Distribution of mercury species and mercury isotope ratios in soils and river suspended matter of a mercury mining area†
Abstract
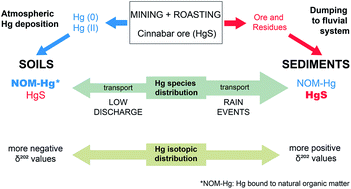
Mercury (Hg) released by mining activities can be dispersed in the environment, where it is subject to species transformations. Hg isotope ratios have been used to track sources in Hg contaminated areas, although it is unclear to what extent variations in δ-values are attributed to distinct Hg species. Hg was mined as Hg sulphide (cinnabar) in Idrija, Slovenia for centuries. Sediments are loaded with mining-residues (cinnabar and calcine), whereas contaminated soils mainly contain Hg bound to natural organic matter (NOM-Hg) related to atmospheric Hg deposition. Hg released from soils and sediments is transported as suspended matter (SM) in the Idrijca river to the Gulf of Trieste (GT), Italy. We determine Hg isotope ratios in river SM, sediments and soils from the Idrijca-catchment to decipher the Hg isotope ratio variability related to Hg species distribution in different grain-size fractions. δ202Hg values of SM collected from tributaries corresponded to those found in soils ranging from −2.58 to 0.19‰ and from −2.27 to −0.88‰, respectively. Speciation measurements reveal that fine fractions (0.45–20 μm) are dominated by NOM-Hg, while larger fractions contain more cinnabar. More negative δ202Hg values were related to higher proportions of NOM-Hg, which are predominant in soils and SM. Rain events increase SM-loads in the river, mainly due to resuspension of coarse grain-size fractions of bottom sediments bearing larger proportions of cinnabar, which leads to more positive δ202Hg values. The large magnitude of variation in δ202Hg and the smaller magnitude of variation in Δ199Hg (−0.37 to 0.09‰) are likely related to fractionation during ore roasting. Soil samples with high NOM-Hg content show more negative δ202Hg values and larger variation of Δ199Hg. More negative δ202Hg values in GT sediments were rather linked to distant sedimentation of soil derived NOM-Hg than to sedimentation of autochthonous marine material. Heterogeneity in the Idrija ore and ore processing likely produce large variations in the Hg isotopic composition of cinnabar and released metallic Hg, which complicate the differentiation of Hg sources. Combining Hg isotope measurements with solid phase Hg speciation reveals that Hg isotope ratios rather indicate different Hg species and are not necessarily symptomatic for Hg pollution sources.
- This article is part of the themed collection: Mercury Biogeochemistry, Exposure, and Impacts


 Please wait while we load your content...
Please wait while we load your content...