Revealing reaction mechanisms of nanoconfined Li2S: implications for lithium–sulfur batteries†
Abstract
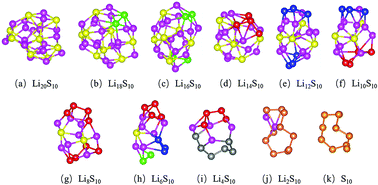
Using Li2S as an active material and designing nanostructured cathode hosts are considered as promising strategies to improve the performance of lithium–sulfur (Li–S) batteries. In this study, the reaction mechanisms during the delithiation of nanoconfined Li2S as an active material, represented by a Li20S10 cluster, are examined by first-principles based calculations and analysis. Local reduction and disproportionation reactions can be observed although the overall delithiation process is an oxidation reaction. Long-chain polysulfides can form as intermediate products; however they may bind to insoluble S2−via Li atoms as mediators. Activating the charging process only requires an overpotential of 0.37 V if using Li20S10 as the active material. Sulfur allotropes longer than cyclo-S8 are observed at the end of the charge process. Although the discharge voltage of Li20S10 is only 1.27 V, it can still deliver an appreciable theoretical energy density of 1480 W h kg−1. This study also suggests that hole polarons, in Li20S10 and intermediate products, can serve as carriers to facilitate charge transport. This work provides new insights toward revealing the detailed reaction mechanisms of nanoconfined Li2S as an active material in the Li–S battery cathode.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
