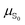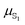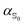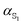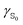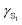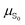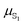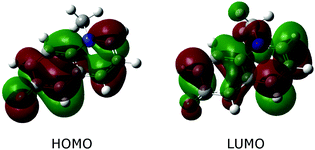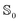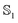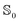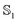Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Evaluating excited state atomic polarizabilities of chromophores†
Esther
Heid
 a,
Patricia A.
Hunt
a,
Patricia A.
Hunt
 *b and
Christian
Schröder
*b and
Christian
Schröder
 *a
*a
aUniversity of Vienna, Faculty of Chemistry, Department of Computational Biological Chemistry, Währingerstraße 19, A-1090 Vienna, Austria. E-mail: christian.schroeder@univie.ac.at; Tel: +43 1 4277 52711
bDepartment of Chemistry, Imperial College London, London, SW7 2AZ, UK. E-mail: p.hunt@imperial.ac.uk
First published on 6th March 2018
Abstract
Ground and excited state dipoles and polarizabilities of the chromophores N-methyl-6-oxyquinolinium betaine (MQ) and coumarin 153 (C153) in solution have been evaluated using time-dependent density functional theory (TD-DFT). A method for determining the atomic polarizabilities has been developed; the molecular dipole has been decomposed into atomic charge transfer and polarizability terms, and variation in the presence of an electric field has been used to evaluate atomic polarizabilities. On excitation, MQ undergoes very site-specific changes in polarizability while C153 shows significantly less variation. We also conclude that MQ cannot be adequately described by standard atomic polarizabilities based on atomic number and hybridization state. Changes in the molecular polarizability of MQ (on excitation) are not representative of the local site-specific changes in atomic polarizability, thus the overall molecular polarizability ratio 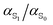 does not provide a good approximation for local atom-specific polarizability changes on excitation. Accurate excited state force fields are needed for computer simulation of solvation dynamics. The chromophores considered in this study are often used as molecular probes. The methods and data reported here can be used for the construction of polarizable ground and excited state force fields. Atomic and molecular polarizabilities (ground and excited states) have been evaluated over a range of functionals and basis sets. Different mechanisms for including solvation effects have been examined; using a polarizable continuum model, explicit solvation and via sampling of clusters extracted from a MD simulation. A range of different solvents have also been considered.
does not provide a good approximation for local atom-specific polarizability changes on excitation. Accurate excited state force fields are needed for computer simulation of solvation dynamics. The chromophores considered in this study are often used as molecular probes. The methods and data reported here can be used for the construction of polarizable ground and excited state force fields. Atomic and molecular polarizabilities (ground and excited states) have been evaluated over a range of functionals and basis sets. Different mechanisms for including solvation effects have been examined; using a polarizable continuum model, explicit solvation and via sampling of clusters extracted from a MD simulation. A range of different solvents have also been considered.
1 Introduction
Electronic excitation of a dye molecule (in solution) will usually lead to a dipole moment change, however it is less well recognized that the polarizability can also change. Excitation induced changes in dipole moment and polarizability will lead to changes to the electrostatic and dispersion interactions between solute and solvent.1 The surrounding solvent is perturbed and is no-longer in equilibrium. The solvent molecules reorientate and relax reaching a new equilibrium state around the electronically excited solute. Polarizability (as well as dipole) effects can be important, particularly in low-polarity solvents, where dispersion interactions are significant or when the solute is highly polarizable. Moreover, excited states are often more polarizable than the ground state, leading to a considerable difference in the ground and excited state polarizabilities. We are interested in a computational description of the changes in polarizability that occur between the ground and excited state of a solute (dye).Time-dependent fluorescence spectroscopy measures the timescale of the solute relaxation, indirectly probing the dynamic properties of the solvent.2–10 The reorganization of the solvent also impacts on the electronic structure of the solute; recently the total (interconnected solute and solvent) response has been directly monitored via optical-Kerr-effect spectroscopy.11–13 The computational description of excitation and relaxation processes often lacks a correct treatment of the solute polarizability and therefore only partly reflects the events probed via experiment.
In a computational approach, one would ideally carry out a large scale ab initio molecular dynamics (MD) simulation with a post Hartree Fock (HF) method. This level of computation is not yet accessible for realistic dyes and a range of methodologies with different levels of approximation are available. Quantum chemical methods naturally include polarization, coumarin 153 has been studied using an AM1(i.e. semiempirical)/MM approach,14 and coumarin 120 has been examined at the HF/MM level.15 More recently, methodologies sampling solute clusters from classical MD trajectories followed by the evaluation of each cluster using a TD-DFT/MM/PCM approach have been reported.16 The next step has been implemented, where a quantum solute region (CAM-B3LYP) is able to polarize the surrounding classical cluster, modeled by a fully polarizable potential (AMOEBA).17 Other variations include running ab initio MD simulations of various flavors.18–20
One of the simplest approaches for studying the dynamic solvent response after electronic excitation is to employ classical MD simulations with a fixed partial charge potential for both the solute and solvent. Excitation of the solute is modeled by modifying the partial charges of the solute to represent the excited state electronic distribution.9,10,21–30 Note that in this case there is no representation of the impact of the changing electronic structure on the force constants of the solute potential (for bonds, angles or torsion angles) or on the van der Waals terms of the solute potential. Moreover, while the dipole moment may be correctly recovered, the change in the polarizability of the solute is ignored.
The natural next step is to include polarization effects, employing polarizable solute and solvent potentials. Polarizable potentials are available for common solvents but are rare for solutes. Efforts have been made to mimic solute polarizability changes at the classical level, for example using artificial solutes (a rigid diatomic) in MD simulations.31–34 A number of model (analytic) theories have been developed employing parameters extracted from experimental spectra.11,35,36 Nevertheless, the effect of a change in solute polarizability on the solvent dynamics has not been well studied.
Providing a simple but robust method for determining the ground and excited state polarizability of key chromophores and including polarizability changes within a MD treatment of solvation dynamics is highly desirable. Including solute polarizability is also advantageous from the perspective of providing more accurate trajectories for the mixed quantum mechanics (QM)/molecular mechanics (MM) approaches that sample solute clusters.
Experimentally, the Frank–Condon polarizability change in the solute can be characterized using Stark spectroscopy.37–42 Relaxed excited state polarizability changes can be probed using time-resolved microwave conductivity.43,44
Computationally, QM methods provide access to both the ground and excited state electronic structure of a chromophore and can be used to evaluate the polarizability. A more accurate computational model requires the use of either an explicit or implicit solvation model. To access the excited state of larger molecules, TD-DFT can be employed. Recently TD-DFT has been used with a linear response (LR) and a corrected linear response (cLR) Polarizable Continuum Model (PCM) formalism.45,46 Although the cLR-PCM approach treats the excited state properties more accurately, it has been shown that for many systems the computationally less expensive LR-PCM approach suffices.45 Thus, the general procedure outlined in ref. 45 can be used to determine the excited state molecular dipole moments and polarizabilities of solute dyes.
The QM methods described above yield molecular polarizabilities, however, a MD treatment of polarizability usually requires atomic polarizabilities. In MD simulations Drude oscillators can be employed to recover polarizability effects. Charged dummy particles (Drude particles) are attached to each non-hydrogen atom via a fictitious harmonic “bond” to mimic the movement of the electron cloud.47 The harmonic force constant kδi is directly connected to the partial charge of the Drude particle qδi and the polarizability αi of the atom i via
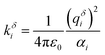 | (1) |
To date atomic polarizabilities employed in MD force fields have been calculated via a designed regression methodology based on experimental data such as the refractive index and molar volume of similar, pure compounds, usually solvents.48–52 However, we are not interested in bulk properties of a solvent, but in the electronic properties of a chromophore in a dilute solution. Moreover, refractive indices and molar volumes are not available for electronically excited states, thus only ground state atomic polarizabilities are accessible. A new methodology for decomposing ground and excited state (solution) molecular polarizabilities into atomic contributions based on a computational approach needs to be developed, i.e. the decomposition cannot be based on experimental observables. In this study we demonstrate a novel and effective computationally based methodology (employing a set of widely available codes) for determining the atomic polarizabilities of solvated molecules.
We consider two well-known molecular probes: N-methyl-6-oxyquinolinium betaine (MQ) and coumarin 153 (C153), Fig. 1. For MQ, calculations of the dipole moment in gas phase9,53,54 and solution9,19 exist, as well as molecular polarizabilities in gas phase.53 For C153, atom-type (i.e. based on the element, such as C and hybridisation state) polarizabilities have also been computed in the gas phase.13 Thus the calculations carried out here will complement the known electronic properties of MQ and C153. More importantly, we will lay the necessary foundations for the general design of polarizable solute force fields.
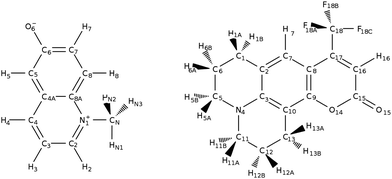 | ||
| Fig. 1 Chromophores and their respective atom labeling used in this study. Left: N-Methyl-6-oxyquinolinium betaine, right: coumarin 153. | ||
In this article we outline and test a QM based methodology for determining atomic excited state polarizabilities for (realistic, i.e. large) dye molecules in solution. The correct choice of functional and basis set, as well as the influence of the solvent on the calculated electronic properties of the solute are investigated.
2 Computational details
All calculations were performed with the Gaussian09 program using density functional theory (DFT).55 For all calculations, the energy convergence threshold was improved to 10−8 a.u., and the density convergence threshold to 10−10 a.u., scf = conver = 10 keyword. An enhanced integration grid was employed (99 radial shells, with 590 angular points per shell), int = ultrafine keyword. Geometry optimization of the ground state was performed with M06-2X, ωB97xD and B3LYP-D3BJ functionals employing a 6-31G(d) basis set, the resulting structures were confirmed as minima by frequency analysis (i.e. no imaginary frequencies). TD-DFT was used with the same functionals/basis sets to compute the first excited (singlet) states.We follow a commonly employed practice of optimizing structures with a modest basis-set and subsequently carrying out single point calculations with a more extensive basis-set. Electronic properties for MQ have been evaluated on the (respective) lower 6-31G(d) level geometry using M06-2X with Sadlej's polarizable pVTZ basis set,56 ωB97xD/aug-cc-pVTZ and B3LYP-D3BJ/6-311G(d,p).
The success of TD-DFT methods can depend on the choice of functional, especially for the calculation of excited states with multi-reference character, or those that involve Rydberg states or when strong charge-transfer occurs, in such cases the amount of exchange included can be highly influential.46,57–59 The excitations examined here are simple π to π* transitions without substantial multireference character and only modest charge transfer, thus are not expected to show strong exchange dependent effects.54,60 To confirm a weak dependence on exchange, the range of functionals outlined above include different levels of exchange; M06-2X (54%), ωB97xD (range sensitive 22–100%) and B3LYP-D3BJ (20%).
Dispersion is important for delocalized π systems, such as the dye molecules examined here. The functionals employed also include a range of mechanisms for modeling dispersion; in the parametrization of the functional (M06),61 as a long range corrected hybrid (ωB97xD)62 or as an ad hoc correction of the B3LYP functional63,64via the method of Grimme (D3BJ).65
Calculations were carried out in the gas phase and with an implicit solvent. A PCM model was employed for water, methanol (MeOH) and ethanol (EtOH).66 The ionic liquid, 1-ethyl-3-methylimidazolium-tetrafluoroborate (C2mimBF4), was modeled via the Universal Solvation Model Based on Solute Electron Density (SMD) method.67,68 In each case, the structure was optimized separately in the gas-phase and solvent environment.
To test the influence of the modest 6-31G(d) basis set on the geometry, we further optimized the structure of MQ employing the M06-2X functional and Sadlej's basis set, both in the gas phase and in PCM (water). No significant differences were found, the root-mean-square deviation of the respective atom coordinates lies under 0.005 Å; further information, including a comparison of the structures can be found in the ESI,† Section 1 and Table S1.
To assess the validity of the implicit solvation model additional calculations were carried out with explicit water molecules. MQ is known to form approximately three hydrogen bonds in the ground state and two hydrogen bonds in the excited state.60 Calculations have been carried out with three explicit water molecules positioned near to the oxygen atom within MQ, two different approaches have been applied. In the first method, a conformation in which MQ formed a cluster with three hydrogen bonds was arbitrarily selected from a previously reported MD simulation of MQ in water.9 The MQ·3H2O cluster was cut and embedded in an implicit (PCM) water solvent and optimized, subsequently the polarizability and dipole moment were computed. In the second method, ten different cluster conformers were extracted from the same MD simulation. MQ and the three water molecules closest to the oxygen atom were cut and the electric structure (polarizability and dipole moment) computed for each frozen conformer (i.e. no geometry optimization was carried out at the QM level).
3 Results and discussion
3.1 Theoretical description
Polarizabilities of the solute ground and excited
and excited 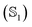 state can be obtained experimentally or computationally via Stark's relation eqn (2) which links the energy E of a state to an applied external electric field F
state can be obtained experimentally or computationally via Stark's relation eqn (2) which links the energy E of a state to an applied external electric field F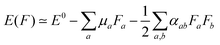 | (2) |
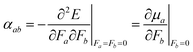 | (3) |
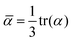 | (4) |
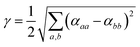 | (5) |
This approach is common for calculating molecular polarizabilities and is valid for both gas phase and implicit solvent calculations of ground or excited state molecules.1,46,69–72 However, atomic polarizabilities are required as input for a polarizable MD potential. The atomic polarizability can be calculated as a derivative of the atomic dipole moment with respect to the electric field analogously to eqn (3) and thus we need to determine the atomic dipole moments.
The total molecular dipole moment (for a neutral molecule) can be evaluated relative to an arbitrary reference point, in this case the coordinate system origin is at the center of mass, eqn (6) where Zi is the nuclear charge, Ri is the nuclear position, ρ(r) is the electron density and r the electronic coordinate:
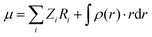 | (6) |
The total dipole moment μ, eqn (6), can also be thought of in terms of a sum of atomic dipole contributions, eqn (7). Atomic charges and atomic dipoles can be defined on each atom (within a non-overlapping atomic integration basin Ωi), eqn (8).
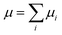 | (7) |
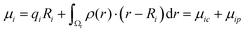 | (8) |
The atomic charge qi includes the nuclear charge and some localized surrounding electron density. Within an atomic basin electron density can polarize and contribute to an atomic dipole moment μip. Thus, qi is not the “normal” atomic charge which is associated with a zero atomic dipole moment. We will now call μic the atomic dipole charge contribution and μip the atomic dipole polarization contribution.
When an electric field is applied to a molecule the distribution of charge is perturbed, electron density can be transferred from one atom to another and the atomic positions Ri can change giving rise to Δμic. The electron density distribution around an atomic site will change and the atomic basin defining the volume of the atom can also change, (resulting in ΔΩi) giving rise to Δμip.73
For each atomic contribution the charge term depends on the absolute position of the atom (while the polarization term does not, since it describes only the relative positions of nucleus and electron cloud). To avoid this dependence, partial charges (on atomic sites i in a neutral molecule) can be converted to a sum of surrounding bond charges qb(ij) where j is an adjacent bonded atomic site and the coordinate contribution to μ is defined relative to the atomic center.74,75
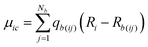 | (9) |
Thus, qb(ij) is the contribution to the net charge of i from the directed bond between the atoms i and j,74Nb is the number of bonds or bond critical points connected to atom i and Rb(ij) is the charge position. This can be at a bond critical point between atom i and j, however Rb(ij) can also simply be mid-way between the atoms, 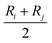 . The net charge on bond points qb(ij) must add up to the atomic dipole charge contribution of the respective atom, and qb(ij) also makes a contribution to atom j.
. The net charge on bond points qb(ij) must add up to the atomic dipole charge contribution of the respective atom, and qb(ij) also makes a contribution to atom j.
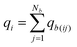 | (10) |
Since qb(ij) describes directed contributions, a reversal of i and j leads to a change in sign, qb(ij) = −qb(ji). If a ring is present in the molecule, the sum of all bond charges in the ring is zero. To determine the bond point charges a set of linear equations is set up (and solved) based on the chemical bonding and the physical restrictions defined. More details and an example are provided in the ESI,† Section 2.
Applying an electric field and employing finite difference methods, the atomic dipole charge and atomic dipole polarizability changes can be evaluated.
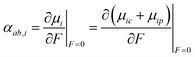 | (11) |
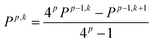 | (12) |
For example, for a specific atom the atomic dipole polarization (μip) is evaluated under a positive (and negative) field in the x-direction delivering μx,ip(Fx) and μx,ip(−Fx) respectively. Then simple finite difference yields the polarization tensor component αxx,ip for that atom, eqn (13).
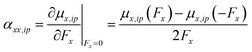 | (13) |
A similar procedure is applied to all the diagonal tensor components and used to determine the average polarizability for that atom 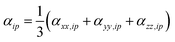 . Subsequently the molecular polarizability can be calculated as the sum of the contributions from each atom,
. Subsequently the molecular polarizability can be calculated as the sum of the contributions from each atom,
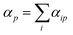 | (14) |
Once the atomic and molecular polarizabilities for the 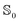 and
and 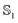 state are known, they can be used to develop a polarizable ground and excited state force field for chromophores.
state are known, they can be used to develop a polarizable ground and excited state force field for chromophores.
3.2 Implementation
Most electronic structure codes will deliver the molecular dipole moment and the molecular polarizability tensor via an analytic or numeric calculation (in G09 use the “polar” keyword). Atomic dipole charges (qi) and the atomic dipole polarization (μip) were obtained from the GDMA code of Misquitta and Stone (multipole moments of rank 1).77,78These quantities are then re-evaluated under the influence of an electric field. A simple vector summation of the atomic bond charges, evaluated with the field on and off, forms the charge transfer component (αic) of the atomic polarizability. The average polarization component (αip) is obtained by finite difference methods; only the diagonal components are needed for each atom. Evaluating αi for k = 1 therefore requires only 6 additional calculations; we applied an electric field of magnitude 0.0008 a.u. in each of the positive and negative x, y and z directions. The molecular polarizability is then obtained by summing the atomic dipole charge transfer and atomic dipole polarizability components. A python script (ESI,† Section 4) was developed to calculate the atomic polarizabilities (i.e. the atomic dipole charge transfer and atomic dipole polarizability) from the atomic coordinates, charges and dipoles.
3.3 Choice of method: functional and basis set
Dipole moments and polarizabilities for MQ in the gas phase and implicit water for different functionals and basis sets are reported in Table 1. TD-DFT has previously been shown to yield accurate electronic properties for MQ, where it was tested against more elaborate methods such as CIS, ROKS and EOM-CCSD.54 The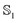 state computed using BLYP was dark and thus T1 was computed in ref. 54. Recently, TD-DFT employing a PCM solvent was successfully used to calculate absorption and emission spectral shapes of MQ, where it was also confirmed via CASSCF/CASPT2 that the first excited state is a single HOMO–LUMO excitation with no multireference character.60 Thus, we expect TD-DFT (in general) to yield an accurate description of both the ground and excited state electronic properties of MQ.
state computed using BLYP was dark and thus T1 was computed in ref. 54. Recently, TD-DFT employing a PCM solvent was successfully used to calculate absorption and emission spectral shapes of MQ, where it was also confirmed via CASSCF/CASPT2 that the first excited state is a single HOMO–LUMO excitation with no multireference character.60 Thus, we expect TD-DFT (in general) to yield an accurate description of both the ground and excited state electronic properties of MQ.
| In vacuo: | ||||||
| M06-2X/Sadlej | 10.8 | 6.9 | 22.0 | 20.5 | 17.6 | 13.4 |
| ωB97xD/aug-cc-pVTZ | 11.0 | 6.8 | 22.0 | 20.3 | 17.7 | 13.5 |
| B3LYP-D3BJ/6-311G(d,p) | 10.1 | 7.2 | 19.8 | 17.5 | 19.0 | 14.2 |
| CASSCF/cc-pVTZ53 | 10.5 | 5.8 | 21.2 | 19.1 | ||
| BLYP-LSD T1/6-311G**54 | 10.2 | 6.8 | ||||
| ωB97xD/aug-cc-pVTZ9 | 11.1 | 7.1 | ||||
| PCM water: | ||||||
| M06-2X/Sadlej | 16.9 | 9.7 | 30.9 | 31.7 | 25.2 | 24.5 |
| ωB97xD/aug-cc-pVTZ | 17.1 | 9.7 | 30.9 | 32.0 | 25.0 | 25.3 |
| B3LYP-D3BJ/6-311G(d,p) | 15.3 | 9.3 | 27.7 | 24.5 | 28.2 | 21.1 |
| BLYP/pVTZ19 | 22 | 14 | ||||
| ωB97xD/aug-cc-pVTZ9 | 16.5 | 8.6 | ||||
The calculated dipole moments and polarizabilities in gas phase correspond well to those reported in literature. In solution slight deviations from the literature values occur. The dipole moments in ref. 19 were calculated from an ab initio trajectory of MQ and explicit water molecules, deviations might therefore stem from the influence of an explicit solvent. In ref. 9, geometry optimization was only carried out in gas phase in the ground state, thus deviations might stem from changes in geometry upon solvation or excitation.
The B3LYP-D3BJ/6-311G(d,p) method produces a low dipole moment for the vacuum ground state, as well as for both solvated states and also yields low polarizabilities, especially for the excited state, when compared to the other methods examined in Table 1. Thus, B3LYP-D3BJ/6-311G(d,p) may be failing to describe the charge-transfer correctly. The same picture arises for the anisotropy of the polarizability. For both the ground and excited state, γ obtained from M06-2X/Sadlej corresponds well to that obtained from ωB97xD/aug-cc-pVTZ, whereas B3LYP-D3BJ/6-311G(d,p) shows much larger deviations. It appears therefore that B3LYP-D3BJ/6-311G(d,p) may not be a suitable method for the calculation of molecular or atomic polarizabilities. We find that M06-2X/Sadlej provides comparable results to the more elaborate ωB97xD/aug-cc-pVTZ level of theory, hence we employ the less expensive M06-2X/Sadlej in all further calculations.
3.4 Influence of the solvent model
Dipole moments and polarizabilities for MQ and C153 in the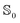 and
and 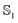 state evaluated (using M06-2X/Sadlej) with different solvents and different solvent models are reported in Table 2. The vacuum polarizability of C153 in the ground and excited state corresponds well to the literature values of 28.2 Å3 and 34.4 Å3, respectively.13 Polarizabilities of MQ were already compared to literature values in the previous subsection.
state evaluated (using M06-2X/Sadlej) with different solvents and different solvent models are reported in Table 2. The vacuum polarizability of C153 in the ground and excited state corresponds well to the literature values of 28.2 Å3 and 34.4 Å3, respectively.13 Polarizabilities of MQ were already compared to literature values in the previous subsection.
| ε | Δα (%) | |||||
|---|---|---|---|---|---|---|
| MQ: | ||||||
| Vacuum | 1 | 10.8 | 6.9 | 21.9 | 20.4 | −7 |
| PCM water | 78.4 | 16.9 | 9.7 | 30.8 | 31.7 | +3 |
| PCM MeOH | 32.6 | 16.7 | 9.6 | 30.5 | 31.3 | +2 |
| PCM EtOH | 24.9 | 16.6 | 9.5 | 30.4 | 31.0 | +2 |
| PCM C2mimBF4 | 12.9 | 16.2 | 9.3 | 29.8 | 30.1 | +1 |
| SMD water | 78.4 | 19.2 | 11.1 | 23.9 | 36.3 | +10 |
| SMD C2mimBF4 | 12.9 | 17.5 | 10.1 | 31.7 | 33.3 | +5 |
| Expl. + PCM opt. | 78.4 | 14.5 | 8.4 | 29.8 | 32.5 | +9 |
| Expl. + PCM rep. | 78.4 | 14.2 | 8.4 | 31.4 | 32.5 | +9 |
| ±1.3 | ±1.0 | ±0.6 | ±0.7 | |||
| C153: | ||||||
| Vacuum | 1 | 7.0 | 12.4 | 31.6 | 34.8 | +10 |
| PCM water | 78.4 | 10.0 | 18.9 | 45.5 | 48.2 | +6 |
| PCM MeOH | 32.6 | 9.9 | 18.7 | 44.9 | 47.6 | +6 |
| PCM EtOH | 24.9 | 9.9 | 18.5 | 44.6 | 47.3 | +6 |
| SMD C2mimBF4 | 12.9 | 10.2 | 19.4 | 46.7 | 49.0 | +5 |
The data (in solution) reported in Table 2 all correspond to default PCM radii (scaled VdW-radii, scaling factor 1.1 for PCM and scaling factor 1.0 for SMD) The influence of the PCM radii was tested and it was found that an increased radius slightly influences dipole moment and polarizability. A larger radius will push the surface charges at the solvent cavity boundary further from the atomic centers, weakening Coulombic interactions and thus will act similarly to a reduction in the dielectric constant of the solvent. Further information and data is provided in the ESI,† Section 5 and Table S2.
In general, the lower the dielectric constant of the solvent, the smaller the absolute dipole moment and polarizability, for both the ground and excited states. Nevertheless, the effect is very small and changes are similar for both states. There is a slight difference between water modeled using PCM vs. SMD probably due to the different parametrization employed for each method. For all the solvents studied using PCM, the ratio between 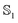 and
and 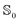 state polarizability remains nearly unchanged at a value of about 1.02 for MQ and 1.06 for C153. Importantly, the similar ratios over a range of different solvents indicate that a single force field (for each chromophore) could be applicable in a wide range of solvents.
state polarizability remains nearly unchanged at a value of about 1.02 for MQ and 1.06 for C153. Importantly, the similar ratios over a range of different solvents indicate that a single force field (for each chromophore) could be applicable in a wide range of solvents.
The vacuum dipole moment and polarizability of MQ are very low and decrease upon excitation, in contrast to the solvent environments where both MQ and C153 exhibit a polarizability increase upon excitation. Thus, these results (for MQ and C153) indicate that gas phase calculations should not be employed to predict polarizability in solution.
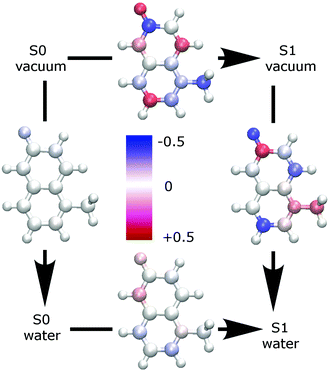
Fig. 2 shows the change of partial charges upon inclusion of a solvent (water) or on excitation. Ground state properties are minimally influenced by an implicit solvent (left panel of Fig. 2), while the excited state properties are more substantially impacted (right panel of Fig. 2).79,80 The inclusion of solvent effects is therefore very important to yield the correct solvated excited state electric properties. Furthermore, changes in the partial charges observed upon excitation in the gas phase (top panel of 2) appear to have too high values which are physically implausible.
Table 2 also lists the dipole moment and polarizability for a cluster of one MQ molecule and three water molecules located close to the oxygen atom evaluated for an optimized geometry (opt.) or from frozen geometries taken as snap-shots from a MD simulation (rep.). The QM calculation reports a whole cluster dipole moment and polarizability, thus to determine the dipole moment and polarizability of the individual molecules we use the CHelpG charges. Dipole moments are calculated classically via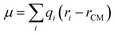 where qi are the respective partial charges and rCM is the position of the center of mass of MQ (without water). The total charge of MQ is not zero, as some of the electron density has moved into the H-bonded water molecules. Thus, because the charge is slightly reduced the dipole moments obtained are estimates (relative to the other values reported). Polarizabilities have been calculated by subtracting three times the polarizability of a single water molecule (in PCM water) from the overall values, and thus these values are also only estimates, (relative to the other values reported).
where qi are the respective partial charges and rCM is the position of the center of mass of MQ (without water). The total charge of MQ is not zero, as some of the electron density has moved into the H-bonded water molecules. Thus, because the charge is slightly reduced the dipole moments obtained are estimates (relative to the other values reported). Polarizabilities have been calculated by subtracting three times the polarizability of a single water molecule (in PCM water) from the overall values, and thus these values are also only estimates, (relative to the other values reported).
The ground and excited state polarizabilities of MQ are similar for the optimized MQ·3H2O cluster, and for the ten averaged MD conformations (within the evaluated uncertainty in α), compared to MQ within an implicit water solvent. Furthermore, the geometry of MQ differs between the ten MD snapshots and the optimized cluster, while the polarizabilities do not. These results support the use of fixed polarizabilities within a MD potential, and indicate that explicit solvent molecules do not strongly influence the overall polarizability in this system. However, an analysis of the precise influence of explicit solvent on the polarizability would require a larger number of snapshots. Alternatively, the cluster polarizability can be dissected into atomic contributions, and summed up over all solute contributions to yield the solute polarizability. The calculation of atomic contributions furthermore enables the detection of site-specific changes in polarizability induced by hydrogen bonds that are lost in the overall molecular property.
3.5 Atomic polarizability
We have established that the M06-2X functional with a Sadlej basis set and PCM (or explicit) solvation model is appropriate and we now proceed to use this methodology for calculating the atomic polarizabilities of MQ, MQ·3H2O and C153. The atomic polarizability was calculated as the change in atomic dipole moment upon applying an external field (here 0.0008 a.u.), where there is an atomic dipole contribution from charge transfer μc arising from a change in partial charge at each atomic site and an atomic dipole polarization contribution μp, arising from the change in inherent dipole moment at each atomic site.The respective atomic polarizabilities for MQ and MQ·3H2O are given in Table 3 (atom labeling is taken from Fig. 1), the corresponding values for C153 are given in the ESI,† Section 6 and Table S3.
| MQ in PCM water | MQ·3H2O in PCM water | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ground state | Excited state | Δα | Ground state | Excited state | Δα | |||||||||
| i | α p,i | α c,i | α i | α p,i | α c,i | α i | Δαi (%) | α p,i | α c,i | α i | α p,i | α c,i | α i | Δαi (%) |
| CN | 0.33 | 0.86 | 1.19 | 0.33 | 0.93 | 1.26 | +6 | 0.33 | 0.86 | 1.19 | 0.33 | 0.93 | 1.26 | +7 |
| HN1 | 0.24 | 0.15 | 0.40 | 0.27 | 0.16 | 0.43 | +9 | 0.24 | 0.15 | 0.39 | 0.27 | 0.16 | 0.43 | +10 |
| HN2 | 0.24 | 0.13 | 0.37 | 0.28 | 0.15 | 0.43 | +17 | 0.22 | 0.13 | 0.36 | 0.28 | 0.15 | 0.41 | +16 |
| HN3 | 0.24 | 0.13 | 0.37 | 0.28 | 0.15 | 0.43 | +17 | 0.22 | 0.13 | 0.36 | 0.28 | 0.15 | 0.41 | +17 |
| N1 | 0.30 | 2.00 | 2.30 | 0.39 | 2.02 | 2.40 | +5 | 0.30 | 1.87 | 2.16 | 0.39 | 2.04 | 2.43 | +12 |
| C2 | 0.59 | 1.36 | 1.96 | 0.74 | 1.42 | 2.16 | +11 | 0.53 | 1.29 | 1.82 | 0.71 | 1.44 | 2.15 | +18 |
| H2 | 0.27 | 0.22 | 0.49 | 0.33 | 0.24 | 0.56 | +15 | 0.25 | 0.21 | 0.46 | 0.33 | 0.24 | 0.55 | +21 |
| C3 | 0.64 | 1.33 | 1.96 | 0.73 | 1.45 | 2.18 | +11 | 0.64 | 1.29 | 1.93 | 1.45 | 0.71 | 2.16 | +12 |
| H3 | 0.30 | 0.22 | 0.50 | 0.33 | 0.22 | 0.55 | +8 | 0.28 | 0.21 | 0.49 | 0.31 | 0.22 | 0.55 | +10 |
| C4 | 0.55 | 1.69 | 2.24 | 0.70 | 1.76 | 2.46 | +10 | 0.52 | 1.59 | 2.09 | 0.70 | 1.78 | 2.47 | +18 |
| H4 | 0.27 | 0.18 | 0.44 | 0.33 | 0.21 | 0.52 | +17 | 0.25 | 0.18 | 0.43 | 0.31 | 0.19 | 0.50 | +18 |
| C4A | 0.39 | 2.30 | 2.70 | 0.49 | 2.49 | 2.98 | +10 | 0.40 | 2.12 | 2.52 | 0.47 | 2.52 | 2.99 | +19 |
| C5 | 0.73 | 1.94 | 2.68 | 0.55 | 1.90 | 2.45 | −8 | 0.67 | 1.76 | 2.43 | 0.53 | 1.90 | 2.43 | +0 |
| H5 | 0.31 | 0.19 | 0.50 | 0.27 | 0.18 | 0.44 | −13 | 0.21 | 0.15 | 0.36 | 0.16 | 0.13 | 0.31 | −15 |
| C6 | 0.36 | 2.12 | 2.46 | 0.34 | 1.93 | 2.27 | −8 | 0.33 | 2.04 | 2.37 | 0.31 | 2.06 | 2.36 | +0 |
| O 6 | 1.48 | 0.73 | 2.21 | 1.20 | 0.68 | 1.88 | −15 | 0.87 | 1.41 | 2.28 | 0.79 | 1.22 | 2.00 | −12 |
| C7 | 0.65 | 1.54 | 2.19 | 0.76 | 1.57 | 2.33 | +7 | 0.59 | 1.39 | 1.99 | 0.76 | 1.60 | 2.19 | +18 |
| H7 | 0.33 | 0.22 | 0.55 | 0.34 | 0.24 | 0.58 | +5 | 0.19 | 0.16 | 0.36 | 0.30 | 0.22 | 0.52 | +46 |
| C8 | 0.59 | 1.73 | 2.31 | 0.58 | 1.81 | 2.39 | +3 | 0.58 | 1.60 | 2.18 | 0.58 | 1.84 | 2.42 | +11 |
| H8 | 0.24 | 0.16 | 0.41 | 0.24 | 0.16 | 0.40 | −2 | 0.24 | 0.16 | 0.40 | 0.24 | 0.16 | 0.40 | +0 |
| C8A | 0.43 | 2.19 | 2.61 | 0.39 | 2.30 | 2.70 | +3 | 0.40 | 2.03 | 2.43 | 0.39 | 2.33 | 2.71 | +12 |
| Total | 9.3 | 21.5 | 30.8 | 9.8 | 21.9 | 31.7 | +3 | 8.4 | 20.7 | 29.0 | 9.2 | 22.7 | 31.9 | +10 |
We first consider MQ in PCM water. The charge transfer αc for the whole molecule is 21.5 Å3 in the 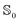 state and 21.9 Å3 in the
state and 21.9 Å3 in the 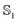 state. The dipole polarizability αp is 9.3 Å3 in the
state. The dipole polarizability αp is 9.3 Å3 in the 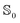 state and 9.8 Å3 in the
state and 9.8 Å3 in the 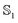 state. Both contribute to the molecular polarizabilities reported in Table 2. Thus, the charge transfer term accounts for most of the molecular polarizability. Dominance by the charge transfer term has been determined previously for other compounds, e.g. nitroanilines.81
state. Both contribute to the molecular polarizabilities reported in Table 2. Thus, the charge transfer term accounts for most of the molecular polarizability. Dominance by the charge transfer term has been determined previously for other compounds, e.g. nitroanilines.81
A closer look at Table 3 and at Fig. 3, which shows the highest occupied (HOMO) and the lowest unoccupied (LUMO) molecular orbital, reveals that the change in polarizability is related to the change of electron density distribution upon excitation. The bottom panel of Fig. 2 also shows the charge redistribution.
Electron density from the phenyl ring, mostly from C5, O6 and C6 is shifted towards the pyridinium part of MQ; C5, O6 and C6 all show a decreased polarizability with respect to both polarization and charge transfer components. The sites gaining electron density, mostly C2 and C4, show an increased polarizability. This finding corresponds with our natural expectation that the electron cloud at an electron rich site is more likely to move upon perturbation than the tightly bound electron cloud around an electron deficient site. These changes in polarizability affect the intrinsic polarization αip to a larger extent but have also a minor contribution from the charge transfer term αic. Overall, αip changes more upon excitation than αic (up to ±29% compared to ±12%), but in absolute terms αic contributes about two thirds to the total atomic polarizability, so that the overall change in atomic polarizability at a site does not exceed ±17% in MQ. The computed change in the molecular polarizability, +2%, does not reflect the local site-specific change in atomic polarizability, ±17%.
The insight obtained from studying the individual atomic polarizabilities is important; the atomic polarizability does not uniformly increase upon excitation. This means an excited state potential cannot be developed by simply scaling the molecular ground state polarizabilities with 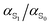 . Polarizability changes are very site and structure specific. Transferability of atomic polarizabilities should also not be taken for granted, especially for specific functional groups within a molecule.
. Polarizability changes are very site and structure specific. Transferability of atomic polarizabilities should also not be taken for granted, especially for specific functional groups within a molecule.
For example, polarizabilities from various designed regression calculations fail to reproduce atomic polarizabilities of strongly charged sites. In MQ, the anionic oxygen O6 (highlighted in bold in Table 3) shows a much larger polarizability (1.48 Å3 from polarization, 2.21 Å3 in total) with respect to literature atomic polarizabilities for this atom type (oxygen), 0.3 to 0.7 Å3.48–52 The failure of atomic polarizabilities from designed regression was already reported for charged molecules,48 but not for charged atom sites. The corollary is that experimental evaluations of transferable atomic atom type polarizabilities via designed regression should only be carried out for very similar molecular classes.
The effects of explicit solvation can be evaluated by considering changes in the ground state atomic polarizability of O6. Three water molecules solvate the O6 site and (the ground state) polarization contribution decreases from 1.48 Å3, to 0.87 Å3, while at the same time the charge transfer polarization increases from 0.73 Å3, to 1.41 Å3. The presence of the hydrogen bonds has increased the charge on the oxygen atom while also hindering the free movement of the electron density and reducing polarization. Thus these results indicate that for an accurate representation of the atomic polarizabilities of solvated molecules, one should include explicit solvent molecules when hydrogen bonding is moderate to strong.
The overall molecular polarizability decreases in the ground state (with three hydrogen bonds) and increases slightly in the excited state (with two hydrogen bonds), so that the overall change for explicit solvation is larger than for isolated MQ. The contributions from polarization and charge transfer for MQ in MQ·3H2O (29.0 Å3 in the ground state and 31.9 Å3 in the excited state) do not correlate directly with the molecular polarizabilities reported in Table 2 (29.8 Å3 and 32.5 Å3 respectively). The latter are only estimates, as they were calculated via α(MQ·3H2O) − 3α(H2O). Thus, the calculation of atomic contributions also ensures the correct distribution of the overall polarizability of a system to the respective atomic sites and individual molecules within a cluster. An accurate decomposition of the cluster polarizability into molecular components is especially important for larger systems, e.g. where a larger number of solvent molecules are included explicitly.
For C153 (ESI,† Section 6 and Table S3), a different picture arises: the atomic polarizabilities for all the aromatic C atoms and the non-aromatic ring C atoms is roughly the same. Only C18, the fluorinated carbon atom has a very low polarizability, which shows the influence of the electronegative fluorine atoms. Upon excitation, the polarization increases; now some of the ring C atoms show a larger increase in polarizability than other atoms, nevertheless the difference is not as extreme as in MQ.
The high-frequency dielectric constant ε∞ of the chromophore in its PCM cavity can be evaluated using the following relation,
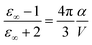 | (15) |
4 Conclusion
The ground and excited state molecular dipole moments and polarizabilities of the fluorescence probes N-methyl-6-oxyquinolinium betaine and coumarin 153 in different solvents have been computed. The ratio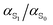 , was found to be approximately 1.02 for MQ, and 1.06 for C153.
, was found to be approximately 1.02 for MQ, and 1.06 for C153.
Use of the exchange–correlation functional M06-2X with the Sadlej polarizable pVTZ basis set was found to be suitable for the calculation of excited state properties, yielding analogous results to the more elaborate ωB97xD/aug-cc-pVTZ method, but at a much lower computational cost.
The influence of the solvent is crucial; for MQ in the gas phase the polarizability is reduced on excitation, while in the solvent the polarizability is increased (on excitation). Thus, employing a gas-phase calculation to predict solution phase polarizabilities can deliver qualitatively incorrect results. Upon the inclusion of explicit water molecules, the atomic polarizability at the oxygen site of MQ decreases significantly. For an accurate representation of atomic polarizabilities it is therefore necessary to include hydrogen bonding partners where appropriate.
Dissection of the overall molecular polarizability of MQ, MQ·3H2O and C153 in PCM water into atomic contributions provided insight into the site specific changes of electric properties upon excitation, as well as the influence of hydrogen bonding on the atomic polarizability. On excitation, the phenyl ring of MQ looses electron density and becomes less polarizable, whereas the pyridinium ring gains electron density and shows increased polarizability at all sites. The polarizability changes of C153 are less site-specific upon excitation. Therefore, the polarizability of some chromophores does not uniformly increase upon excitation and the transferability of atomic polarizabilities should be treated carefully. Furthermore, the change of atomic polarizability upon excitation is not reflected in the molecular ratio 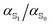 at many sites.
at many sites.
To sum up, the quantum mechanical calculation of atomic polarizabilities provides a good description of molecular electronic structure in the systems studied and enables the set-up of accurate ground and excited state polarizable force fields, through the calculation of atomic polarizability components.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the Austrian Science Fund FWF in the context of Project No. FWF-P28556-N34. Some of the simulations of C153 were performed using the facilities of the Imperial College High Performance Computing Service. E. H. is recipient of a DOC Fellowship of the Austrian Academy of Sciences at the Institute of Computational Biological Chemistry. We thank Prof. Anthony Stone, Cambridge University, UK, for his valuable advice.References
- F. C. Grozema, R. Telesca, H. T. Jonkman, L. D. A. Siebbeles and J. G. Snijders, J. Chem. Phys., 2001, 115, 10014 CrossRef CAS.
- M. Maroncelli and G. R. Fleming, J. Chem. Phys., 1987, 86, 6221 CrossRef CAS.
- M. Maroncelli, J. Mol. Liq., 1993, 57, 1 CrossRef CAS.
- R. Jimenez, G. R. Fleming, P. V. Kumar and M. Maroncelli, Nature, 1994, 369, 471 CrossRef CAS.
- S. Vajda, R. Jimenez, S. J. Rosenthal, V. Fidler, G. R. Fleming and E. W. Castner, Jr., J. Chem. Soc., Faraday Trans., 1995, 91, 867 RSC.
- E. L. Mertz, V. A. Tikhomirov and L. I. Krishtalik, J. Phys. Chem. A, 1997, 101, 3433 CrossRef CAS.
- M. Sajadi, M. Weinberger, H.-A. Wagenknecht and N. P. Ernsting, Phys. Chem. Chem. Phys., 2011, 13, 17768 RSC.
- M. Sajadi, F. Berndt, C. Richter, M. Gerecke, R. Mahrwald and N. P. Ernsting, J. Phys. Chem. Lett., 2014, 5, 1845 CrossRef CAS PubMed.
- E. Heid, S. Harringer and C. Schröder, J. Chem. Phys., 2016, 145, 164506 CrossRef PubMed.
- E. Heid and C. Schröder, J. Chem. Phys., 2016, 145, 164507 CrossRef PubMed.
- F. Terenziani and A. Painelli, Chem. Phys., 2003, 295, 35 CrossRef CAS.
- S. Park, J. Kim and N. F. Scherer, Phys. Chem. Chem. Phys., 2012, 14, 8116–8122 RSC.
- X. Sun, B. M. Ladanyi and R. M. Stratt, J. Phys. Chem. B, 2015, 119, 9129 CrossRef CAS PubMed.
- F. Cichos, R. Brown and P. A. Bopp, J. Chem. Phys., 2001, 114, 6834 CrossRef CAS.
- K. Ando, J. Chem. Phys., 1997, 107, 4585–4596 CrossRef CAS.
- N. De Mitri, S. Monti, G. Prampolini and V. Barone, J. Chem. Theory Comput., 2013, 9, 4507–4516 CrossRef CAS PubMed.
- D. Loco, É. Polack, S. Caprasecca, L. Lagardère, F. Lipparini, J.-P. Piquemal and B. Mennucci, J. Chem. Theory Comput., 2016, 12, 3654–3661 CrossRef CAS PubMed.
- I. Timrov, M. Micciarelli, M. Rosa, A. Calzolari and S. Baroni, J. Chem. Theory Comput., 2016, 12, 4423–4429 CrossRef CAS.
- C. Allolio, M. Sajdi, N. P. Ernsting and D. Sebastiani, Angew. Chem., Int. Ed., 2013, 52, 1813 CrossRef.
- A. Petrone, G. Donati, P. Caruso and N. Rega, J. Am. Chem. Soc., 2014, 136, 14866 CrossRef CAS.
- P. V. Kumar and M. Maroncelli, J. Chem. Phys., 1995, 103, 3038 CrossRef CAS.
- R. M. Stratt and M. Maroncelli, J. Phys. Chem., 1996, 100, 12981 CrossRef CAS.
- B. M. Ladanyi and M. Maroncelli, J. Chem. Phys., 1998, 109, 3204 CrossRef CAS.
- F. Cichos, R. Brown, U. Rempel and C. von Borczyskowski, J. Phys. Chem. A, 1999, 103, 2506 CrossRef CAS.
- L. R. Martins, A. Tamashiro, D. Laria and M. S. Skaf, J. Chem. Phys., 2003, 118, 5955 CrossRef CAS.
- L. R. Martins and M. S. Skaf, Chem. Phys. Lett., 2003, 370, 683 CrossRef CAS.
- F. Ingrosso, B. M. Ladanyi, B. Mennucci, M. D. Elola and J. Tomasi, J. Phys. Chem. B, 2005, 109, 3553 CrossRef CAS.
- E. A. Nikitina, A. V. Odinokov, F. V. Grigoriev, M. V. Basilevsky, A. A. Khlebunov, V. A. Sazhnikov and M. V. Alfimov, J. Phys. Chem. B, 2007, 111, 3953 CrossRef CAS PubMed.
- K. E. Furse and S. A. Corcelli, J. Chem. Theory Comput., 2009, 5, 1959 CrossRef CAS.
- V. Barone, J. Bloino, S. Monti, A. Pedone and G. Prampolini, Phys. Chem. Chem. Phys., 2011, 13, 2160 RSC.
- B. D. Bursulaya, D. A. Zichi and H. J. Kim, J. Phys. Chem., 1995, 99, 10069 CrossRef CAS.
- D. Jeong, Y. Shim, M. Y. Choi and H. J. Kim, J. Phys. Chem. B, 2007, 111, 4920 CrossRef CAS PubMed.
- D. W. Small, D. V. Matyushov and G. A. Voth, J. Am. Chem. Soc., 2003, 125, 7470 CrossRef CAS PubMed.
- T. Ishida, J. Phys. Chem. B, 2005, 109, 18558–18564 CrossRef CAS PubMed.
- D. V. Matyushov, J. Chem. Phys., 2001, 115, 8933–8941 CrossRef CAS.
- D. V. Matyushov and M. D. Newton, J. Phys. Chem. A, 2001, 105, 8516–8532 CrossRef CAS.
- W. Liptay, Angew. Chem., Int. Ed. Engl., 1969, 8, 177–188 CrossRef CAS.
- W. Liptay, H.-J. Schlosser, B. Dumbacher and S. Hünig, Z. Naturforsch., A: Astrophys., Phys. Phys. Chem., 1968, 23, 1613–1625 CAS.
- L. M. P. Beekman, R. N. Frese, G. J. S. Fowler, R. Picorel, R. J. Cogdell, I. H. M. van Stokkum, C. N. Hunter and R. van Grondelle, J. Phys. Chem. B, 1997, 101, 7293–7301 CrossRef CAS.
- L. M. P. Beekman, M. Steffen, I. H. M. van Stokkum, J. D. Olsen, C. N. Hunter, S. G. Boxer and R. van Grondelle, J. Phys. Chem. B, 1997, 101, 7284–7292 CrossRef CAS.
- G. U. Bublitz, R. Ortiz, S. R. Marder and S. G. Boxer, J. Am. Chem. Soc., 1997, 119, 3365–3376 CrossRef CAS.
- G. U. Bublitz, R. Ortiz, C. Runser, A. Fort, M. Barzoukas, S. R. Marder and S. G. Boxer, J. Am. Chem. Soc., 1997, 119, 2311–2312 CrossRef CAS.
- M. P. De Haas and J. M. Warman, Chem. Phys., 1982, 73, 35–53 CrossRef CAS.
- J. M. Warman, G. H. Gelinck, J. J. Piet, J. W. A. Suykerbuyk, M. P. de Haas, B. M. W. Langeveld-Voss, R. A. J. Janssen, D. H. Hwang, A. B. Holmes, M. Remmers, D. Neher, K. Mullen and P. Bauerle, Proc. SPIE, 1997, 3145, 142–149 CrossRef CAS.
- S. Budza, M. Medved, B. Mennucci and D. Jacquemin, J. Phys. Chem. A, 2014, 118, 5652 CrossRef PubMed.
- M. Medveď, S. Budzák and T. Pluta, Theor. Chem. Acc., 2015, 134, 78 CrossRef.
- V. M. Anisimov, G. Lamoureux, I. V. Vorobyov, N. Huang, B. Roux and A. D. MacKerell, J. Chem. Theory Comput., 2005, 1, 153 CrossRef PubMed.
- C. E. S. Bernardes, K. Shimizu, J. C. Lopes, P. Marquetand, E. Heid, O. Steinhauser and C. Schröder, Phys. Chem. Chem. Phys., 2016, 18, 1665 RSC.
- K. Bica, C. Schröder, M. Deetlefs and K. R. Seddon, Phys. Chem. Chem. Phys., 2013, 15, 2703 RSC.
- Y. K. Kang and M. S. Jhon, Theor. Chim. Acta, 1982, 61, 41 CrossRef CAS.
- K. J. Miller, J. Am. Chem. Soc., 1990, 112, 8543 CrossRef CAS.
- Y. Gu and T. Yan, J. Phys. Chem. A, 2013, 117, 219 CrossRef CAS PubMed.
- M. Sajadi, Y. Ajaj, I. Ioffe, H. Weingärtner and N. P. Ernsting, Angew. Chem., Int. Ed., 2010, 49, 454 CrossRef CAS PubMed.
- C. Allolio and D. Sebastiani, Phys. Chem. Chem. Phys., 2011, 13, 16395 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Wallingford CT, 2009 Search PubMed.
- A. J. Sadlej, Theor. Chim. Acta, 1991, 79, 123 CrossRef CAS.
- M. Savarese, É. Brémond and C. Adamo, Theor. Chem. Acc., 2016, 135, 99 CrossRef.
- D. Jacquemin, V. Wathelet, E. A. Perpète and C. Adamo, J. Chem. Theory Comput., 2009, 5, 2420–2435 CrossRef CAS PubMed.
- D. Jacquemin, E. A. Perpète, I. Ciofini, C. Adamo, R. Valero, Y. Zhao and D. G. Truhlar, J. Chem. Theory Comput., 2010, 6, 2071–2085 CrossRef CAS PubMed.
- A. Petrone, J. Cerezo, F. J. Avila Ferrer, G. Donati, R. Improta, D. Rega and F. Santoro, J. Phys. Chem. A, 2015, 119, 5426 CrossRef CAS PubMed.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215 CrossRef CAS.
- J.-D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615 RSC.
- A. D. Becke, J. Chem. Phys., 1993, 98, 1372–1377 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- X. Song and D. Chandler, J. Chem. Phys., 1998, 108, 2594 CrossRef CAS.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2009, 113, 6378 CrossRef CAS PubMed.
- V. S. Bernales, A. V. Marenich, R. Contreras, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2012, 116, 9122 CrossRef CAS PubMed.
- L. F. Pašteka, M. Melicherík, P. Neogrády and M. Urban, Mol. Phys., 2012, 110, 2219 CrossRef.
- P. K. Mukherjee, T. Minato and D. P. Chong, Int. J. Quantum Chem., 1983, 23, 447 CrossRef CAS.
- P. Karamanis, D. Bégué and C. Pouchan, J. Chem. Phys., 2007, 127, 094706 CrossRef PubMed.
- S. Ferdous and J. B. Lagowski, J. Polym. Sci., Part B: Polym. Phys., 2007, 45, 1983 CrossRef CAS.
- K. E. Laidig and R. F. W. Bader, J. Chem. Phys., 1990, 93, 7213 CrossRef CAS.
- T. A. Keith, Atomic Response Properties, Wiley-VCH Verlag GmbH & Co. KGaA, 2007, pp. 61–94 Search PubMed.
- A. Krawczuk-Pantula, D. Pérez, K. Stadnicka and P. Macchi, Trans. Am. Crystallogr. Assoc., 2011, 42, 1 Search PubMed.
- M. Medved, M. Stachová, D. Jacquemin, J.-M. André and E. A. Perpète, J. Mol. Struct. THEOCHEM, 2007, 847, 39 CrossRef CAS.
- A. J. Stone, J. Chem. Theory Comput., 2005, 1, 1128 CrossRef CAS PubMed.
- A. J. Misquitta and A. J. Stone, J. Chem. Phys., 2006, 124, 024111 CrossRef PubMed.
- D. Jacquemin, V. Wathelet, E. A. Perpète and C. Adamo, J. Chem. Theory Comput., 2009, 5, 2420 CrossRef CAS PubMed.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999 CrossRef CAS PubMed.
- M. in het Panhuis, R. W. Munn and P. L. A. Popelier, J. Chem. Phys., 2004, 120, 11479 CrossRef CAS PubMed.
- J. L. P. Lustres, S. A. Kovalenko, M. Mosquera, T. Senyushkina, W. Flasche and N. P. Ernsting, Angew. Chem., Int. Ed., 2005, 44, 5635 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Basis set dependence, definition of bond charges, Romberg differentiation, python script to calculate atomic polarizabilities, influence of the cavity radius, atomic polarizabilities of coumarin 153, all tables in atomic units. See DOI: 10.1039/c7cp08549d |
| This journal is © the Owner Societies 2018 |