Retardation behavior of hydration of calcium sulfate hemihydrate (bassanite) induced by sodium trimetaphosphate (STMP)
Abstract
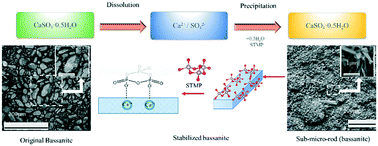
The sub-micro crystals of a bassanite intermediate were isolated through the introduction of sodium trimetaphosphate (STMP) during the hydration process of bulk bassanite. The particle size and morphology of precipitates were first checked by scanning electron microscopy. The particle size was found to be significantly reduced with increments of STMP, and the majority of the precipitates at a >80 mM STMP addition level had sub-micro size. The length, height, and width of the crystals were determined to be ∼1 μm, ∼150 nm, and ∼300 nm, respectively, by atomic force microscopy. Surface structural information at the molecular level was then obtained by diffuse reflectance infrared Fourier transform spectroscopy. Afterwards, the crystal structure evolution with different amounts of STMP was determined by wide angle X-ray diffraction. Both analytical results show that the final precipitated sub-micro rods (100 mM STMP) are almost all bassanite rather than gypsum. Moreover, the multipeak deconvolution of the WAXD patterns presents a quantitative analysis of the weight percent of bassanite in the final precipitates. The final concentration of bassanite is close to 100% (100 mM STMP). Based on these results, the hydration process of bulk bassanite can be described as the dissolution of original bulk bassanite, the formation of bassanite sub-micro rods, and finally the self-assembly of the sub-micro rods along the c-axis to form gypsum micro-crystals without the additive.


 Please wait while we load your content...
Please wait while we load your content...