Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Supramolecular interaction of non-racemic benzimidazolium based ion pairs with chiral substrates†
Salma
Mumtaz
ab,
Israel
Cano
 *b,
Nargis
Mumtaz
a,
Ahmed
Abbas
c,
Jairton
Dupont
*b,
Nargis
Mumtaz
a,
Ahmed
Abbas
c,
Jairton
Dupont
 *bd and
Humaira Yasmeen
Gondal
*bd and
Humaira Yasmeen
Gondal
 *a
*a
aDepartment of Chemistry, University of Sargodha, Sargodha, 40100, Pakistan. E-mail: hygondal@yahoo.com
bGSK Carbon Neutral Laboratory for Sustainable Chemistry, University of Nottingham, NG7 2GA, Nottingham, UK. E-mail: israel.canorico@nottingham.ac.uk
cH.E.J. Research Institute of Chemistry, ICCBS, University of Karachi, Karachi, 75270, Pakistan
dLaboratory of Molecular Catalysis, Institute of Chemistry, UFRGS, Av. Bento Gonçalves, 9500, Porto Alegre 91501-970, RS, Brazil. E-mail: jairton.dupont@ufrgs.br
First published on 23rd July 2018
Abstract
A series of novel benzimidazolium-based non-racemic ionic liquids (ILs) was synthesized from low-cost chiral terpenoid alcohols and fully characterized by the use of a wide variety of techniques, such as DSC, ESI-MS, ATR FT-IR, polarimetry as well as 1H and 13C NMR spectroscopy. The ILs were investigated as chiral shift agents for the chiral recognition of racemic mixtures of Mosher's acid potassium salt by 19F NMR spectroscopy, leading to high splitting values of the CF3 signal. Supramolecular interactions between salt and H–C2 of chiral benzimidazolium cation are responsible for the chiral recognition, as was demonstrated by experimental evidences. Indeed, the enantiomeric excess value of enantioenriched substrates depends mainly on the strength of the contact ion pairs.
Introduction
Over the past few years, ionic liquids (ILs) have attracted much interest due to their exceptional physical and chemical properties, such as reasonable thermal stability, lack of measurable vapor pressure, high conductivity, appropriate viscosity, catalytic activity and ease recovery and recyclability.1 In particular, chiral ILs deserve a special attention due to their unique ability to act as organocatalysts, coordinating ligands and/or solvents for enantioselective transformations.2–4 These compounds can be prepared by asymmetric synthesis, but protocols based on the use of inexpensive and pre-existing chiral substrates derived from the chiral pool are more attractive to scale-up the synthesis. Consequently, most reported chiral ILs are obtained from naturally available chiral moieties, such as amino acids,5 carbohydrates,6 terpenoids and alkaloids.7 Chiral ILs exhibit applications in ligand exchange chromatography,8 stereoselective polymerization,9 liquid and gas chromatography,10 capillary electrophoresis,10e,11 and as organocatalysts, coordinating ligands and/or solvents for asymmetric synthesis.3,4,12 In addition, chiral ILs have also proven to be very effective as chiral shift agents in NMR spectroscopy for the discrimination of racemic mixtures.4,12,13 These analytical chiral separations are accomplished by in situ generation of temporary diastereomeric adducts through hydrogen bonds, halogen bonding or ionic, dipole–dipole, ion-dipole, van der Waals and π–π stacking interactions.14 The first application of chiral ILs as shift agents in NMR spectroscopy was described by Wasserscheid et al. in 2002, where an ephedrine derived IL was used to determine the enantiomeric excess (ee) of a racemic mixture of Mosher's acid sodium salt by a simple integration of NMR signals.15 Since then, many examples have been reported employing chiral ILs as shift reagents,4,12,13 displaying moderate to high enantio-discrimination levels. However, little is known about the supramolecular interaction between racemic substrates and the non-racemic IL ion pair.Therefore, in order to contribute towards the understanding upon the supramolecular interactions that can be established between racemic substrates and non-racemic ILs, we have designed and prepared a new family of chiral ILs and explored their potential use as chiral recognition agents. Herein we report the synthesis of a new family of benzimidazolium-based chiral ILs prepared from commonly available and inexpensive chiral terpene alcohols such as (+)-menthol, (+)-fenchyl alcohol and (+)-isopinocampheol (Fig. 1). The obtained enantiomerically pure ILs were characterized by DSC, ATR FT-IR, ESI-MS, polarimetry along with 1H and 13C NMR spectroscopy. In addition, the new ILs were studied as chiral shift agents for the chiral recognition of racemic mixtures of Mosher's acid potassium salt. Experimental studies enabled us to elucidate the nature of the interaction between the non-racemic ILs and the racemic Mosher's acid potassium salt. Finally, the ILs demonstrated their ability for the determination of the enantiomeric excess of enantioenriched samples.
 | ||
| Fig. 1 Natural secondary alcohols 1a: (+)-menthol; 1b: (+)-fenchyl alcohol; 1c: (+)-isopinocampheol. | ||
Results and discussion
Synthesis and characterization of the new benzimidazolium-based chiral ILs
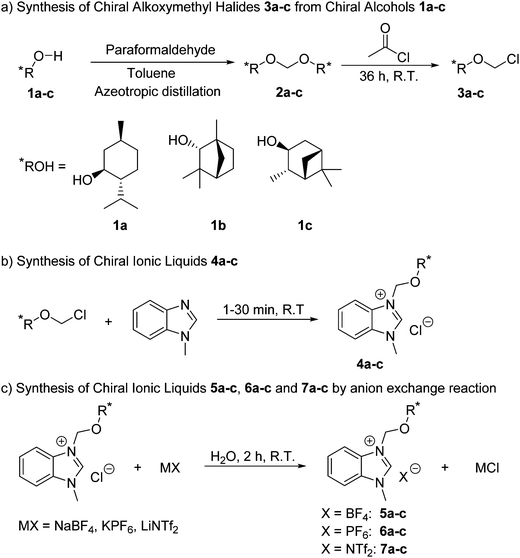
The benzimidazolium-based chiral ILs 4–7 were obtained through a three step synthetic route (Scheme 1 and Sections 2 and 3, ESI†). The reaction of paraformaldehyde with the chiral alcohols 1a–c generated the corresponding acetals 2a–c, which were reacted in situ with acetyl chloride to give the alkoxymethyl halides 3a–c (Scheme 1a).16 Next, the non-racemic ILs 4a–c were synthesized in good yields (Scheme 1b) by reaction of 3a–c with 1-methylbenzimidazole at room temperature (RT). This step is very fast since the reaction mixture was immediately transformed in a semisolid which was further stirred for 30 min to ensure the complete reaction. Finally, the anion exchange by reaction with sodium tetrafluoroborate (NaBF4), potassium hexafluorophosphate (KPF6) and lithium bistrifluoromethanesulfonimidate (LiNTf2) produced the non-racemic ILs 5–7a–c (Scheme 1c).The obtained non-racemic ILs 4–7 were completely characterized by Fourier-Transform Infrared (ATR FT-IR) spectroscopy, electrospray ionization mass spectrometry (ESI-MS), and 1H and 13C NMR spectroscopy (for further details see ESI†), whereas their melting point was studied by differential scanning calorimetry (DSC). Table 1 displays the most important physical properties of 4–7.
| Entry | Non-racemic IL | Physical stateb | Yield (%) | Mpc (°C) | [α]20D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (deg mL g−1 dm−1) d (deg mL g−1 dm−1) |
|---|---|---|---|---|---|
| a Uncertainties of measured values: yield, ±1%; mp, ±0.5 °C; [α]20D, ±2%. b Physical state at 20 °C. c Mp: melting point determined by DSC. d [α]20D: c = 4 mg mL−1, MeOH, 20 °C. | |||||
| 1 | 4a | White solid | 97 | 127 | +136.5 |
| 2 | 5a | White solid | 99 | 132 | +147.5 |
| 3 | 6a | White solid | 99 | 123 | +94.5 |
| 4 | 7a | Light brown oil | 99 | — | +96.5 |
| 5 | 4b | White solid | 95 | 124 | +39.8 |
| 6 | 5b | White solid | 98 | 122 | +38.2 |
| 7 | 6b | White solid | 99 | 147 | +45.0 |
| 8 | 7b | Light brown oil | 99 | — | +22.5 |
| 9 | 4c | White solid | 93 | 122 | +37.8 |
| 10 | 5c | White solid | 98 | 124 | +41.0 |
| 11 | 6c | White solid | 99 | 124 | +55.0 |
| 12 | 7c | Light brown oil | 98 | — | +30.0 |
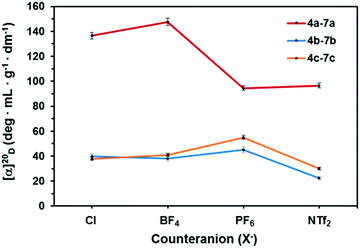
All synthesized ILs are non-racemic, as it is confirmed by their specific rotation ([α]20D). We observed a trend in the [α]20D values of ILs depending on the chiral building block ((+)-menthol, 1a; (+)-fenchyl alcohol, 1b; (+)-isopinocampheol, 1c) employed for their synthesis. In the case of (+)-menthol ILs 4a–7a, there is a large difference between the specific rotation of Cl− and BF4− based salts and those of PF6− and NTf2− ILs (Fig. 2). However, for 4b–7b and 4c–7c the [α]20D values of Cl− based salts were found to be similar to those of BF4− and lower than PF6− based non-racemic ILs, whereas NTf2− compounds showed the lowest values of [α]20D (Fig. 2). On the other hand, DSC revealed a melting point (mp) in the range of 122–147 °C for 4–6 (Table 1), while all NTf2 ILs are liquid at RT.
Chiral recognition of racemic mixtures of Mosher's acid potassium salt by the new benzimidazolium-based chiral ILs
The potential for chiral recognition of synthesized ILs was evaluated through the study of the diastereomeric interaction between 4–7 and a racemic mixture of Mosher's acid potassium salt. For this purpose, various mixtures of Mosher's acid potassium salt and the corresponding chiral IL were analyzed by 19F NMR spectroscopy in order to observe splitting of CF3 signal. Each chiral IL was mixed with the racemic mixture of salt in a specific deuterated solvent at RT, sonicated for 3–5 seconds if required and then filtered.Different concentrations of 6b regarding the racemic salt were tested in order to find the optimal amount of chiral IL required for maximum splitting of CF3 signal of racemic Mosher's acid potassium salt. The influence of 6b concentration on the degree of CF3 signal splitting is represented graphically in Fig. 3. Two equiv. of 6b was the best ratio to obtain the maximum splitting (23.7 Hz, Fig. 3), whereas the extent of the splitting decreased for higher concentrations of 6b. The influence of IL concentration on the magnitude of chemical shift difference is well known and has been previously described by several authors.5d,15,17
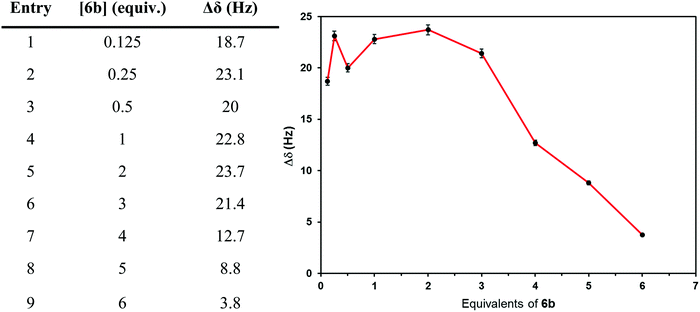 | ||
| Fig. 3 19F NMR chemical shift (recorded in toluene-d8) of racemic Mosher's acid potassium salt in the presence of different amounts of 6b. | ||
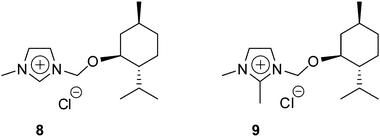
The nature of the interaction between the chiral ILs and the racemic Mosher's acid potassium salt was investigated by experimental studies. The racemic salt could interact with the IL through hydrogen bonding with the H–C2 of the enantiopure imidazolium cation.18 However, an interaction by π–π stacking between the phenyl groups of both compounds is also likely.4b Therefore, we prepared the chiral ILs 8 and 9 in order to investigate the type of interaction between the different species in solution (Fig. 4).
Benzimidazolium-based chiral IL 4a led to a splitting of 19.6 Hz in toluene (Fig. 5), whereas the use of 8 resulted in a similar discrimination (Fig. 5, 19.9 Hz). Consequently, the formation of the diastereomeric adduct is not attributable to the π–π stacking interactions between the phenyl groups of racemic substrate and chiral IL. Interestingly, no splitting was observed with 9 (Fig. 5), for which the hydrogen H–C2 was replaced by a methyl group. This proves that the chiral IL interact with the Mosher's acid potassium salt through hydrogen bonding with the hydrogen atom located in C2 position of the imidazolium ring. This is one of the rare cases in which clear experimental evidences of the supramolecular interactions responsible for chiral recognition are observed.
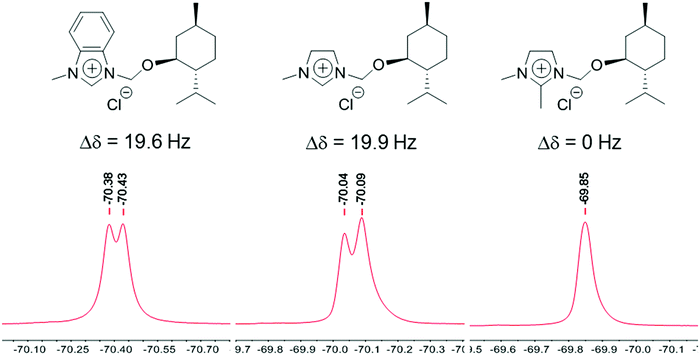 | ||
| Fig. 5 19F NMR investigation of the interaction between the non-racemic ILs and the racemic Mosher's acid potassium salt by the use of different imidazolium-based ILs. | ||
Next, we studied the role of solvents and nature of chiral ILs on the extent of splitting of CF3 signal, since the strength of ion pairs are dependent on the polarity of the solvent employed.19 The experiments were conducted using different deuterated solvents but splitting was only observed in CDCl3 and toluene-d8 (Table 2), while no splitting was detected when more polar solvents such as D2O, MeOD and CD3CN were used. Consequently, it was realized that the solvent strongly affects the formation of the diastereomeric adduct and a reduction in its polarity probably increase the strength of the hydrogen bonding (and contact ion pair)20 and thus the interaction between the molecules of IL and Mosher's acid derivative.18 In most cases, changing the solvent to toluene leads to an increase in splitting of the 19F NMR signal of the racemic Mosher's acid potassium salt in comparison with CDCl3 (Table 2). For instance, chiral ILs 4–5a and 5–6b produced a splitting >8 units higher in toluene than those obtained in CDCl3 (entries 1 and 2). Similarly, the use of chiral ILs 5c and 6c resulted in a splitting of 15.3 and 8.6 Hz in toluene, respectively, whereas no splitting was observed when CDCl3 was utilized as solvent (entries 10 and 11).
| Entry | Non-racemic IL | Precursor alcohol | X | Δδb (Hz) | |
|---|---|---|---|---|---|
| CDCl3 | Toluene | ||||
| a Reagents and conditions: non-racemic IL (2 equiv.), racemic Mosher's acid potassium salt (1 equiv.), deuterated solvent (0.6 mL). NS: no splitting. b Uncertainty for Δδ: ±2%. | |||||
| 1 | 4a | (+)-Menthol | Cl | 9.0 | 19.6 |
| 2 | 5a | (+)-Menthol | BF4 | 10.6 | 19.0 |
| 3 | 6a | (+)-Menthol | PF6 | 11.6 | 11.3 |
| 4 | 7a | (+)-Menthol | NTf2 | NS | NS |
| 5 | 4b | (+)-Fenchol | Cl | 7.2 | 15.0 |
| 6 | 5b | (+)-Fenchol | BF4 | 9.7 | 19.0 |
| 7 | 6b | (+)-Fenchol | PF6 | 12.3 | 23.7 |
| 8 | 7b | (+)-Fenchol | NTf2 | NS | NS |
| 9 | 4c | (+)-Isopinocampheol | Cl | 11.3 | 11.5 |
| 10 | 5c | (+)-Isopinocampheol | BF4 | NS | 15.3 |
| 11 | 6c | (+)-Isopinocampheol | PF6 | NS | 8.6 |
| 12 | 7c | (+)-Isopinocampheol | NTf2 | NS | NS |
The magnitude of the splitting is also affected by the type of anion forming the chiral IL used, and it appears to be related with the strength of the contact ion pair. In general, Cl−, BF4− and PF6− based salts form relatively strong ion pairs21 and provided a good discrimination, whereas no splitting was observed for hydrophobic ILs prepared with NTf2− as counteranion, which possess a weaker cation–anion interaction (entries 4, 8 and 12). Regarding the cation, chiral ILs derived from (+)-menthol and (+)-fenchol led to a similar splitting of the 19F NMR signal (entries 1–8), while the values observed for ILs prepared from (+)-isopinocampheol were lower (entries 9–12). In this context, the maximum splitting of CF3 signal was obtained for 6b (derived from (+)-fenchol), with Δδ = 23.7 Hz (entry 7). This could be due to the higher steric impediment produced by the proximity of methyl groups of fenchol to the benzimidazolium ring in comparison with menthol and isopinocampheol.
The high chiral discrimination induced by ILs 4–7 encouraged us to evaluate their use for the determination of the enantiomeric excess of enantioenriched samples of Mosher's acid salt. Two samples with approximately R/S = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and R/S = 4
1 and R/S = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were prepared through the addition of (R)-Mosher's acid salt to the corresponding racemic salt. The integration of CF3 signals observed in the 19F NMR spectra provided the almost exact ratio of each enantiomer in the samples (Fig. 6). Consequently, the ILs described herein can be successfully employed to determine the ee value of chiral samples.
1 were prepared through the addition of (R)-Mosher's acid salt to the corresponding racemic salt. The integration of CF3 signals observed in the 19F NMR spectra provided the almost exact ratio of each enantiomer in the samples (Fig. 6). Consequently, the ILs described herein can be successfully employed to determine the ee value of chiral samples.
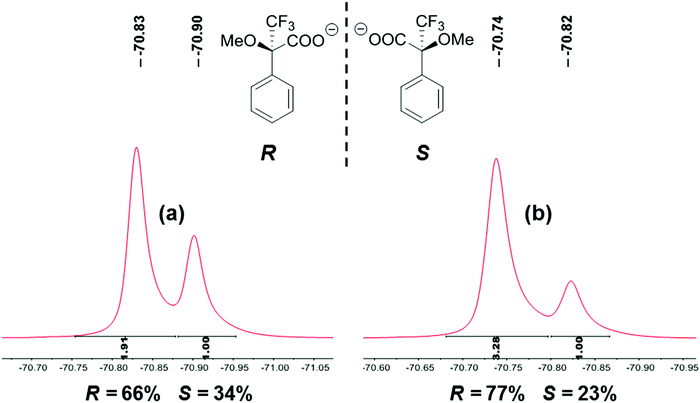 | ||
Fig. 6
19F NMR spectra and determination of % ee values of enantioenriched samples of Mosher's acid potassium salt. (a) R/S = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (b) R/S = 4 1, (b) R/S = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
Conclusions
In summary, we have successfully designed and prepared a new family of twelve benzimidazolium-based non-racemic ILs through a three step synthetic methodology under solvent-free conditions. An exhaustive characterization was carried out by different techniques, which enabled us to confirm the structures of obtained ILs. The non-racemic ILs were found to be efficient chiral shift agents for the chiral recognition of racemic mixtures of Mosher's acid potassium salt by 19F NMR spectroscopy. In addition, experimental studies showed that the formation of the diastereomeric adduct is attributable to the interaction of the IL with the Mosher's acid potassium salt through hydrogen bonding with the more acidic hydrogen located in C2 position of the imidazolium ring. The effectiveness of the chiral recognition is directly related with the strength of the IL contact ion pair. Finally, the ILs demonstrated their potential for the determination of the enantiomeric excess of enantioenriched samples of Mosher's acid salt. In conclusion, these new benzimidazolium-based non-racemic ILs have shown their merit as chiral agents for enantiorecognition, which may inspire the design of new ILs with applications in chiral separation techniques.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Salma Mumtaz acknowledges HEC Pakistan for financial support under IRSIP program. Prof. Jairton Dupont gratefully thank support from the EPSRC. Dr Israel Cano acknowledges financial support from the European Community through a Marie Skłodowska-Curie Individual Fellowships (IF-EF; Programme/Call: H2020-MSCA-IF-2015; Proposal No: 704710-Sdchirnanocat).References
- (a) T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef PubMed; (b) P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772–3789 CrossRef; (c) R. Sheldon, Chem. Commun., 2001, 2399–2407 RSC; (d) N. Jain, A. Kumar, S. Chauhan and S. M. S. Chauhan, Tetrahedron, 2005, 61, 1015–1060 CrossRef; (e) D. Rooney, J. Jacquemin and R. Gardas, Top. Curr. Chem., 2009, 290, 185–212 CrossRef PubMed; (f) S. Aparicio, M. Atilhan and F. Karadas, Ind. Eng. Chem. Res., 2010, 49, 9580–9595 CrossRef; (g) D. D. Irge, Am. J. Phys. Chem., 2016, 5, 74–79 CrossRef.
- (a) C. Baudequin, J. Baudoux, J. Levillain, D. Cahard, A.-C. Gaumont and J.-C. Plaquevent, Tetrahedron: Asymmetry, 2003, 14, 3081–3093 CrossRef; (b) J. Ding and D. W. Armstrong, Chirality, 2005, 17, 281–292 CrossRef PubMed.
- (a) A. Paczal and A. Kotschy, Monatsh. Chem., 2007, 138, 1115–1123 CrossRef; (b) S. Luo, L. Zhang and J.-P. Cheng, Chem. – Asian J., 2009, 4, 1184–1195 CrossRef PubMed; (c) S. Payra, A. Saha and S. Banerjee, Curr. Organocatal., 2017, 4, 4–32 CrossRef; (d) A. Singh and H. K. Chopra, Curr. Org. Synth., 2017, 14, 488–510 CrossRef.
- (a) C. Baudequin, D. Bregeon, J. Levillain, F. Guillen, J.-C. Plaquevent and A.-C. Gaumont, Tetrahedron: Asymmetry, 2005, 16, 3921–3945 CrossRef; (b) K. Bica and P. Gaertner, Eur. J. Org. Chem., 2008, 3235–3250 CrossRef; (c) T. Payagala and D. W. Armstrong, Chirality, 2012, 24, 17–53 CrossRef PubMed.
- (a) H. Ohno and K. Fukumoto, Acc. Chem. Res., 2007, 40, 1122–1129 CrossRef PubMed; (b) D. K. Bwambok, S. K. Challa, M. Lowry and I. M. Warner, Anal. Chem., 2010, 82, 5028–5037 CrossRef PubMed; (c) X. Chen, X. Li, A. Hu and F. Wang, Tetrahedron: Asymmetry, 2008, 19, 1–14 CrossRef; (d) L. Gonzalez, B. Altava, M. Bolte, M. I. Burguete, E. García-Verdugo and S. V. Luis, Eur. J. Org. Chem., 2012, 4996–5009 CrossRef; (e) M. B. A. Rahman, K. Jumbri, M. Basri, E. Abdulmalek, K. Sirat and A. B. Salleh, Molecules, 2010, 15, 2388–2397 CrossRef PubMed.
- (a) V. Kumar, C. Pei, C. E. Olsen, S. J. C. Schäffer, V. S. Parmar and S. V. Malhotra, Tetrahedron: Asymmetry, 2008, 19, 664–671 CrossRef; (b) O. N. V. Buu, A. Aupoix, N. D. T. Hong and G. Vo-Thanh, New J. Chem., 2009, 33, 2060–2072 RSC; (c) Z. Zhou, J. Qiu, L. Xie, F. Du, G. Xu, Y. Xie and Q. Ling, Catal. Lett., 2014, 144, 1911–1918 CrossRef; (d) A. Costa, A. Forte, K. Zalewska, G. Tiago, Z. Petrovski and L. C. Branco, Green Chem. Lett. Rev., 2015, 8, 8–12 CrossRef.
- (a) R. A. F. Matos and C. K. Z. Andrade, Tetrahedron Lett., 2008, 49, 1652–1655 CrossRef; (b) D. Nageshwar, D. M. Rao and P. V. R. Acharyulu, Synth. Commun., 2009, 39, 3357–3368 CrossRef; (c) J. Feder-Kubis, M. Kubicki and J. Pernak, Tetrahedron: Asymmetry, 2010, 21, 2709–2718 CrossRef; (d) T. Heckel, A. Winkel and R. Wilhelm, Tetrahedron: Asymmetry, 2013, 24, 1127–1133 CrossRef.
- (a) Q. Liu, K. Wu, F. Tang, L. Yao, F. Yang, Z. Nie and S. Yao, Chem. – Eur. J., 2009, 15, 9889–9896 CrossRef PubMed; (b) Y. Yan-Xia, L. Jing and J. Xin-Yu, J. Cent. South Univ., 2013, 20, 1173–1177 CrossRef.
- (a) T. Biedroń and P. Kubisa, Polym. Int., 2003, 52, 1584–1588 CrossRef; (b) H.-y. Ma, X.-h. Wan, X.-f. Chen and Q.-f. Zhou, Chin. J. Polym. Sci., 2003, 21, 265–270 Search PubMed; (c) T. Biedroń and P. Kubisa, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 3454–3459 CrossRef.
- (a) A. Berthod, L. He and D. W. Armstrong, Chromatographia, 2001, 53, 63–68 CrossRef; (b) Z. Zhou, X. Li, X. Chen and X. Hao, Anal. Chim. Acta, 2010, 678, 208–214 CrossRef PubMed; (c) L. M. Yuan, Y. Han, Y. Zhou, X. Meng, Z. Y. Li, M. Zi and Y. X. Chang, Anal. Lett., 2006, 39, 1439–1449 CrossRef; (d) X. Sun, J. Xu, X. Zhao, Y. Zhai and J. Xing, Chromatographia, 2013, 76, 1013–1019 CrossRef; (e) C. P. Kapnissi-Christodoulou, I. J. Stavrou and M. C. Mavroudi, J. Chromatogr. A, 2014, 1363, 2–10 CrossRef PubMed.
- (a) Y. Francois, A. Varenne, E. Juillerat, D. Villemin and P. Gareil, J. Chromatogr. A, 2007, 1155, 134–141 CrossRef PubMed; (b) J. Yu, L. Zuo, H. Liu, L. Zhang and X. Guo, Biomed. Chromatogr., 2013, 27, 1027–1033 CrossRef PubMed; (c) J. Zhang, Y. Du, Q. Zhang, J. Chen, G. Xu, T. Yu and X. Hua, J. Chromatogr. A, 2013, 1316, 119–126 CrossRef PubMed; (d) J. Zhang, Y. Du, Q. Zhang and Y. Lei, Talanta, 2014, 119, 193–201 CrossRef PubMed.
- A. Winkel, P. V. G. Reddy and R. Wilhelm, Synthesis, 2008, 999–1016 Search PubMed.
- M. L. Patil and H. Sasai, Chem. Rec., 2008, 8, 98–108 CrossRef PubMed.
- G. K. E. Scriba, J. Chromatogr. A, 2016, 1467, 56–78 CrossRef PubMed.
- P. Wasserscheid, A. Bösmann and C. Bolm, Chem. Commun., 2002, 200–201 RSC.
- S. Mumtaz, S. W. Khan, J. H. Zaidi, A. Iqbal, Z. M. Cheema, K. M. Khan and S. Perveen, Lett. Org. Chem., 2013, 10, 578–583 CrossRef.
- (a) H. Clevier, L. Boulanger, N. Audic, L. Toupet, M. Mauduit and J.-C. Guillemin, Chem. Commun., 2004, 1224–1225 RSC; (b) B. Altava, D. S. Barbosa, M. I. Burguete, J. Escorihuela and S. V. Luis, Tetrahedron: Asymmetry, 2009, 20, 999–1003 CrossRef; (c) R. Jayachandra and S. R. Reddy, RSC Adv., 2016, 6, 39758–39761 RSC.
- A. Winkel and R. Wilhelm, Eur. J. Org. Chem., 2010, 5817–5824 CrossRef.
- M. Zanatta, A. L. Girard, N. M. Simon, G. Ebeling, H. K. Stassen, P. R. Livotto, F. P. dos Santos and J. Dupont, Angew. Chem., Int. Ed., 2014, 53, 12817–12821 CrossRef PubMed.
- L. Santos, B. Neto, C. Consorti, C. Pavam, W. Almeida, F. Coelho, J. Dupont and M. Eberlin, J. Phys. Org. Chem., 2006, 19, 731–737 CrossRef.
- H. K. Stassen, R. Ludwig, A. Wulf and J. Dupont, Chem. – Eur. J., 2015, 21, 8324–8335 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental procedures, synthetic procedures, characterization of new compounds, and 1H NMR and 13C NMR spectra. See DOI: 10.1039/c8cp03881c |
| This journal is © the Owner Societies 2018 |