Kinetic Monte Carlo simulations of water ice porosity: extrapolations of deposition parameters from the laboratory to interstellar space
Abstract
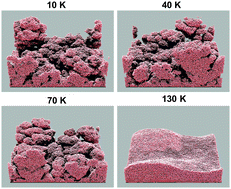
Dust grains in cold, dense interstellar clouds build up appreciable ice mantles through the accretion and subsequent surface chemistry of atoms and molecules from the gas. These mantles, of thicknesses on the order of 100 monolayers, are primarily composed of H2O, CO, and CO2. Laboratory experiments using interstellar ice analogues have shown that porosity could be present and can facilitate diffusion of molecules along the inner pore surfaces. However, the movement of molecules within and upon the ice is poorly described by current chemical kinetics models, making it difficult either to reproduce the formation of experimental porous ice structures or to extrapolate generalized laboratory results to interstellar conditions. Here we use the off-lattice Monte Carlo kinetics model MIMICK to investigate the effects that various deposition parameters have on laboratory ice structures. The model treats molecules as isotropic spheres of a uniform size, using a Lennard-Jones potential. We reproduce experimental trends in the density of amorphous solid water (ASW) for varied deposition angle, rate and surface temperature; ice density decreases when the incident angle or deposition rate is increased, while increasing temperature results in a more-compact water ice. The models indicate that the density behaviour at higher temperatures (≥80 K) is dependent on molecular rearrangement resulting from thermal diffusion. To reproduce trends at lower temperatures, it is necessary to take account of non-thermal diffusion by newly-adsorbed molecules, which bring kinetic energy both from the gas phase and from their acceleration into a surface binding site. Extrapolation of the model to conditions appropriate to protoplanetary disks, in which direct accretion of water from the gas-phase may be the dominant ice formation mechanism, indicate that these ices may be less porous than laboratory ices.
- This article is part of the themed collection: Theory, experiment, and simulations in laboratory astrochemistry


 Please wait while we load your content...
Please wait while we load your content...