Toward high-efficiency, hysteresis-less, stable perovskite solar cells: unusual doping of a hole-transporting material using a fluorine-containing hydrophobic Lewis acid†
Abstract
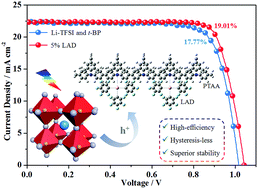
Perovskite solar cells (PSCs) have demonstrated high power conversion efficiency (PCE) but inferior long-term stability and remarkable hysteresis. To date, the most efficient PSCs have a n–i–p device architecture and use Li-TFSI/t-BP as standard bi-dopants for the hole-transporting layer (HTL). However, such dopants not only induce deleterious effects on stability but also significantly affect the hysteresis of PSCs. Here, we demonstrate that a fluorine-containing hydrophobic Lewis acid dopant (LAD) can be employed as an effective dopant for PTAA to realize an exceptional fill factor of 0.81 and a record PCE as high as 19.01%, the highest value among the ever reported PSCs based on a novel dopant in the HTL, versus 17.77% for the control device with Li-TFSI/t-BP doped PTAA. To the best of our knowledge, this is the first case in which a PSC based on a novel dopant for the HTL shows higher efficiency than a PSC based on the state-of-the-art bi-dopants Li-TFSI/t-BP. Besides, the LAD-based PSC displays lower J–V hysteresis and much better long-term stability of up to 70 days under air exposure without encapsulation. We believe that this work will pave a new avenue for high-efficiency, hysteresis-less and stable PSCs exploring hydrophobic dopants as alternatives to hydrophilic Li-TFSI/t-BP.


 Please wait while we load your content...
Please wait while we load your content...