geology of a polluting element, International Geology Reviews, 1997, 39, 905–923 CrossRef.
- D. Kocman, S. J. Wilson, H. M. Amos, K. H. Telmer, F. Steenhuisen, E. M. Sunderland, R. P. Mason, P. Outridge and M. Horvat, Supplementary materials: toward an assessment of the global inventory of present-day mercury releases to freshwater environments, Int. Res. J. Publ. Environ. Health, 2017, 14, 138 CrossRef PubMed.
- W. C. Kerfoot, S. Harting, R. Rossmann and J. A. Robbins, Anthropogenic copper inventories and mercury profiles from Lake Superior: Evidence for mining impacts, J. Great Lake. Res., 1999, 25(4), 663–682 CrossRef CAS.
- U.S.EPA (U.S. Environmental Protection Agency), Landmark settlement will reduce air pollution from northern Michigan smelter: heightened levels of mercury found in blood of local Native Americans, 1994, www.justice.gov/opa/pr/Pre-96/January95/58.txt.html Search PubMed.
- C. C. Gilmour, E. A. Henry and R. Mitchell, Sulfate stimulation of mercury methylation in freshwater sediments, Environ. Sci. Technol., 1992, 26, 2281–2287 CrossRef CAS.
- C. J. Watras, K. A. Morrison, O. Regnell and T. K. Kratz, The methylmercury cycle in Little Rock Lake during experimental acidification and recovery, Limnol. Oceanogr., 2006, 51(1), 257–270 CrossRef CAS.
- K. T. Konstantinidis, N. Isaac, J. Fett, S. Simpson, D. T. Long and T. L. Marsh, Microbial diversity and resistance to copper in metal-contaminated lake sediment, Microb. Ecol., 2003, 45, 191–202 CrossRef CAS PubMed.
- D. F. Grigal, Inputs and outputs of mercury from terrestrial watersheds: a review, Environ. Rev., 2002, 10, 1–39 CrossRef CAS.
- W. F. Fitzgerald and C. H. Lamborg, Geochemistry of mercury in the environment. Treatise on Geochemistry, Environ. Geochem. Health, 2004, 9, 107–149 CAS.
- P. Lorey and C. Driscoll, Historical trends of mercury deposition in Adirondack lakes, Environ. Sci. Technol., 1999, 33, 718–722 CrossRef CAS.
- N. C. Kamman and D. R. Engstrom, Historical and present fluxes of mercury to Vermont and New Hampshire lakes inferred from 210Pb dated sediment cores, Atmos. Environ., 2002, 36, 1599–1609 CrossRef CAS.
- B. A. Monson, D. F. Staples, S. P. Bhavsar, T. M. Holsen, C. S. Schrank, S. K. Moses, D. J. McGoldrick, S. M. Backus and K. A. Williams, Spatiotemporal trends of mercury in walleye and largemouth bass from the Laurentian Great Lakes region, Ecotoxicology, 2011, 20, 1555–1567 CrossRef CAS PubMed.
- J. G. Wiener, D. C. Evers, D. A. Gay, H. A. Morrison and K. A. Williams, Mercury contamination in the Laurentian Great Lakes region: introduction and overview, Environ. Pollut., 2011, 161, 243–251 CrossRef PubMed.
- D. V. C. Weseloh, D. J. Moore, C. E. Hebert, S. R. de Solla, B. M. Braune and D. J. McGoldrick, Current concentrations and spatial and temporal trends in mercury in Great Lakes herring gull eggs., 1974–2009, Ecotoxicology, 2011, 20(7), 1644–1658 CrossRef CAS PubMed.
- C. A. Eagles-Smith, J. T. Ackerman and J. J. Willacker, et al., Spatial and temporal patterns of mercury concentrations in freshwater fish across the Western United States and Canada, Sci. Total Environ., 2016, 568, 1171–1184 CrossRef CAS PubMed.
- T. M. Grieb, G. L. Bowie, C. T. Driscoll, S. P. Gloss, C. L. Schofield and D. B. Porcella, Factors affecting mercury accumulation in fish in the upper michigan peninsula, 1990, Environment. Toxicol. Chem., 1990, 9(7), 919–930 CrossRef CAS.
- C. J. Watras, K. A. Morrison and J. S. Host, Concentration of mercury species in relationship to other site-specific factors in the surface waters of northern Wisconsin lakes, Limnol. Oceanogr., 1995, 40(3), 556–565 CrossRef CAS.
- C. R. Hammerschmidt, W. F. Fitzgerald, C. H. Lamborg, P. H. Balcom and P. T. Visscher, Biogeochemistry cycling of methylmercury in lakes and tundra watersheds of Arctic Alaska, Environ. Sci. Technol., 2004, 40, 1204–1211 CrossRef.
- P. Selvendiran, C. T. Driscoll, M. R. Montesdeoca, H. Choi and T. M. Holsen, Mercury dynamics and transport in two Adirondack lakes, Limnol. Oceanogr., 2009, 54(2), 413–427 CrossRef CAS.
- H. Hsu-Kim, K. H. Kucharzyk, T. Zhang and M. A. Deshusses, Mechanisms regulating mercury bioavailability for methylating microorganisms in the aquatic environment: a critical review, Environ. Sci. Technol., 2013, 47(6), 2441–2456 CrossRef CAS PubMed.
- J. M. Benoit, C. C. Gilmour, A. Heyes, R. P. Mason and C. L. Miller, Geochemical and biological controls over methylmercury production and degradation in aquatic ecosystems, in Biogeochemistry of environmentally important trace elements, ed. Y. Cai and O. C. Braids, American Chemical Society, 2003, vol. 835, Washington, D.C.pp. 262–297 Search PubMed.
- A. M. Graham, G. R. Aiken and C. C. Gilmour, Dissolved organic matter enhances microbial mercury methylation under sulfide conditions, Environ. Sci. Technol., 2012, 46(5), 2715–2723 CrossRef CAS PubMed.
- C. J. Watras, K. A. Morrison, O. Regnell and T. K. Kratz, The methylmercury cycle in Little Rock Lake during acidification and recovery, Limnol. Oceanogr., 2006, 51(1), 257–270 CrossRef CAS.
- C. McDonald, B. Baker-Muhich, T. Fitz, P. Garrison, J. Petchenik, P. Rasmussen, R. Thiboldeaux, W. Walker and C. Watras, Taconite iron mining in Wisconsin: A review, Wisconsin Department of Natural Resources, Bureau of Science Services, 2013 Search PubMed.
- C. J. Watras and K. A. Morrison, The response of two remote, temperate lakes to changes in atmospheric mercury deposition, sulfate, and the water cycle, Can. J. Fish. Aquat. Sci., 2008, 65, 100–116 CrossRef CAS.
- MDEQ/WB-09/038, An assessment of environmental selenium levels around Empire and Tilden Mines, Selenium Monitoring Work Group, Marquette County, Michigan, 2009 Search PubMed.
- K. L. Cole, M. B. Davis, M. B. Stearns, F. Walker and K. Guntenspergen, Historical landcover changes in the Great Lakes Region, in The Land Use History of North America (LUHNA), USGS, 1998, ch. 6, https://landcover.usgs.gov/luhna/chap6.php Search PubMed.
- J. R. Thompson, D. N. Carpenter, C. V. Cogbill and D. R. Foster, 2013. Four centuries of change in Northeastern United States forests, PLoS One, 2013, 8(9), e72540, DOI:10.1371/journal.pone.0072540.
- J. Jeong, N. R. Urban and S. Green, Release of copper from mine tailings on the Keweenaw Peninsula, J. Great Lakes Res., 1999, 25, 721–734 CrossRef CAS.
- R. C. Harris, et al., Whole-ecosystem study shows rapid fish-mercury response to changes in mercury deposition, Proc. Natl. Acad. Sci. U. S. A., 2007, 104(42), 16586–16591 CrossRef CAS PubMed.
- R. F. Lepak, R. Yin, D. P. Krabbenhof, J. M. Ogorek, J. F. DeWild, T. M. Holsen and J. P. Hurley, Use of stable isotope signatures to determine mercury sources in the Great Lakes, Environ. Sci. Technol. Lett., 2015, 2(12), 335–341 CrossRef CAS.
- G. A. Briggs, Plume rise: A critical survey, Air Resources Atmospheric Turbulence and Diffusion Lab., Oak Ridge, Tenn., 1969 Search PubMed.
- Blueprint record. C&H Hecla Smelter Company Collection. MS-005. Box 291. Michigan Tech Archives & Copper Country Historical Collections.
- Blueprint record. C&H Hecla Smelter Company Collection. MS-005. Box 152. Michigan Tech Archives & Copper Country Historical Collections.
| This journal is © The Royal Society of Chemistry 2018 |