Mercury methylation in stormwater retention ponds at different stages in the management lifecycle†
Abstract
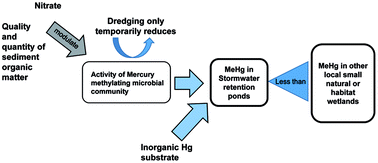
Stormwater retention ponds effectively manage erosion, flooding, and pollutant loadings, but are also sources of methylmercury (MeHg), a bioaccumulative neurotoxin which is produced by anaerobic aquatic microorganisms. Stormwater retention ponds have a 10–15 year working life, after which they are dredged and reflooded. In this study, we related MeHg biogeochemistry to the different stages of the management lifecycle. In a new, a dredged, and a mature stormwater retention pond, we measured MeHg and inorganic mercury (IHg) concentrations, and the potential for MeHg formation (Kmeth), during the early summer, peak summer, and fall of 2013. In our study sites, MeHg concentrations appear to be driven by mercury (Hg) methylation, indicated by significant correlations between Kmeth values and MeHg concentrations and the percent of Hg present as MeHg. Relationships between Hg variables and ancillary biogeochemistry suggest that Hg methylation is carried out by sulfate reducing bacteria, but that the process is modulated by the supply of IHg substrate, sediment total and labile organic carbon, and possibly competition with nitrate reducers. Wetlands at different points in the management lifecycle differ in terms of their MeHg biogeochemistry. The organic matter-poor new wetland had low MeHg production (mean Kmeth 0.014 per day) and sediment concentrations (mean 0.015 ng g−1), while the mature wetland both produced and accumulated MeHg about five times more actively. Methylmercury production capacity was only temporarily reduced in the reflooded sediments of the dredged wetland, which experienced rapid increases in Kmeth values from low (mean 0.015 per day) immediately after dredging, to values similar to those in the mature wetland after five months. This pattern may have been related to recolonization of the sediments with mercury methylators or increased microbial activities in response to the addition of fresh organic matter. Additional studies should focus on the applicability of these patterns to stormwater retention ponds in other areas, and particularly investigate the effects of stormwater pond dredging on their microbial ecology and MeHg biogeochemistry.
- This article is part of the themed collection: Mercury Biogeochemistry, Exposure, and Impacts


 Please wait while we load your content...
Please wait while we load your content...