Asymmetric triphenylamine–phenothiazine based small molecules with varying terminal acceptors for solution processed bulk-heterojunction organic solar cells†
Abstract
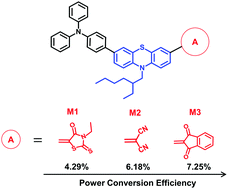
Three compounds consisting of the electron-donating triphenylamine–phenothiazine conjugate backbone and each of the electron-withdrawing groups 3-ethylrhodanine, malononitrile and 1,3-indandione have been synthesized and used as donors in blends with [6,6]-phenyl-C70-butyric acid methyl ester (PC71BM) for organic solar cell devices. After improvements of the active layer structure by a selected donor-to-acceptor weight ratio and a two-step solvent and thermal annealing, the organic solar cells showed power conversion efficiency (PCE) values in the range of 4.79–7.25%. The highest PCE was obtained for the bulk heterojunction device with the indandione compound, which can be attributed to its better absorption profile, higher crystallinity, more balanced electron and hole transport, higher charge collection efficiency and reduced recombination, in comparison with the photovoltaic cells from the other two compounds. DFT-calculated characteristics, absorption spectra and cyclic voltammetry of the compounds, along with X-ray diffraction patterns of the blend films, are used to validate the photovoltaic results.


 Please wait while we load your content...
Please wait while we load your content...