Open Access Article
Open Access ArticleTunable Prussian blue analogues for the selective synthesis of propargylamines through A3 coupling†
Carlos
Marquez
 ,
Francisco G.
Cirujano
,
Francisco G.
Cirujano
 ,
Cédric
Van Goethem
,
Cédric
Van Goethem
 ,
Ivo
Vankelecom
,
Dirk
De Vos
,
Ivo
Vankelecom
,
Dirk
De Vos
 * and
Trees
De Baerdemaeker
* and
Trees
De Baerdemaeker
 *
*
Centre for Surface Chemistry and Catalysis, KU Leuven, Celestijnenlaan 200F, 3001 Leuven, Belgium. E-mail: dirk.devos@kuleuven.be; trees.debaerdemaeker@kuleuven.be
First published on 4th April 2018
Abstract
M1[Co(CN)6]2/3-type Prussian blue analogues (M1–Co PBAs) were studied as catalysts for the synthesis of propargylamines via A3 coupling of phenylacetylene, benzaldehyde and piperidine. Cu0.86Zn0.14–Co PBA was the best catalyst for the reaction by combining the high conversion obtained with Cu–Co PBA with the excellent selectivity obtained with Zn–Co PBA.
The reaction between a terminal alkyne, a secondary amine and an aldehyde, also known as the A3 coupling, is a multicomponent reaction with a high atom efficiency and water as the only by-product. Therefore, it is considered a green process for the synthesis of pharmaceutical intermediates or final products such as bioactive propargylamines.1–5 In order to carry out the reaction in substantially short times and with high selectivity to the propargylamine product (the A3 product) the use of a catalyst – normally containing transition metals – is necessary.6–12 To simplify catalyst recovery, heterogenization of the active phase is desired.6,13–17
Prussian blue analogues (PBAs), in many cases also referred to as double metal cyanides (DMCs), are cyanide-bridged transition metal coordination polymers with the general formula M1u[M2(CN)n]v·xH2O (hereafter abbreviated as “M1–M2 PBA”). PBAs are easily synthesized by a precipitation reaction between aqueous solutions of the cyanometalate complex, [M2(CN)6]u−, and an M1 salt.18 Even though PBAs were among the first reported coordination polymers, their use as catalyst only dates back to the 1960s.18 Recently, several studies have focused on the expansion of the catalytic applications of PBAs. This can be achieved by virtue of the multiple possible variations in the active metal as well as by changes in synthesis procedures (e.g. using alcohols and other organic additives). For example, Zn–Co PBA based materials are well-known epoxide polymerization catalysts19,20 and have also been used as catalyst for the activation of alkynes in hydroamination reactions (C–N bond formation),21 and for copolymerization of CO2 and epoxides.22,23 Moreover, mixed metal PBAs (Fe2+,Cu2+–Co PBA) have been employed as solid catalysts for the aerobic oxidation of oximes to carbonyl compounds.24 In this work, we have synthesized a series of PBAs based on earth-abundant divalent metals (Fe, Co, Ni, Cu and Zn) and investigated their potential for the synthesis of propargylamines via C–H activation in the A3 coupling reaction of phenylacetylene, benzaldehyde and piperidine (Scheme 1). To the best of our knowledge, this is the first time that PBAs are applied for C–H activation of phenylacetylene in multicomponent reactions.
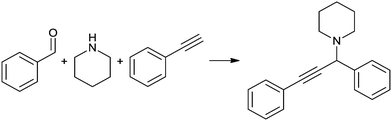 | ||
| Scheme 1 A3 coupling between phenylacetylene, benzaldehyde and piperidine to produce the corresponding propargylamine. | ||
A series of PBAs were synthesized by modifying previously reported procedures25,26 through addition of an aqueous solution of K3[Co(CN)6] to an aqueous solution of a M1Cl2·xH2O salt (FeCl2·4H2O, CoCl2, NiCl2·6H2O, CuCl2·2H2O or ZnCl2) containing PTMEG and tert-butanol. ICP analyses of selected PBA samples show that the M1/Co ratio obtained is higher than the stoichiometric 1.5, indicating that a slight excess of M1 is present in the structure (Table S1†). This is expected, considering that with a 10 to 1 ratio M1Cl2·xH2O to K3[Co(CN)6], an excess M1Cl2·xH2O was used during the synthesis. The crystallinity of the samples was confirmed by powder X-ray diffraction (PXRD, Fig. S1†). All samples show reflections corresponding to a cubic phase typical for metal hexacyanocobaltates.27–29 As expected for the different M1s, the FTIR spectra of the samples show a blue shift in the position of the CN stretching band compared to K3[Co(CN)6] (Fig. S2†).26,30,31 The textural properties of the PBAs vary depending on M1, as evidenced by N2 physisorption (Table S2 and Fig. S3†). Although this type of material is usually microporous in nature, the N2 isotherms of Fe–Co, Co–Co and Cu–Co PBA also exhibit a hysteresis loop around p/p0 = 0.8, indicative of the presence of mesopores. The acid properties of selected samples were studied with pyridine adsorption followed by FTIR spectroscopy (Fig. S4 and Table S2†). The bands at 1450, 1490 and 1610 cm−1 are attributed to pyridine adsorbed on Lewis acid sites.32 No band was observed around 1540 cm−1, which indicates that there are no Brønsted acid sites in the samples.32
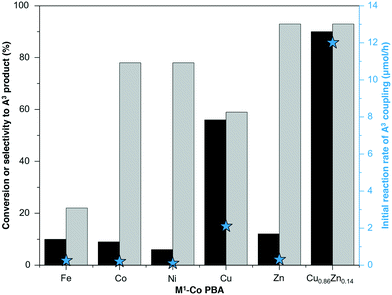
All synthesized PBA samples were investigated both in terms of activity and selectivity to the A3 product. The reaction rate was particularly sensitive to the nature of the M1 metal (Fig. 1 and S5†). For the bimetallic M1–Co PBAs, the highest reaction rate was obtained with Cu–Co PBA, exhibiting an activity one order of magnitude higher than the other PBAs. These results are in line with the reported excellent catalytic activity of Cu sites for this type of reaction.14–17,33,34 In the case of Fe–Co PBA, the activity for the A3 coupling reaction was low and the predominant reaction was the reduction of benzaldehyde to benzyl alcohol, most likely following a Meerwein–Ponndorf–Verley (MPV) mechanism due to the presence of 2-butanol as solvent and potential reductant. Even though the use of Fe as catalytic site has been reported for both A3 coupling and MPV reactions,12,35,36 this specific Fe site favors the reduction of benzaldehyde over the C–H activation of phenylacetylene under these reaction conditions (Fig. S6†).
On the one hand, the highest phenylacetylene conversion was obtained with Cu–Co PBA (Fig. 1). On the other hand, the selectivity towards the A3 product was higher using Zn–Co PBA compared to the other metals. In the latter case, just a small amount of acetophenone was produced. This trend is also maintained when the selectivity is assessed at the same phenylacetylene conversion (Fig. S7†). In light of this, a series of CuxZn1−x–Co multi-metal PBA complexes with different Cu/Zn ratios was prepared with the aim of tuning the catalytic performance of the system.
Elemental analysis showed that the multi-metal samples, CuxZn1−x–Co PBA, contain a much larger amount of Cu than Zn, compared to the initial Cu/Zn molar ratios used during synthesis (Table S1†). High resolution X-ray diffraction measurements and Pawley fitting (Fig. S8†) allowed the refinement of the lattice parameters of the sample Cu0.86Zn0.14–Co PBA, which was found to crystallize in the cubic space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m – just like Cu–Co PBA and Zn–Co PBA (Fig. S9 and S10†). As expected given the high Cu content, the lattice parameters of Cu0.86Zn0.14–Co PBA are in between those of Cu–Co PBA and Zn–Co PBA, but much closer to those of Cu–Co PBA (Table S3†). Similarly, the rest of the multi-metal samples show reflections corresponding to a cubic phase (Fig. S11†). Other physicochemical properties of the multi-metal samples (ν(C
m – just like Cu–Co PBA and Zn–Co PBA (Fig. S9 and S10†). As expected given the high Cu content, the lattice parameters of Cu0.86Zn0.14–Co PBA are in between those of Cu–Co PBA and Zn–Co PBA, but much closer to those of Cu–Co PBA (Table S3†). Similarly, the rest of the multi-metal samples show reflections corresponding to a cubic phase (Fig. S11†). Other physicochemical properties of the multi-metal samples (ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), Lewis acidity, textural properties) are also intermediate between those of Cu–Co PBA and Zn–Co PBA (Table S2 and Fig. S3, S4 and S12†). Furthermore, HAADF-STEM images (Fig. 2) of the sample Cu0.86Zn0.14–Co PBA confirm the formation of a single PBA phase and the close proximity between Zn and Cu, as no segregated Zn-rich or Cu-rich phases are observed.
N), Lewis acidity, textural properties) are also intermediate between those of Cu–Co PBA and Zn–Co PBA (Table S2 and Fig. S3, S4 and S12†). Furthermore, HAADF-STEM images (Fig. 2) of the sample Cu0.86Zn0.14–Co PBA confirm the formation of a single PBA phase and the close proximity between Zn and Cu, as no segregated Zn-rich or Cu-rich phases are observed.
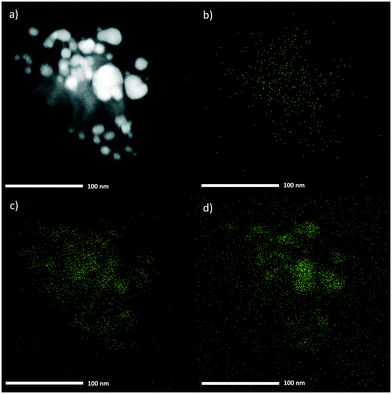 | ||
| Fig. 2 HAADF-STEM image (a) and EDX composition mapping for Zn (b), Co (c) and Cu (d) of the sample Cu0.86Zn0.14–Co PBA. | ||
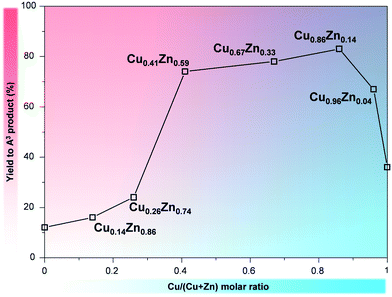
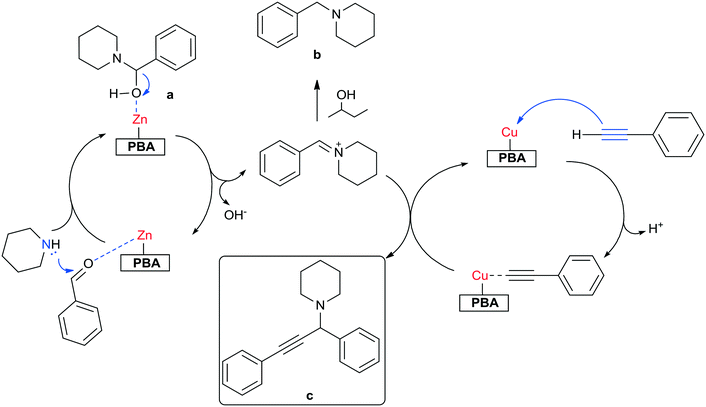
The incorporation of Cu into the Zn–Co PBA increased the catalytic activity of the solid for the A3 coupling reaction in comparison to the original Zn–Co PBA (Fig. 3). Remarkably, at higher Cu content, the simultaneous presence of Cu and Zn in the structure yielded catalysts exhibiting activity superior to that of Cu–Co PBA. In fact, the TOF obtained with the most active multi-metal sample (Cu0.86Zn0.14–Co PBA) was almost five times higher than the one obtained with Cu–Co PBA (Table 1). This suggests a synergistic effect when combining Zn and Cu in the same crystalline framework. Furthermore, the selectivity to the A3 product is also increased with respect to the Cu–Co PBA, from 60% to 93% at 90% conversion of phenylacetylene (Fig. S7†). This increase is attributed to the presence of Zn sites in the PBA structure: they could facilitate the formation of the iminium ion, while also helping in the C–C bond formation between the iminium ion and the Cu-coordinated alkyne (Scheme 2).37
| Cu | Zn | Cu0.86Zn0.14 | |
|---|---|---|---|
| a Turnover frequency based on initial rates of the A3 coupling reaction expressed as mmol of A3 product formed per mmol of M1 (Cu, Zn or Cu + Zn) per h. b Turnover frequency for the hydration of phenylacetylene (0.05 mmol) at 383 K for 6 h expressed as mmol of acetophenone formed per mmol of M1 (in the absence of piperidine and benzaldehyde) per h. c Turnover frequency for the piperidine (0.05 mmol) and benzaldehyde (0.05 mmol) coupling at 383 K for 24 h expressed as mmol of 1-benzylpiperidine and α-phenyl-1-piperidinemethanol formed per mmol of M1 in the absence of phenylacetylene per h. | |||
| TOF A3 couplinga | 0.43 | 0.052 | 2.1 |
| TOF phenylacetylene hydrationb | 0.18 | 0.0025 | 0.055 |
| TOF benzaldehyde–piperidine couplingc | 0.24 | 0.43 | 0.40 |
To further prove this hypothesis, additional reactions were performed first in the absence of, and then with only phenylacetylene in the reaction mixture (Table 1). Results show that Zn2+ sites seem to facilitate the coupling between benzaldehyde and piperidine to form α-phenyl-1-piperidine-methanol and 1-benzylpiperidine, with Zn–Co PBA exhibiting a TOF almost double the TOF of Cu–Co PBA for this reaction. The product 1-benzylpiperidine is believed to be formed by reduction of the iminium ion via a hydrogen-transfer mechanism involving the 2-butanol solvent.38 In contrast, Cu sites appear to enable the activation of phenylacetylene. The TOF obtained with the Cu–Co PBA was two orders of magnitude higher than the one obtained with Zn–Co PBA for the hydration of phenylacetylene. Contrary to previous reports13,39 claiming that the formation of the iminium ion between the aldehyde and the amine occurs almost spontaneously above 353 K, our results show that a specific site – Zn2+ in this case – is needed for this reaction to occur considerably. Without Zn, the rapid activation of phenylacetylene (on Cu sites) yields considerable amounts of acetophenone, whereas without Cu in the structure, this activation takes place too slowly and the coupling of phenylacetylene, piperidine and benzaldehyde does not occur substantially. Blank experiments of the reactions under the same conditions did not produce detectable amounts of any product, also confirming this hypothesis.
Moreover, the selectivity to the A3 product was very different when the Cu active sites were in the form of soluble species (Table S4†). In the case of the homogeneous Cu(OAc)2, CuCl2 and Cu(ClO4)2 catalysts, only 1,4-diphenylbuta-1,3-diyne (from the homocoupling of phenylacetylene) was detected as a product after 24 h, which is not formed when PBAs are used as catalyst. This result suggests that under these reaction conditions, the Cu2+ centers in the PBA framework are stable and maintain their +2 oxidation state.15 Additionally, Cu0.86Zn0.14–Co PBA exhibited a similar selectivity and a higher conversion than the homogenous ZnCl2 salt. A comparable activity was obtained with respect to other heterogeneous Cu-containing catalysts reported in literature, such as of [Cu(2-pymo)2] and Cu nanoparticles supported on graphene under similar reaction conditions.15,40 In aprotic solvents (both non polar like toluene and dioxane, and polar and highly coordinating such as DMSO) the yields of the A3 product decreased (Table S5†). The best catalytic performance of the Cu0.86Zn0.14–Co PBA was obtained with 2-butanol as a solvent at 383 K. Protic, polar solvents have been found to improve the rate of A3 coupling reaction, presumably by facilitating initial iminium ion formation due to the stabilization of charged activation states.41
The yield of A3 product vs. time is plotted in Fig. S13† for Cu0.86Zn0.14–Co PBA. The kinetics of the A3 coupling reaction are still a matter of debate. It has been reported that the phenylacetylene conversion vs. time plot should follow a trend similar to pseudo second-order kinetics because the production of the propargylamine depends on the concentration of both phenylacetylene and the iminium ion.15 However, more recent studies fitted the data by a first-order kinetics equation with respect to phenylacetylene.42 Analysis of the conversion of phenylacetylene at different reaction times reveals that the best fit (highest coefficient of determination, R2) was obtained when the data were fitted by first-order in the alkyne (Fig. S14†). This result was further supported by the evaluation of the variation of the reaction rate with respect to the concentration of phenylacetylene (Fig. S15†). When the concentration of phenylacetylene was doubled, the rate of the reaction also doubled, which suggests that the reaction order with respect to this reactant is equal to one. In contrast, when the concentration of piperidine was varied instead (Fig. S16†), a lower reaction rate for the A3 coupling was observed as the initial concentration of piperidine was increased. This not only shows that the reaction order is not equal to one with respect of piperidine, but also suggests inhibition caused by piperidine due to strong adsorption on Cu2+ sites or formation of Cu complexes.43–47
Finally, the heterogeneity of the catalyst was studied by a hot filtration test. As shown in Fig. S17,† the hot filtrate shows no appreciable activity after stirring for an additional 20 h, indicating that there is no leaching of active species from the catalyst. This was further corroborated by elemental analyses performed after reaction (Table S1†). Remarkably, recycling tests show that Cu0.86Zn0.14–Co PBA largely maintains its activity after five runs (80% yield after fifth run vs. 85% for the fresh catalyst), even though there is a phase change when compared to the pristine sample (Fig. S17†). However, no notable changes were observed in the FTIR spectrum of Cu0.86Zn0.14–Co PBA after one reaction cycle (Fig. S18†).
Conclusions
The catalytic performance of a series of PBAs was evaluated for the synthesis of propargylamines through A3 coupling. These materials show the possibility of tuning their catalytic performance by virtue of the multiple possible variations in the active metal. Here, the combination of Zn and Cu yielded a series of synergistic CuxZn1−x–Co multi-metal PBA complexes, with Cu0.86Zn0.14–Co PBA proving to be an active, selective and recyclable heterogeneous catalyst for the reaction.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No. 641887 (project acronym: DEFNET). F. G. C. acknowledges the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No. 750391 (project acronym SINMOF) for financial support. F. W. O. Vlaanderen (Research Foundation Flanders) is thanked for project funding (D. D. V.: research projects; C. V. G. and I. V.: grant no. G.0256.14N) and a Postdoctoral Fellowship (T. D. B.). D. D. V. thanks KU Leuven for the Metusalem grant CASAS. Funding for the TEM through Hercules project AKUL/13/19 is kindly acknowledged. The authors are grateful to prof. Andrew L. Goodwin, Hanna L. B. Boström, Emily M. Reynolds (University of Oxford) and the beamline staff of I11 (Diamond Light Source) for collecting the high resolution XRD data during Block Allocation Group beamtime (EE13284). The authors thank Michael T. Wharmby (DESY) for useful discussions and prof. Jin Won Seo (KU Leuven) for TEM support.References
- C. Wei and C.-J. Li, J. Am. Chem. Soc., 2003, 125, 9584–9585 CrossRef CAS PubMed.
- J. Dulle, K. Thirunavukkarasu, M. C. Mittelmeijer-Hazeleger, D. V. Andreeva, N. Raveendran Shiju and G. Rothenberg, Green Chem., 2013, 15, 1238–1243 RSC.
- V. A. Peshkov, O. P. Pereshivko and E. V. Van der Eycken, Chem. Soc. Rev., 2012, 41, 3790–3807 RSC.
- S. Sarkar, A. Banerjee and B. K. Patel, in Multicomponent Re-actions: Synthesis of Bioactive Heterocycles, ed. K. L. Ameta and A. Dandia, CRC Press, Boca Raton, 2017, ch. 6, pp. 139–182 Search PubMed.
- W.-J. Yoo, L. Zhao and C.-J. Li, Aldrichimica Acta, 2011, 44, 43–54 CAS.
- S. Cheng, N. Shang, C. Feng, S. Gao, C. Wang and Z. Wang, Catal. Commun., 2017, 89, 91–95 CrossRef CAS.
- S. Sakaguchi, T. Mizuta, M. Furuwan, T. Kubo and Y. Ishii, Chem. Commun., 2004, 1638–1639 RSC.
- L. C. Akullian, M. L. Snapper and A. H. Hoveyda, Angew. Chem., Int. Ed., 2003, 42, 4244–4247 CrossRef CAS PubMed.
- Y. Kuninobu, Y. Inoue and K. Takai, Chem. Lett., 2006, 35, 1376–1377 CrossRef CAS.
- K. Namitharan and K. Pitchumani, Eur. J. Org. Chem., 2010, 3, 411–415 CrossRef.
- W.-W. Chen, H.-P. Bi and C.-J. Li, Synlett, 2010, 3, 475–479 Search PubMed.
- W.-W. Chen, R. V. Nguyen and C.-J. Li, Tetrahedron Lett., 2009, 50, 2895–2898 CrossRef CAS.
- T. R. Mandlimath and K. I. Sathiyanarayanan, RSC Adv., 2016, 6, 3117–3125 RSC.
- M. Gholinejad, F. Saadati, S. Shaybanizadeh and B. Pul-lithadathil, RSC Adv., 2016, 6, 4983–4991 RSC.
- I. Luz, F. X. Llabrés i Xamena and A. Corma, J. Catal., 2012, 285, 285–291 CrossRef CAS.
- G. H. Dang, H. Q. Lam, A. T. Nguyen, D. T. Le, T. Truong and N. T. S. Phan, J. Catal., 2016, 337, 167–176 CrossRef CAS.
- P. Rania, P. F. Siril and R. Srivastava, Mol. Catal., 2017, 433, 100–110 CrossRef.
- P. Valvekens and D. De Vos, in New Materials for Catalytic Applications, ed. V. I. Parvulescu and E. Kemnitz, Elsevier, Amsterdam, 2016, ch. 1, pp. 1–12 Search PubMed.
- J. Milgrom, US Pat., 3278457, 1966 Search PubMed.
- R. J. Herold, US Pat., 3278457, 1966 Search PubMed.
- A. Peeters, P. Valvekens, R. Ameloot, G. Sankar, C. E. A. Kirschhock and D. De Vos, ACS Catal., 2013, 3, 597–607 CrossRef CAS.
- X. H. Zhang, S. Chen, X. M. Wu, X. K. Sun, F. Liu and G. R. Qi, Chin. Chem. Lett., 2007, 18, 887–890 CrossRef CAS.
- J. Sebastian and D. Srinivas, Appl. Catal., A, 2014, 482, 300–308 CrossRef CAS.
- A. García-Ortiz, A. Grirrane, E. Reguera and H. García, J. Catal., 2014, 311, 386–392 CrossRef.
- C. Marquez, M. Rivera-Torrente, P. P. Paalanen, B. M. Weckhuysen, F. G. Cirujano, D. De Vos and T. De Baerde-maeker, J. Catal., 2017, 354, 92–99 CrossRef CAS.
- I. Kim, J. T. Ahn, C.-S. Ha, C. S. Yang and I. Park, Polymer, 2003, 44, 3417–3428 CrossRef CAS.
- D. F. Mullica, W. O. Milligan, G. W. Beall and W. L. Reeves, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1978, 34, 3558–3561 CrossRef.
- A. Ludi, H. U. Guedel and M. Ruegg, Inorg. Chem., 1970, 9, 2224–2227 CrossRef CAS.
- G. W. Beall, W. O. Milligan, J. Korp and I. Bernal, Inorg. Chem., 1997, 16, 2715–2718 CrossRef.
- X.-H. Zhang, Z.-J. Huab, S. Chenc, F. Liua, X.-K. Suna and G.-R. Qi, Appl. Catal., A, 2007, 325, 91–98 CrossRef CAS.
- J. Fernandez Bertran, J. Blanco Pascual and E. Reguera Ruiz, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, 685–689 Search PubMed.
- E. P. Perry, J. Catal., 1963, 2, 371–379 CrossRef.
- M. J. Albaladejo, F. Alonso, Y. Moglie and M. Yus, Eur. J. Org. Chem., 2012, 16, 3093–3104 CrossRef.
- M. J. Albaladejo, F. Alonso and M. J. González-Soria, ACS Catal., 2015, 5, 3446–3456 CrossRef CAS.
- D. Schröder and H. Schwarz, Angew. Chem., Int. Ed. Engl., 1990, 29, 910–912 CrossRef.
- A. Naik, T. Maji and O. Reiser, Chem. Commun., 2010, 46, 4475–4477 RSC.
- R. R. Mondal, S. Khamarui and D. K. Maiti, ACS Omega, 2016, 1, 251–263 CrossRef CAS.
- A. Peeters, L. Claes, I. Geukens, I. Stassen and D. De Vos, Appl. Catal., A, 2014, 469, 191–197 CrossRef CAS.
- S. B. Park and H. Alper, Chem. Commun., 2005, 1315–1317 RSC.
- S. Frindy, A. El Kadib, M. Lahcini, A. Primo and H. García, Catal. Sci. Technol., 2016, 6, 4306–4317 CAS.
- G. A. Price, A. K. Brisdon, K. R. Flower, R. G. Pritchard and P. Quayle, Tetrahedron Lett., 2014, 55, 151–154 CrossRef CAS.
- Q. Li, A. Das, S. Wang, Y. Chen and R. Jin, Chem. Commun., 2016, 52, 14298–14301 RSC.
- C.-M. Fu and A. M. Schaffer, Ind. Eng. Chem. Prod. Res. Dev., 1985, 24, 68–75 CrossRef CAS.
- R. T. Pflaum and W. W. Brandt, J. Am. Chem. Soc., 1954, 76, 6215–6219 CrossRef CAS.
- P. Lahtinen, E. Lankinen, M. Leskelä and T. Repo, Appl. Catal., A, 2005, 295, 177–184 CrossRef CAS.
- N. Zhao, L. Liu, F. Wang, J. Li and W. Zhang, Adv. Synth. Catal., 2014, 356, 2575–2579 CrossRef CAS.
- X. Qi, R. Bai, L. Zhu, R. Jin, A. Lei and Y. Lan, J. Org. Chem., 2016, 81, 1654–1660 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details (synthesis of the PBAs, characterization, catalytic testing), additional characterization (ICP, high resolution XRD patterns and Pawley fitting, N2 physisorption isotherms) and catalytic data. See DOI: 10.1039/c8cy00073e |
| This journal is © The Royal Society of Chemistry 2018 |