Advances in liquid metals for biomedical applications
Abstract
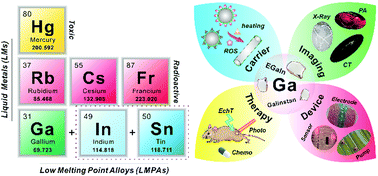
To date, liquid metals have been widely applied in many fields such as electronics, mechanical engineering and energy. In the last decade, with a better understanding of the physicochemical properties such as low viscosity, good fluidity, high thermal/electrical conductivity and good biocompatibility, gallium and gallium-based low-melting-point (near or below physiological temperature) alloys have attracted considerable attention in bio-related applications. This tutorial review introduces the common performances of liquid metals, highlights their featured properties, as well as summarizes various state-of-the-art bio-applications involving carriers for drug delivery, molecular imaging, cancer therapy and biomedical devices. Challenges for the clinical translation of liquid metals are also discussed.


 Please wait while we load your content...
Please wait while we load your content...