Three different types of bridging ligands in a 3d–3d′–3d′′ heterotrimetallic chain†
Abstract
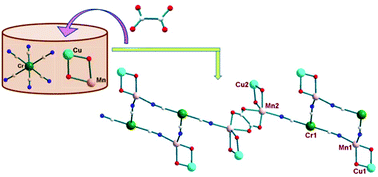
A one-pot synthesis of a 3d–3d′–3d′′ heterotrimetallic coordination polymer with double diphenoxido, single cyanido and bis-bidentate oxalate as alternating bridges which exhibits an overall antiferromagnetic behaviour has been developed.


 Please wait while we load your content...
Please wait while we load your content...