Open Access Article
Open Access ArticlePolymorphism of a porous hydrogen bond-assisted ionic organic framework†
Dániel Vajk
Horváth
 a,
Tamás
Holczbauer
a,
Tamás
Holczbauer
 *ab,
Laura
Bereczki
*ab,
Laura
Bereczki
 b,
Roberta
Palkó
b,
Roberta
Palkó
 a,
Nóra Veronika
May
a,
Nóra Veronika
May
 b,
Tibor
Soós
b,
Tibor
Soós
 a and
Petra
Bombicz
a and
Petra
Bombicz
 b
b
aInstitute of Organic Chemistry, Research Centre for Natural Sciences, Hungarian Academy of Sciences, Magyar tudósok körútja 2, 1117 Budapest, Hungary. E-mail: holczbauer.tamas@ttk.mta.hu
bChemical Crystallography Research Laboratory, Research Centre for Natural Sciences, Hungarian Academy of Sciences, Magyar tudósok körútja 2, 1117 Budapest, Hungary
First published on 2nd March 2018
Abstract
The polymorphism of a porous, non-covalently bonded ionic organic framework is reported. The framework is constructed by hydrogen bonding and anion⋯π interactions. In a solvatomorphic lattice, pyridine takes part in the framework formation. The role of molecular rigidity in framework construction is proven by analogous non-porous crystals, where polymorphism also appears.
Solid structures with large volume areas have a broad range of applications such as sensing,1 drug delivery,2 heterogeneous catalysis,3,4 separation,5 storage,6etc.7 We describe the polymorphism and solvatomorphism of porous cationic molecular crystals constructed by the assistance of C–H⋯Br− and Br−⋯π interactions. The rigid molecular conformation achieved by C–H⋯π intramolecular interactions is a condition of the constructed framework, which is proven by comparing structures of related more flexible molecules crystallising into different non-porous architectures. Polymorphism occurs in the case of both porous and non-porous packing arrangements (Scheme 1, Table 1).
| 1a | 1b | 1c | 2a | 2b | 2c | |
|---|---|---|---|---|---|---|
| a Without THF. b Without pyridine/with pyridine. c V void/(ρcalc × Vunitcell). d Because of the high void volumes, Platon SQUEEZE software was used during the refinement. | ||||||
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21/n | P21/c | Fddd | Pnna | P21/n |
| a [Å] | 12.2542(11) | 15.0148(17) | 12.4240(8) | 22.071(3) | 24.343(3) | 17.465(3) |
| b [Å] | 12.3204(11) | 9.6841(11) | 23.0450(15) | 25.991(3) | 15.987(4) | 15.707(3) |
| c [Å] | 12.4610(11) | 25.874(3) | 15.1970(8) | 28.554(4) | 17.571(5) | 25.981(4) |
| α [°] | 91.981(7) | 90 | 90 | 90 | 90 | 90 |
| β [°] | 106.699(7) | 100.421(3) | 99.050(7) | 90 | 90 | 110.398(3) |
| γ [°] | 93.107(7) | 90 | 90 | 90 | 90 | 90 |
| V unit cell [Å3] | 1796.9(3) | 3700.1(7) | 4296.9(4) | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 380(4) 380(4) |
6838(3) | 6680(2) |
| V void [Å3] | 0 | 80 | 335 | 6864a | 2666a | 2398/1221a,b |
| [%] | 2% | 8% | 42%a | 39%a | 36%/18%a,b | |
| Porec [cm3 g−1] | 0 | 0.018 | 0.069 | 0.556a | 0.433a | 0.358/0.181a,b |
| KPI [%] | 68.6 | 66.4 | 61.6 | 41.2a | 46.2a | 56.7/47.5a,b |
| Z′ for 1 or 2 | 1 | 1 | 1 | 1/4 | 1/2 | 1 |
| Final R indices [I > 2σ(I)] | R 1 = 0.0471, wR2 = 0.1044 | R 1 = 0.0681, wR2 = 0.1306 | R 1 = 0.0787, wR2 = 0.1945 | R 1 = 0.2302, wR2 = 0.5471 | R 1 = 0.1047, wR2 = 0.2801 | R 1 = 0.1276, wR2 = 0.3386 |
| R indices (all data) | R 1 = 0.0777, wR2 = 0.1257 | R 1 = 0.1207, wR2 = 0.1512 | R 1 = 0.1205, wR2 = 0.2219 | R 1 = 0.2968, wR2 = 0.5847 | R 1 = 0.1862, wR2 = 0.3288 | R 1 = 0.2138, wR2 = 0.3937 |
The design of highly ordered porous architectures has attracted wide interest owing to their broad application. They are chemically diverse materials whose macroscopic properties can be tailored. The discovery of metal organic frameworks (MOFs)7–10 opened a new branch of research. Most recently, liquid phase MOFs11 were reported. Covalent organic frameworks (COFs)12,13 are crystalline porous polymers. Porous hydrogen-bonded organic frameworks (HOFs) have come to the forefront of interest in recent years.14–16 The basic building blocks of HOFs mainly consist of two parts: a scaffold and hydrogen bonding interaction sites.17
A well-orchestrated interplay of intermolecular forces, molecular inflexibility and the presence of symmetry characterize the non-covalently bonded organic frameworks.
Most of the organic frameworks described are electronically neutral. The design principles and applications of ionic metal–organic frameworks (iMOFs)18 and ionic covalent organic frameworks (iCOFs)19 were recently published. The ions inside the channels of the skeletons can be utilized for specific interactions with guest molecules, like increased efficiency and selectivity in ion exchange. During the development of ionicity of iMOFs, the role of the metal ion or cluster and the choice of the ligand are decisive. iCOFs with ionic linkers and neutral knots are reported.19 Two types of ionic frameworks can be distinguished by the nature of the backbone: anionic and cationic.
The long-range periodicity in crystals of HOFs is a product of the directionally specific short-range intermolecular interactions. Understanding molecular recognition principles is important to control the self-assembly of HOFs.20 It is beneficial to prevent π⋯π stacking, which is against void formation.14 A diversity of hydrogen bonds and supramolecular interactions of carboxylic, hydroxyl, amine and amide groups often serve as hydrogen-bonded motifs of HOFs.5 A useful method to increase the strength of the hydrogen bond is to incorporate charged sites to assist hydrogen bonding of the building blocks. The interaction between an electron deficient arene and an anion has been recognised recently as a non-covalent interaction.21,22
Polymorphism is only occasionally observed in the family of porous frameworks. Free energy differences between polymorphic forms are usually small.23 Polymorphs differ in their relative thermodynamic stabilities which are influenced by temperature and pressure. The polymorphic transformation changes the adsorption properties of a framework, and polymorphism is associated with the so-called “breathing effect”.24 Polymorphs differ in their crystal packing arrangements and/or in the conformation of the molecules – there are different supramolecular interactions and crystallographic symmetries in the lattices of different polymorphic forms.23
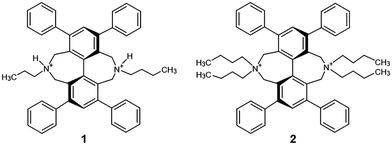
An iCOF formed from 4,4′,4′′,4′′′-(pyrene-1,3,6,8-tetrayl)tetraaniline (PyTTA) as a neutral knot with an organic cationic bromide linker was reported very recently.19 Stable anion⋯π interaction complexes of naphthalene diimides (NDI) with chloride and bromide in the gas phase were reported not long ago.25 These two achievements suggested that hydrogen bond-assisted ionic organic frameworks can be prepared by the application of a modified Maruoka type organocatalyst containing a diazadibenzo[ef,kl]heptalene skeleton. Maruoka achieved outstanding efficiency and enantioselectivity in alkylation reactions with chiral phase-transfer catalysts owing to a BINOL backbone (Fig. S38 and Table S8, ESI†).26,27 The main molecular structural characteristics of ammonium salt catalysts are their large aromatic moieties, structural rigidity and marked axial chirality. The quaternary ammonium cation part of the catalyst has now been duplicated, and the aromatic moiety moved from the edge of the molecule to the centre. In the two newly synthesized Maruoka-type phase-transfer catalysts reported here, the two reaction centres of the symmetrical molecules were formed by the ring closure of rigid, seven-membered rings in the last step of the synthesis. One or two butyl groups (1 and 2) are attached to the saturated ring nitrogen (Scheme 1). The molecules are fully substituted with apolar phenyl and alkyl groups. The synthesized model compounds were used in framework construction experiments and can also be a promising new family of Maruoka-type catalysts with further modifications. The 3D open pore framework structure is advantageous in catalytic activity.
Both compounds 1 and 2 were synthesised (Scheme S1, ESI†) starting from the inexpensive chalcone 3 and dimethyl-1,3-acetonedicarboxylate 4. Michael addition and subsequent condensation of the dinucleophile 4 with the dielectrophile 3 gave the cyclohexanone derivative 5. This compound was transformed into the tetrasubstituted iodobenzene 7via the tosylhydrazone intermediate 6 in a Bamford–Stevens type one pot reaction, followed by in situ oxidation with 50% yield. The polysubstituted iodo compound 7 gave the biphenyl 8via the Ullmann homocoupling reaction. The ester functional group was reduced to 9 and brominated to give the tetrabromo compound 10. This intermediate was subjected to ring closure with butylamine or dibutylamine to give compound 1 or 2.
The 5,11-dibutyl-1,3,7,9-tetraphenyl-4,5,6,10,11,12-hexahydro-5,11-diazadibenzo[ef,kl]heptalene (1) molecule is substituted by only one butyl substituent at each N atom. By single crystal X-ray structure determination, the neutral dibutyl derivative shows dimorphism (1a: P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , 1b: P21/n). In the partially flexible molecules, one of the butyl groups is disordered in both structures. It is attributed to the remaining flexibility of the semi-rigid molecule, due to the lack of anchoring intramolecular interactions of the substituents, because of the uneven butyl to phenyl (2
, 1b: P21/n). In the partially flexible molecules, one of the butyl groups is disordered in both structures. It is attributed to the remaining flexibility of the semi-rigid molecule, due to the lack of anchoring intramolecular interactions of the substituents, because of the uneven butyl to phenyl (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) ratio. The external surface of 1 is highly apolar. C–H⋯π interactions stabilize the lattices. In its HCl salt, 5,11-dibutyl-1,3,7,9-tetraphenyl-4,5,6,10,11,12-hexahydro-5,11-diazadibenzo[ef,kl]heptalene-5,11-diium-chloride (1c: P21/n), the residual space among the flexible substituents attached to a stiff scaffold allows parts of the molecule: one butyl and two terminal phenyl groups, to be disordered. N–H⋯Cl− and C–H⋯Cl− interactions are present in the lattice. Neither the dimorphs of the neutral molecule (1a, 1b) nor its HCl salt (1c) are able to build a framework structure. The flexibility of the molecule (1) does not support the formation of an organic framework structure.
4) ratio. The external surface of 1 is highly apolar. C–H⋯π interactions stabilize the lattices. In its HCl salt, 5,11-dibutyl-1,3,7,9-tetraphenyl-4,5,6,10,11,12-hexahydro-5,11-diazadibenzo[ef,kl]heptalene-5,11-diium-chloride (1c: P21/n), the residual space among the flexible substituents attached to a stiff scaffold allows parts of the molecule: one butyl and two terminal phenyl groups, to be disordered. N–H⋯Cl− and C–H⋯Cl− interactions are present in the lattice. Neither the dimorphs of the neutral molecule (1a, 1b) nor its HCl salt (1c) are able to build a framework structure. The flexibility of the molecule (1) does not support the formation of an organic framework structure.
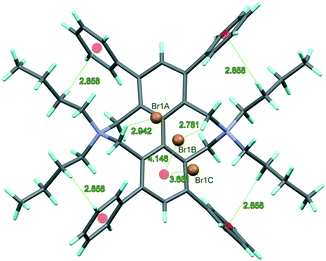
In spite of the general expectations, the introduction of the second butyl group on the ring nitrogen reduces the flexibility of the 5,5,11,11-tetrabutyl-1,3,7,9-tetraphenyl-4,5,6,10,11,12-hexahydro-5,11-diazadibenzo[ef,kl]heptalene-5,11-diium bromide (2) molecule with increasing steric crowding. The molecule is strained by four C–Hβex⋯π intramolecular interactions between each butyl and the neighbouring terminal phenyl substituents (butyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phenyl, 4
phenyl, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) (Fig. 1).
4) (Fig. 1).
The crystal lattices of 2 are highly ordered porous solid-state architectures formed by supramolecular self-assembly via non-covalent interactions.
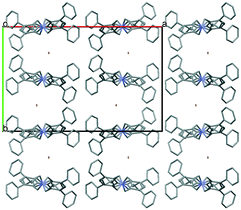
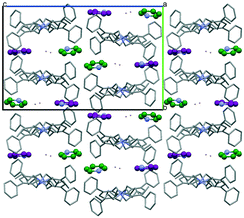
Simple recrystallization of the HBr salt from tetrahydrofuran results in two polymorphic frameworks (2a: Fddd, Fig. 2, and 2b: Pnna, Fig. 3). Void volumes are 2a: 42% and 2b: 39%, respectively, in the highly porous systems. The rigid, conformational highly similar molecules pack differently in the dimorphic architectures.
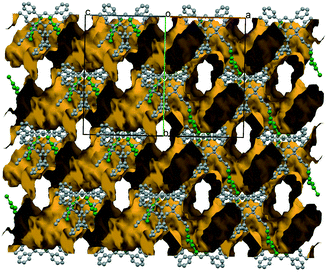 | ||
| Fig. 2 The 3D sponge-like crystal structure of 2a (Fddd, 42%), one of the iHOF dimorphs. The bromide anions are coloured green. The channels are filled with disordered THF. | ||
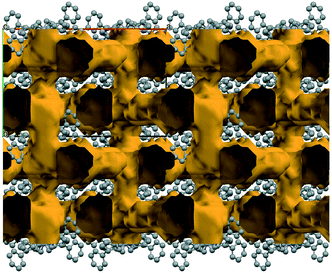 | ||
| Fig. 3 The framework structure of 2b (Pnna, 39%), the other member of the iHOF dimorphs. The bromide anions are coloured green. The channels are filled with disordered THF. | ||
The organic frameworks are assisted by the interactions formed between Cαin–H and Br− as charge-assisted hydrogen bonds, as well as by anionic Br−⋯π interactions with the electron deficient central phenyl ring (Fig. 1).
By applying additional solvent pyridine to THF, a novel highly porous structure appears, whose void volume is 36%. In this solvatomorphic form (2c: P21/n; Fig. 4 and 5), pyridine molecules become part of the cationic framework with C–HPh-ex⋯π secondary interactions as neutral linkers between the cationic knots. It is in contrast to published iCOFs with ionic linkers and neutral knots.
Conformational symmetry appears, showing two (2a) or one (2b) twofold symmetry axis in the molecule. The lattice symmetry and the molecular symmetry of the rigid scaffold decrease correspondingly, having 1/4 (2a, Fddd), 1/2; (2b, Pnna) and 1 (2c, P21/n) structural unit in the asymmetric unit of the polymorphs and the solvatomorphic form.
In conclusion, the presented crystal structures illustrate the polymorphism of a porous, hydrogen bond-assisted ionic organic framework, iHOF (2a and 2b). The framework is constructed by C–H⋯halogenide ions and the recently described anion⋯π interactions. A solvatomorph of the framework is prepared (2c), where the pyridine molecules take part in the formation of the framework. The role of the molecular rigidity in framework construction is demonstrated by the non-porous crystal structures of related more flexible molecules (1a–1c), where polymorphism also appears (1a and 1b).
This work was supported by the National Research, Development and Innovation Office-NKFIH through OTKA K115762, K116150 and K124544, as well as by the J. Bolyai Research Scholarship of the Hungarian Academy of Sciences (TH).Conflicts of interest
There are no conflicts to declare.Notes and references
- T. Kusukawa and M. Fujita, J. Am. Chem. Soc., 2002, 124, 13576–13582 CrossRef CAS PubMed.
- M. Vallet-Regí, F. Balas and D. Arcos, Angew. Chem., Int. Ed., 2007, 46, 7548–7558 CrossRef PubMed.
- J. S. Seo, D. Whang, H. Lee, S. I. Jun, J. Oh, Y. J. Jeon and K. Kim, Nature, 2000, 404, 982–986 CrossRef CAS PubMed.
- J. Lee, A. Hu and W. Lin, J. Am. Chem. Soc., 2002, 124, 12948–12949 CrossRef PubMed.
- D. N. Dybtsev, H. Chun, S. H. Yoon, D. Kim and K. Kim, J. Am. Chem. Soc., 2004, 126, 32–33 CrossRef CAS PubMed.
- R. E. Morris and P. S. Wheatley, Angew. Chem., Int. Ed., 2008, 47, 4966–4981 CrossRef CAS PubMed.
- M. J. Prakash and M. S. Lah, Chem. Commun., 2009, 3326–3341 RSC.
- O. M. Yaghi and G. Li, Angew. Chem., Int. Ed. Engl., 1995, 34, 207–209 CrossRef CAS.
- L. Zhu, X.-Q. Liu, H.-L. Jiang and L.-B. Sun, Chem. Rev., 2017, 117, 8129–8176 CrossRef CAS PubMed.
- G. Mehlana, G. Ramon and S. A. Bourne, Microporous Mesoporous Mater., 2016, 231, 21–30 CrossRef CAS.
- R. Gaillac, P. Pullumbi, K. A. Beyer, K. W. Chapman, D. A. Keen, T. D. Bennett and F.-X. Coudert, Nat. Mater., 2017, 16, 1149–1154 CrossRef CAS PubMed.
- M. Dogru and T. Bein, Chem. Commun., 2014, 50, 5531–5546 RSC.
- C. S. Diercks and O. M. Yaghi, Science, 2017, 355, 923–930 CrossRef CAS PubMed.
- Y.-F. Han, Y.-X. Yuan and H.-B. Wang, Molecules, 2017, 22, 266–300 CrossRef PubMed.
- H. Wang, Z. Bao, H. Wu, R.-B. Lin, W. Zhou, T.-L. Hu, B. Li, J. C.-G. Zhaoa and B. Chen, Chem. Commun., 2017, 53, 11150–11153 RSC.
- W. Yan, X. Yu, T. Yan, D. Wu, E. Ning, Y. Qi, Y.-F. Han and Q. Li, Chem. Commun., 2017, 53, 3677–3680 RSC.
- F. M. A. Noa, S. A. Bourne, H. Su and L. R. Nassimbeni, Cryst. Growth Des., 2017, 17, 4647–4654 Search PubMed.
- A. Karmakar, A. V. Desai and S. K. Ghosh, Coord. Chem. Rev., 2016, 307, 313–341 CrossRef CAS.
- N. Huang, P. Wang, M. A. Addicoat, T. Heine and D. Jiang, Angew. Chem., Int. Ed., 2017, 56, 4982–4986 CrossRef CAS PubMed.
- G. Resnati, E. Boldyreva, P. Bombicz and M. Kawano, IUCrJ, 2015, 2, 675–690 CrossRef CAS PubMed.
- X. Lucas, A. Bauzá, A. Frontera and D. Quinonero, Chem. Sci., 2016, 7, 1038–1050 RSC.
- M. Giese, M. Albrecht and K. Rissanen, Chem. Commun., 2016, 52, 1778–1795 RSC.
- P. Bombicz, Crystallogr. Rev., 2017, 23, 118–151 CrossRef.
- D. Aulakh, J. R. Varghese and M. Wriedt, Inorg. Chem., 2015, 54, 8679–8684 CrossRef CAS PubMed.
- R. E. Dawson, A. Hennig, D. P. Weimann, D. Emery, V. Ravikumar, J. Montenegro, T. Takeuchi, S. Gabutti, M. Mayor, J. Mareda, C. A. Schalley and S. Matile, Nat. Chem., 2010, 2, 533–538 CrossRef CAS PubMed.
- M. Kitamura, S. Shirakawa and K. Maruoka, Angew. Chem., Int. Ed., 2005, 44, 1549–1551 CrossRef CAS PubMed.
- S. Shirakawa, K. Liu and K. Maruoka, J. Am. Chem. Soc., 2012, 134, 916–919 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1813220–1813225. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ce00041g |
| This journal is © The Royal Society of Chemistry 2018 |