Wearable energy sources based on 2D materials
Abstract
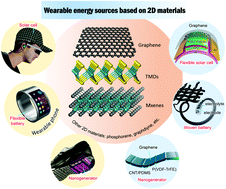
Wearable energy sources are in urgent demand due to the rapid development of wearable electronics. Besides flexibility and ultrathin thickness, emerging 2D materials present certain extraordinary properties that surpass the properties of conventional materials, which make them advantageous for high-performance wearable energy sources. Here, we provide a comprehensive review of recent advances in 2D material based wearable energy sources including wearable batteries, supercapacitors, and different types of energy harvesters. The crucial roles of 2D materials in the wearable energy sources are highlighted. Based on the current progress, the existing challenges and future prospects are outlined and discussed.
- This article is part of the themed collection: 2D nanomaterials: graphene and transition metal dichalcogenides


 Please wait while we load your content...
Please wait while we load your content...