Pressure-induced abnormal ionic–polaronic–ionic transition sequences in AgBr
Abstract
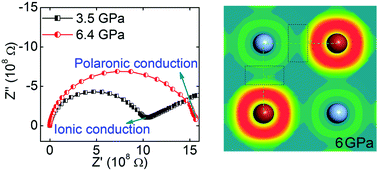
The electrical transport behavior of the superionic conductor AgBr was systematically studied under high pressure up to 30.0 GPa with electrochemical impedance spectra measurements and first-principles calculations. From impedance spectra measurements, a pressure-induced abnormal ionic–polaronic–ionic transition was found. Herein, the ionic to polaronic transition at 5.0 GPa occurs with the absence of a structural phase transition. At 8.6 GPa, the ionic state of AgBr can be reactivated after a structural phase transition. Previous structural studies based on X-ray diffraction data cannot provide strong evidence to support the ionic–polaronic transition in AgBr at 5.0 GPa. In this paper, based on first-principles calculations, a localized-electron-soup model was proposed to explain the physical origin of the ionic–polaronic transition. In this model, more localized electrons around the Br atoms are pressed into interstitial spaces and, simultaneously, polarons are formed between Ag+ ions and the localized electron background at 5.0 GPa. Therefore, the diffusion of Ag+ ions is effectively screened by the movement of the localized electron background from its equilibrium position, much like beans completely trapped in a cup of thick soup.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...