Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A new approach for sustained and efficient H2 photoproduction by Chlamydomonas reinhardtii†
Sergey
Kosourov
 *,
Martina
Jokel
*,
Martina
Jokel
 ,
Eva-Mari
Aro
,
Eva-Mari
Aro
 and
Yagut
Allahverdiyeva
and
Yagut
Allahverdiyeva
 *
*
Molecular Plant Biology, Department of Biochemistry, University of Turku, Turku, FI-20014, Finland. E-mail: serkos@utu.fi; allahve@utu.fi; Tel: +35 8451577800 Tel: +35 8503506181
First published on 17th April 2018
Abstract
Sustained H2 photoproduction is demonstrated in green algae under a train of strong white light pulses interrupted by longer dark phases. The devised protocol relies on the presence of the [FeFe]-hydrogenase in algal chloroplasts, which is activated within a few seconds after the establishment of anaerobiosis. H2 photoproduction proceeds for up to 3 days with the maximum rate occurring in the first 6 hours.
Broader contextMolecular hydrogen (H2) is an ideal energy carrier for a sustainable low-carbon economy. The unicellular green alga Chlamydomonas reinhardtii is capable of producing H2 by splitting water with energy from sunlight. H2 photoproduction in algal cells is driven by the [FeFe]-hydrogenase enzyme(s), which interacts with the photosynthetic electron transport chain at the level of ferredoxin, thus linking the water-splitting reaction at photosystem II (PSII) to the reduction of protons to H2. Since [FeFe]-hydrogenases are extremely O2 sensitive, H2 photoproduction in green algae is difficult to sustain due to the simultaneous release of O2 in the PSII oxygen-evolving complex. This article examines a breakthrough protocol for sustaining efficient H2 photoproduction in algae by transferring the growing cultures from continuous light to a train of strong light pulses superimposed on darkness or low background illumination. This novel protocol represents a way of redirecting the photosynthetic electron flow to the hydrogenase instead of CO2 fixation and biomass formation, thus increasing the overall H2 photoproduction yield. |
Photobiological water splitting to molecular hydrogen (H2) and oxygen (O2), also known as direct water biophotolysis, has been considered as one of the most promising and environmentally friendly approaches for generating bulk quantities of clean H2 biofuel.1 Many species of cyanobacteria and eukaryotic green algae, including the model organism Chlamydomonas reinhardtii, are capable of catalyzing this reaction.2,3 In green algae, water biophotolysis proceeds in two steps:
| 2H2O → 4H+ + O2 + 4e− Step 1 |
| 4H+ + 4e− → 2H2 Step 2 |
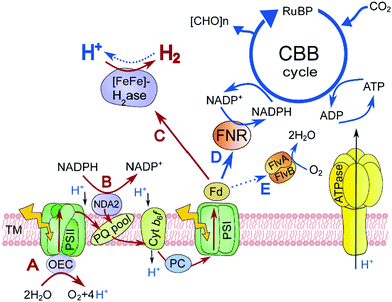
H2 photoproduction in C. reinhardtii also occurs through a mechanism independent of water oxidation. In the indirect process, the reductants derived from the degradation of stored organic substrates, such as starch and proteins, are incorporated into the PETC by a type II NADPH dehydrogenase (Nda2) at the level of the plastoquinone (PQ) pool,6 thus bypassing the water splitting at PSII (Scheme 1, a pathway from B to C). Similar to the direct process, this pathway requires PSI activity to donate electrons to the H2ase. Since both pathways are linked to the H2ase via PSI and Fd (Scheme 1, the C pathway), their contribution to the overall H2 production yield in algal cultures may vary depending on physiological conditions.
Efficient H2 photoproduction in green algae occurs in the light after a period of dark anaerobic incubation.7 The reaction is transient due to a rapid, within seconds, inhibition of H2ase by O2, which is co-produced in the water-splitting reaction. One of the approaches to achieve sustained H2 photoproduction in C. reinhardtii cultures is to deprive them of sulfur.8 Sulfur-deprivation prevents the efficient repair of the light-damaged D1 reaction center protein of PSII, thus leading to a gradual loss of the water-splitting activity in algal cells over time. As a consequence, the actively respiring algae establish an anaerobic environment in the sealed photobioreactor, induce the H2ase enzyme(s) and continuously produce H2 gas for several days.9 Although the loss of active PSII centers sustains H2 photoproduction in algae, it also downregulates the direct, water oxidation-dependent flow of electrons to the H2ase, resulting in low overall efficiency of the process. Sulfur-deprivation requires extensive and time-consuming centrifugations, which make this protocol difficult for application even in laboratory scale projects (yet a few alternatives have been suggested10,11).
In the current work, we demonstrate that efficient H2 photoproduction can be sustained in growing C. reinhardtii cultures for at least three days by switching the algal suspensions from continuous light to a train of short strong light pulses superimposed on either darkness or permanent low light illumination. The protocol is very simple, non-damaging to algae and reproducible even under strict autotrophic conditions.
Theoretical considerations
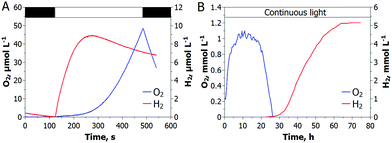
At the current state, H2 photoproduction in algal cultures is only possible via a temporal separation of the O2 evolving and H2 producing reactions. C. reinhardtii cultures, dark-adapted in anoxic conditions, produce H2 upon exposure to light, before the onset of O2 evolution (Fig. 1A), while sulfur-deprived cells show the opposite behavior (Fig. 1B). Although the maximum specific H2 photoproduction activity is higher in dark-adapted cells than in sulfur-deprived algae, the latter produce H2 much longer and yield more H2 gas. For sustaining the H2 production process in dark-adapted algae, one could suggest the low light/high cell density condition that prevents O2 accumulation in cultures due to active respiration, but at the expense of efficiency.12 Alternatively, H2 production can be driven at high light intensities by funneling photosynthetic electrons to the H2ase (Scheme 1, the C pathway), instead of the Calvin–Benson-Bassham (CBB) cycle (Scheme 1, the D pathway), with simultaneous control of the intracellular O2 level. Although Rubisco deficiency has been reported to promote H2 evolution in green algae, the yield of H2 in the Rubisco-deficient mutant culture was not particularly high, most probably due to the downregulation of the photosynthetic electron flow to the H2ase in this strain.13 Nevertheless, the partial inactivation of the CBB cycle did improve the H2 photoproduction yield.14The H2ase enzyme, induced in algae under dark anaerobic conditions,15,16 acts as an alternative electron sink upon illumination and promotes the activity of oxygenic photosynthesis by eliminating the accumulation of excess electrons in PETC.17 The light activation of the CBB cycle requires time affecting photosynthetic productivity under fluctuating light.18 We propose that a train of very short light pulses should arrest the algal photosynthesis in the H2 photoproduction stage, provided the duration of each light pulse is short enough to minimize the electron flow to the CBB cycle and to prevent O2 accumulation. To test this hypothesis, we subjected C. reinhardtii to a train of short (1–5 s) light pulses interrupted by longer (3–9 s) dark phases. These experiments were subsequently repeated under low background illumination (3 μmol photons m−2 s−1) in place of dark phases.
Materials and methods
All experiments were performed with unstressed, actively growing C. reinhardtii cultures either on TAP (photomixotrophic growth) or on a modified TAP medium without acetate (photoautotrophic growth). CC-124, CC-4533 and CC-5128 (hydEF) strains were pre-grown under a 14 h photoperiod at 75 μmol photons m−2 s−1 photosynthetic active radiation (PAR) and 25 °C. H2 photoproduction was analyzed during the active period of photosynthesis, within 5 to 10 h from the beginning of the photoperiod. No centrifugation steps were applied. Growing algal cultures were pipetted into a gas-tight 23 mL GC vial equipped with H2 and O2 microsensors (H2-NP and OX-NP, Unisense A/S) connected to an amplifier. The electrodes were pierced inside the vial through a Teflon-coated rubber septum. Cells in the vial were sparged with argon (Ar) for 2–3 min in the dark, followed by incubation in the dark for another 1–5 min. Subsequently, a train of light pulses was applied to the culture and the H2 and O2 levels were monitored by the OxyHydrogen software via the STM32F103 microcontroller board connected to a high precision 24-bit ADC (ADS1256, Texas Instruments). The white LED light pulses (420 μmol photons m−2 s−1) were synchronized through the same microcontroller board. The gas exchange was measured by membrane inlet mass spectrometry (MIMS) using a modified DW1 (Hansatech Instruments) electrode chamber as previously described.19The long-term H2 photoproduction experiments were performed with a 10 mL cell suspension in 70 mL gas-tight vials under an Ar atmosphere. The pulses of white light (280 μmol photons m−2 s−1) interrupted by dark periods or the constant light of the same intensity were provided by the growth chamber (AlgaeTron AG 130-ECO, PSI). The vials were continuously shaken and H2 production yields were measured using a gas chromatograph (Clarus 500, PerkinElmer) equipped with a thermal conductivity detector and a molecular sieve 5A column (60/80 mesh). The total Chl content and hydrogenase activity were measured as described previously.9
The average energy of the incident light in the PAR (400–700 nm) region was determined at the surface of the liquid with the STS-VIS spectrometer (Ocean Optics, Inc.). Light energy to hydrogen energy conversion efficiency (LHCE) was calculated using eqn (1), which considers the partial pressure of H2 gas in the vial headspace at the moment of calculation:20
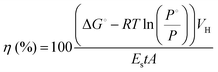 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 J mol−1 at 25 °C and 1 atm), R is the universal gas constant, T is the absolute temperature, P° and P are the standard and observed H2 pressures (atm), VH is the amount of H2 photoproduced (mol), ES is the energy of the incident light radiation (J m−2 s−1), A is the illuminated surface area (m2) and t is the sum of the illumination periods (s).
200 J mol−1 at 25 °C and 1 atm), R is the universal gas constant, T is the absolute temperature, P° and P are the standard and observed H2 pressures (atm), VH is the amount of H2 photoproduced (mol), ES is the energy of the incident light radiation (J m−2 s−1), A is the illuminated surface area (m2) and t is the sum of the illumination periods (s).
For protein analysis, cells were harvested and rapidly frozen in lysis buffer (50 mM Tris pH 8, 2% SDS, 10 mM EDTA, protease inhibitors from Sigma). After thawing, the total protein fraction was isolated and separated in a 12% SDS-PAGE without urea, transferred to a polyvinylidene difluoride membrane (Millipore) and blocked with a 5% blotting grade blocker (Bio-Rad). The samples were loaded on an equal protein basis as determined using a Direct Detect® infrared spectrometer (Merck) and visualized as control with Coomassie Brilliant Blue (Bio-Rad). The accumulation of HydA1/A2 was analyzed by using a specific antibody (Agrisera). As a secondary antibody, anti-rabbit horseradish peroxidase was used in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dilution and HydA1/A2 was visualized with ECL.
000 dilution and HydA1/A2 was visualized with ECL.
Results and discussion
A train of light pulses sustains H2 production in algal cultures
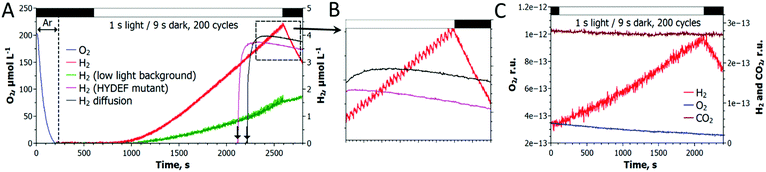
As shown in Fig. 2, a train of 1 s light pulses interrupted by 9 s dark periods induces continuous H2 photoproduction in algal cultures. The procedure shows reproducibility even at a very low cell density (6–7 mg total Chl L−1) and in the absence of acetate (Fig. 3) but requires pre-established anaerobic conditions. Trace quantities of H2 could be observed almost immediately after starting the light pulse illumination of anaerobic cultures, and thereafter the H2 level gradually increased with time. The experiments performed in the DW1/AD electrode chamber (Hansatech Instruments) but under high light intensity (∼800 μmol photons m−2 s−1) pulses produced similar results. The H2ase-deficient hydEF mutant did not show the presence of H2 gas throughout the experiment (Fig. 2A, magenta line), as expected. H2 photoproduction also occurs in algae exposed to pulses superimposed on low background illumination (Fig. 2A, green line).During the H2 production phase, no net O2 evolution could be detected by either the O2 electrode (Fig. 2A) or MIMS (Fig. 2C). The exposure of algae to a similar train of light pulses in DUAL-PAM, but at a background of measuring light, demonstrated a slight decline of the PSII photochemical efficiency in the course of the experiment (Fig. S1, ESI†). The exposure of the pulse-illuminated cells to continuous light induced O2 evolution, occurring with some delay (Fig. S2, ESI†). The accumulation of O2 could also be observed on shortening the dark phase to ≤3 s between the light pulses (Fig. S3, ESI†), thus confirming the presence of functional PSII in algal cells under the illumination system applied here.
Gas exchange measurements performed with MIMS showed no signs of CO2 fixation upon a standard train of light pulses (Fig. 2C). CO2 fixation occurred only upon accumulation of O2 in the cultures as a consequence of shortening the dark phase between light pulses (Fig. S3, ESI†). This provides compelling evidence that in the newly established protocol, the algal cells function as a biocatalyst funneling photosynthetic electrons directly to the H2ase without the activation of the CBB cycle.
Pulse-illumination shows the presence of H2 uptake in algae
As shown in Fig. 2B, transient H2 production peaks regularly appear upon pulse-illumination of C. reinhardtii, whilst noticeable H2 consumption takes place between the light pulses. The amplitude of the sawtooth wave, which occurs both in photoheterotrophic (Fig. 2B) and photoautotrophic (Fig. 3A, inset) cultures, became more pronounced in the course of H2 accumulation in the system. This behavior can be explained by the dependence of the H2 uptake reaction on the H2 partial pressure,21 as well as by the gradual induction of the H2ase activity in cells (Fig. 3B). The involvement of passive processes in the overall H2 uptake, such as a leak of H2 from the system or H2 consumption by the microsensor, seems to be very minor since (i) the H2 trace in cell-free media declines much slower (Fig. 2A, black line) and (ii) the active H2 consumption does not occur in the hydEF mutant with disrupted H2ase (Fig. 2A, magenta line). The H2 uptake reaction in the dark is very strong, and at the current state we do not know whether H2 uptake occurs simultaneously with H2 release in the light. A switch of pulse-illumination to continuous low light, however, did not lead to any noticeable H2 consumption in the cultures (Fig. 2A, green line).Since the reaction balance catalyzed by the reversible H2ase is shifted towards H2 release in the course of pulse-illumination, the involvement of the oxyhydrogen reaction in H2 uptake is very unlikely or its contribution to the process is minor. A similar conclusion could be applied also to H2 uptake during the dark phase after the period of pulse illumination (Fig. 2 and 3). Otherwise, flavodiiron proteins might be involved in the oxyhydrogen reaction by donating electrons to O2 under illumination (Scheme 1, the E pathway).22,23 In principle, H2 uptake in algae may occur without O2 consumption:
| H2 + 2OH− → 2H2O + 2e− |
Yet, in such a case, neither the final electron acceptor nor any intermediate players are known. The occurrence of H2 uptake in the green alga, Scenedesmus sp. was first demonstrated more than 70 years ago.24 Since that time only a little follow-up progress has been made in resolving the metabolic pathways participating in the H2 uptake reaction. H2 oxidation has been proposed to provide reducing equivalents for CO2 fixation, but the reaction requires either a very low level of O2 (up to 1%) or light illumination in complete anaerobiosis for ATP re-generation.25
The absence of CO2 fixation either during a train of light pulses or during the dark phase after termination of the protocol (Fig. 2C) suggests that H2 uptake in algae exposed to pulse-illumination and thereafter is not linked to CO2 reduction. The presence of the H2 consumption pathway was also confirmed in sulfur-deprived C. reinhardtii cells,21,26 harbouring the inactivated Rubisco enzyme.13,27 Since H2 uptake in both cases occurs upon a shift to darkness, the process seems to be driven by the same catabolic pathway. It is clear that more research is needed to completely understand the mechanism(s) of H2 consumption in green algae, yet the elimination of this process should dramatically improve the H2 photoproduction yield in algal cultures.
Pulse-illumination demonstrates a fast activation of [FeFe]-hydrogenase by anaerobiosis
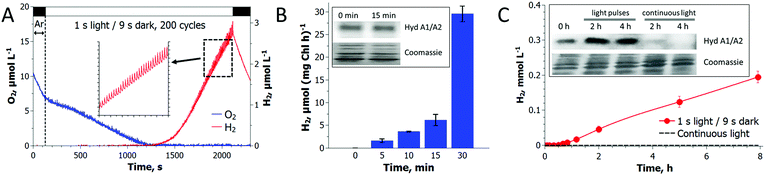
Recently, Liran and co-authors28 concluded that the entire pool of cellular H2ase remains active in air-grown cells, thus allowing algae to produce H2 even under aerobic conditions, and in particular, on switch from low to high light conditions. On the other hand, there is extensive literature showing the extreme sensitivity of algal H2ase to molecular O2. For resolving this contradiction, Liran and co-authors suggested the existence of anaerobic niches inside the cells with a high rate of local respiration that protects the H2ase from O2 inactivation.Our experimental data show that the activation of the H2ase enzyme in air-grown cells and the production of H2 (Fig. 3) occur only after the establishment of anaerobiosis in the culture. As shown in Fig. 3A, the photoautotrophic C. reinhardtii culture is capable of spontaneous establishment of anaerobiosis in the medium under the pulse-illumination if the initial level of O2 is lowered to below 10 μmol L−1 by Ar purging. Algae start producing H2 almost immediately after consuming the residual O2 in the chamber. The reaction, thus, requires strong anaerobiosis and it does not occur in an aerobic environment. The cells pre-grown in air contain HydA1/A2 proteins and the amount does not increase within 15 min of the pulse-illumination (Fig. 3B, inset). Nevertheless, the H2ase activity (measured in the presence of reduced methyl viologen) rises gradually during this time (Fig. 3B) and correlates with the induction of H2 photoproduction in the cells (Fig. 3A). The amount of the HydA1/A2 proteins increases later (Fig. 3C, inset). During the long-term cultivation under the train of light pulses, we could detect the rise of H2ase in the cells, but continuous high light causes the opposite effect (Fig. 3C). In the latter case, no H2 production is observed. These experimental data prove that algae express H2ase during aerobic growth under moderate light. H2ase activation, however, requires strong anaerobiosis, which contradicts the suggestion of Liran and co-authors28 about the functional [FeFe]-H2ase enzyme in an aerobic environment.
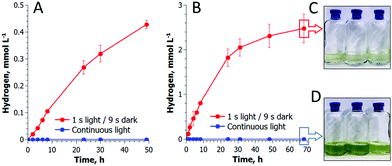
A pulse-illumination protocol sustains H2 production for at least 70 hours and proves the competition between H2 photoproduction and CO2 fixation
Long-term experiments performed with C. reinhardtii cultures in small anaerobic vials demonstrated that pulse-illuminated algae are capable of producing H2 continuously for at least 3 days (Fig. 4). The reaction occurs in the absence of acetate and at an extremely low cell density (Fig. 4A), indicating that the self-shading in the suspension is not a reason for the induction of H2 production in algal cells. C. reinhardtii produces H2 more efficiently during the first 6 h after which the rate gradually declines. The maximum specific rate exceeds the rate of H2 photoproduction in sulfur-deprived algae21,29 and in the best case reaches up to 25 μmol H2 (mg Chl h)−1. Under light conditions typical for the original sulfur-deprivation protocol (∼200 μmol photons m−2 s−1),8,27 pulse-illuminated cultures yield above 3 mmol H2 L−1 during the first 48 h (Fig. S4, ESI†), which is very close to the H2 yield in sulfur-deprived algae.9 However, due to a much shorter illumination time (Fig. S4, ESI†), the pulse-illuminated cultures produce H2 more efficiently than the sulfur-deprived cells (0.5% vs. 0.24%,30 respectively). Sulfur-deprived algae also need an extra 24–48 h (without H2 production) for PSII inactivation, which is not considered in LHCE calculations. Moreover, the pulse-illuminated algae are capable of producing H2 at a maximum conversion efficiency of 1.6–1.7% (2–2.2% if the upper H2 gas combustion energy is assumed) during the first 8 h.It is important to note that algae do not accumulate biomass under pulse-illumination (Fig. 4C), in contrast to continuous light (Fig. 4D). The inhibition of biomass accumulation under a train of light pulses suggests the successful diversion of photosynthetic reductants from carbon fixation to H2 photoproduction. These experimental data, thus, bring additional evidence that the re-direction of the photosynthetic electron flow to the [FeFe]-H2ase enzyme does improve the H2 photoproduction activity in algal cells.31
Conclusions
This research demonstrates that H2 photoproduction in green algae can be sustained by a simple shift in the light conditions of growing algal cultures from continuous illumination to a train of light pulses interrupted by longer dark phases. In a low O2 environment, such pulse-illuminated algae can spontaneously establish anaerobiosis and produce H2 for up to three days. The appearance of H2 gas in the cultures, almost immediately after the establishment of anaerobiosis, points to an important role of the [FeFe]-H2ase enzyme(s) in algal energy metabolism under anaerobic conditions. In addition, the pulse illumination protocol provides strong evidence that CO2 fixation competes with the [FeFe]-H2ase enzyme for the photosynthetic electrons and demonstrates a direct means of eliminating this competition. All the findings together provide new opportunities for metabolic engineering and construction of efficient cell factories with a capacity to re-direct photosynthetic electrons to targeted metabolic pathways and biofuel products, instead of biomass.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support from the Kone Foundation, the NordForsk NCoE program “NordAqua” (project # 82845) and the Academy of Finland FCoE program (# 307335).Notes and references
- W. Lubitz, E. J. Reijerse and J. Messinger, Energy Environ. Sci., 2008, 1, 15–31 CAS.
- V. A. Boichenko, E. Greenbaum and M. Seibert, in Molecular to Global Photosynthesis: Photoconversion of Solar Energy, ed. J. Barber and M. D. Archer, Imperial College Press, London, 2004, pp. 397–451 Search PubMed.
- M. Timmins, S. R. Thomas-Hall, A. Darling, E. Zhang, B. Hankamer, U. C. Marx and P. M. Schenk, J. Exp. Bot., 2009, 60, 1691–1702 CrossRef CAS PubMed.
- Y. Allahverdiyeva, E. M. Aro and S. N. Kosourov, in Bioenergy Research: Advances and Applications, ed. F. X. Vijai, K. Gupta, M. Tuohy, C. P. Kubicek and J. Saddler, Elsevier, Amsterdam, 2014, pp. 367–387 Search PubMed.
- M. L. Ghirardi, A. Dubini, J. Yu and P.-C. Maness, Chem. Soc. Rev., 2009, 38, 52–61 RSC.
- F. Jans, E. Mignolet, P.-A. Houyoux, P. Cardol, B. Ghysels, S. Cuiné, L. Cournac, G. Peltier, C. Remacle and F. Franck, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 20546–20551 CrossRef CAS PubMed.
- H. Gaffron and J. Rubin, J. Gen. Physiol., 1942, 26, 219–240 CrossRef CAS PubMed.
- A. Melis, L. Zhang, M. Forestier, M. L. Ghirardi and M. Seibert, Plant Physiol., 2000, 122, 127–136 CrossRef CAS PubMed.
- S. Kosourov, A. Tsygankov, M. Seibert and M. L. Ghirardi, Biotechnol. Bioeng., 2002, 78, 731–740 CrossRef CAS PubMed.
- T. V. Laurinavichene, I. V. Tolstygina, R. R. Galiulina, M. L. Ghirardi, M. Seibert and A. A. Tsygankov, Int. J. Hydrogen Energy, 2002, 27, 1245–1249 CrossRef CAS.
- P. Lindberg and A. Melis, Planta, 2008, 228, 951–961 CrossRef CAS PubMed.
- A. Scoma, L. Durante, L. Bertin and F. Fava, New Phytol., 2014, 204, 890–900 CrossRef CAS PubMed.
- A. Hemschemeier, S. Fouchard, L. Cournac, G. Peltier and T. Happe, Planta, 2008, 227, 397–407 CrossRef CAS PubMed.
- T. S. Pinto, F. X. Malcata, J. D. Arrabaça, J. M. Silva, R. J. Spreitzer and M. G. Esquível, Appl. Microbiol. Biotechnol., 2013, 97, 5635–5643 CrossRef CAS PubMed.
- T. Happe and A. Kaminski, Eur. J. Biochem., 2002, 269, 1022–1032 CrossRef CAS PubMed.
- M. Forestier, P. King, L. Zhang, M. Posewitz, S. Schwarzer, T. Happe, M. L. Ghirardi and M. Seibert, Eur. J. Biochem., 2003, 270, 2750–2758 CrossRef CAS PubMed.
- B. Ghysels, D. Godaux, R. F. Matagne, P. Cardol and F. Franck, PLoS One, 2013, 8, e64161 CAS.
- P. J. Graham, B. Nguyen, T. Burdyny and D. Sinton, Sci. Rep., 2017, 7, 1–11 CrossRef PubMed.
- H. Mustila, P. Paananen, N. Battchikova, A. Santana-Sanchez, D. Muth-Pawlak, M. Hagemann, E. M. Aro and Y. Allahverdiyeva, Plant Cell Physiol., 2016, 57, 1468–1483 CAS.
- S. Kosourov, G. Murukesan, M. Seibert and Y. Allahverdiyeva, Algal Res., 2017, 28, 253–263 CrossRef.
- S. N. Kosourov, K. A. Batyrova, E. P. Petushkova, A. A. Tsygankov, M. L. Ghirardi and M. Seibert, Int. J. Hydrogen Energy, 2012, 37, 8850–8858 CrossRef CAS.
- Y. Allahverdiyeva, M. Suorsa, M. Tikkanen and E. M. Aro, J. Exp. Bot., 2015, 66, 2427–2436 CrossRef CAS PubMed.
- M. Jokel, S. Kosourov, N. Battchikova, A. A. Tsygankov, E. M. Aro and Y. Allahverdiyeva, Plant Cell Physiol., 2015, 56, 1598–1607 CrossRef CAS PubMed.
- H. Gaffron, J. Gen. Physiol., 1942, 26, 241–267 CrossRef CAS PubMed.
- C. Catalanotti, W. Yang, M. C. Posewitz and A. R. Grossman, Front. Plant Sci., 2013, 4, 1–17 Search PubMed.
- A. Scoma and A. Hemschemeier, Algal Res., 2017, 26, 341–347 CrossRef.
- L. Zhang, T. Happe and A. Melis, Planta, 2002, 214, 552–561 CrossRef CAS PubMed.
- O. Liran, R. Semyatich, Y. Milrad, H. Eilenberg, I. Weiner and I. Yacoby, Plant Physiol., 2016, 172, 264–271 CrossRef CAS PubMed.
- L. Giannelli, A. Scoma and G. Torzillo, Biotechnol. Bioeng., 2009, 104, 76–90 CrossRef CAS PubMed.
- M. L. Ghirardi, Indian J. Biochem. Biophys., 2006, 43, 201–210 CAS.
- S. Rumpel, J. Siebel, C. Farès, J. Duan, E. Reijerse, T. Happe, W. Lubitz and M. Winkler, Energy Environ. Sci., 2014, 1–10 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Photochemical activity, additional H2 and O2 photoproduction curves and additional MIMS data. See DOI: 10.1039/c8ee00054a |
| This journal is © The Royal Society of Chemistry 2018 |