Amine-functionalized Zn(ii) MOF as an efficient multifunctional catalyst for CO2 utilization and sulfoxidation reaction†
Abstract
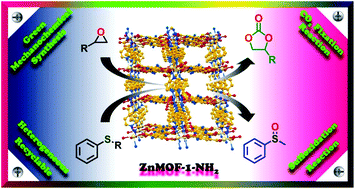
Herein, a zinc(II)-based 3D mixed ligand metal organic framework (MOF) was synthesized via versatile routes including green mechanochemical synthesis. The MOF {[Zn(ATA)(L)·H2O]}n (ZnMOF-1-NH2) has been characterized by various physico-chemical techniques, including SCXRD, and composed of the bipyridyl-based Schiff base (E)-N′-(pyridin-4-ylmethylene)isonicotinohydrazide (L) and 2-aminoterephthalic acid (H2ATA) ligands as linkers. The MOF material has been explored as a multifunctional heterogeneous catalyst for the cycloaddition of alkyl and aryl epoxides with CO2 and sulfoxidation reactions of aryl sulfides. The influence of various reaction parameters is examined to optimize the performance of the catalytic reactions. It is found that solvent-free catalytic reaction conditions offer good catalytic conversion in the case of cyclic carbonates, and for sulfoxide, good conversion and selectivity are achieved in the presence of DCM as a solvent medium under ambient reaction conditions. The chemical and thermal stability of the catalyst are excellent and it is active for up to four catalytic cycles without significant loss in activity. Furthermore, based on the catalytic activity and structural evidence, a plausible mechanism for both catalytic reactions is proposed.


 Please wait while we load your content...
Please wait while we load your content...