An electron transfer driven magnetic switch: ferromagnetic exchange and spin delocalization in iron verdazyl complexes†
Abstract
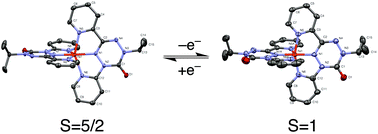
The verdazyl ‘pincer’ ligand, 1-isopropyl-3,5-dipyridyl-6-oxoverdazyl (dipyvd), coordinates iron to form a series of pseudooctahedral coordination compounds [Fe(dipyvd)2]n+ (n = 0–3). In the case where n = 2, the molecular geometry and physical and spectral properties are consistent with a low spin (S = 0) iron(II) ion coordinated by two ferromagnetically coupled radical ligands. Upon one electron reduction, the room temperature effective magnetic moment of the complex jumps from μeff = 2.64 to μeff = 5.86 as a result of spin crossover of the iron atom combined with very strong ferromagnetic coupling of the remaining ligand centered unpaired electron with the metal center. The sign of the exchange is opposite to that observed in other high spin iron/radical ligand systems and appears to be a result of delocalization of the ligand unpaired electron across the whole molecule. The large change in magnetic properties, combined with a delocalized electronic structure and accessible redox potentials, suggests the utility of this and related systems in the development of novel molecular spintronic devices.


 Please wait while we load your content...
Please wait while we load your content...
