A novel route with a Cu(ii)-MOF-derived structure to synthesize Cu/Cu2O NPs@graphene: the electron transfer leads to the synergistic effect of the Cu(0)–Cu(i) phase for an effective catalysis of the Sonogashira cross-coupling reactions †
Abstract
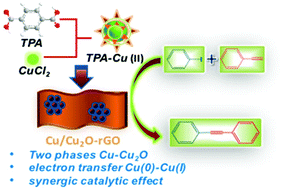
A uniformly dispersed two-phase Cu/Cu2O NPs@graphene was obtained via an innovative “take” and “off” synthesis strategy with a Cu-MOF-derived jacket structure. The electron transfer between the Cu phase and Cu2O phase leads to the synergistic effect of the Cu(0)–Cu(I) phase for an efficient catalysis of the Sonogashira cross-coupling reactions.


 Please wait while we load your content...
Please wait while we load your content...