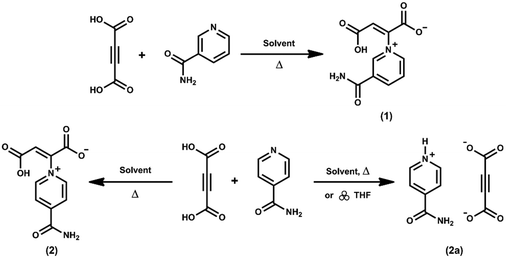
Formation of multi-component crystals with a series of pyridinium-carboxyacrylate zwitterions†
Jean
Lombard
 ,
Leigh
Loots
,
Leigh
Loots
 ,
Tanya
le Roex
,
Tanya
le Roex
 and
Delia A.
Haynes
and
Delia A.
Haynes
 *
*
Department of Chemistry and Polymer Science, Stellenbosch University, P. Bag X1, Matieland, 7602, Republic of South Africa. E-mail: dhaynes@sun.ac.za
First published on 8th December 2017
Abstract
The reaction between acetylenedicarboxylic acid and nicotinamide or isonicotinamide produces two new zwitterionic molecules, namely (Z)-2-(3-carbamoylpyridin-1-ium-1-yl)-3-carboxyacrylate (1) and (Z)-2-(4-carbamoylpyridin-1-ium-1-yl)-3-carboxyacrylate (2), as well as a salt by-product, 4-carbamoylpyridinium acetylenedicarboxylate (2a). The solid-state behaviour of the zwitterions has been investigated by altering variables such as the solvent(s) used for synthesis and crystallisation, and the temperature at which these occur. Zwitterions 1 and 2, as well as a previously reported zwitterion, (Z)-2-(4-cyanopyridin-1-ium-1-yl)-3-carboxyacrylate (3), were combined with various small organic co-formers in an attempt to form multi-component crystals via both mechanochemical and solution methods. Three new hydrated salts were formed with melamine (1·melamine, 2·melamine, and 3·melamine), all of which display extensive charge-assisted hydrogen-bonding networks. Zwitterions 1 and 2 also form the characteristic R22(8) hydrogen-bond motif between the amide group and melaminium. Based on these observed trends in hydrogen bonding, further crystallisation experiments were attempted with molecules with similar hydrogen bonding capability to melamine. Although single crystals were not obtained, six potential salts have been identified based on PXRD, NMR and IR data. Compared to the number of co-formers used, the discovery of only three salts per zwitterion is unusual – a Cambridge Structural Database analysis indicates that this may be due to the robust nature of the hydrogen bonds between zwitterions themselves.
Introduction
Multi-component crystals, in which two or more different molecules are included in one crystalline material,1 are of increasing interest to solid-state chemists in many areas, including the development of new functional materials and the pharmaceutical industry. For example, formation of a multi-component crystal of a drug molecule is often linked to a change in physicochemical properties,2 such as solubility,3,4 stability,5 tabletability,6 and many others linked to the practical application of these materials. Multi-component crystals include co-crystals, salts, and solvates, but many other categories have also been suggested, such as salt solvates, co-crystal salts, co-crystal solvates, and co-crystal salt solvates.7 These terms are still under dispute, especially the term co-crystal – with respect to its necessity, specificity, elegance, and even spelling.7–9 While confusion can be avoided by simply calling all of these systems multi-component crystals, more specific terms do aid in accurately describing a material. For this reason, we will continue with the following definitions: co-crystals contain two or more neutral molecules combined into one crystalline material. If proton transfer takes place, the resulting material is a salt. When one of the components played the role of the solvent in the reaction, the product is referred to as a solvate.Multi-component crystals are usually formed after some thought, using the principles of crystal engineering, is put into the possible intermolecular interactions that molecules could form with one another.10 It is thus important to understand trends in intermolecular interactions if we want to be able to design useful salts, co-crystals and solvates.
Our group recently reported the synthesis and crystal structures of a series of pyridyl-derived zwitterions, consisting of a dicarboxylic acid backbone with a pyridine derivative branching out from the backbone, resulting in a characteristic T-shape.11 Some of these zwitterions have been found to show interesting inclusion abilities,12 conversion between a variety of forms13 and polymorphic behaviour.14 As a continuation of this work, two new zwitterions with the potential to form multi-component crystals have been synthesised, with the aim of linking zwitterions together via hydrogen bonding of a co-former to the foot of the T-shaped zwitterion. Specifically, (Z)-2-(3-carbamoylpyridin-1-ium-1-yl)-3-carboxy-acrylate (1) and (Z)-2-(4-carbamoylpyridin-1-ium-1-yl)-3-carboxyacrylate (2) (Scheme 1) have been synthesised. These zwitterions were chosen as they have an extra hydrogen-bonding group attached to the pyridinium ring in the form of an amide. Amides are known to form a number of robust hydrogen bonding motifs,15 and the introduction of a second molecule with appropriate complimentary functional groups could allow the hydrogen bonding network to propagate in more dimensions, potentially resulting in interesting supramolecular architectures such as host–guest systems.
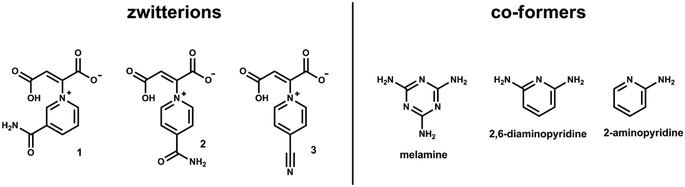 | ||
| Scheme 1 Structures of the three zwitterions discussed in this paper, as well as the three co-formers that were successfully combined with each zwitterion. | ||
Herein we describe the solid-state structures of zwitterions 1 and 2, as well as structures obtained when the zwitterions were combined with other small organic molecules (Scheme 1). A third zwitterion, reported previously by our group,14 shows similar behaviour when combined with these co-formers, and the structures of the resulting salts are also reported. The structures are compared to gain insight into the trends in hydrogen bonding motifs, and to establish the potential use of these zwitterions in forming further solid-state supramolecular structures.
Results and discussion
Synthesis and crystallisation of zwitterions
Zwitterions 1 and 2 were synthesised in a straightforward manner: the combination of acetylenedicarboxylic acid (ADC) and the pyridine derivative (nicotinamide/isonicotinamide) in a particular solvent or solvent system, with mild heating and stirring for a few minutes, yields reasonably sized, colourless crystals after 24 hours (Scheme 2, see Experimental section for details). Salt 2a can be formed using the same starting materials and synthetic method as for zwitterion 2, but a different combination of solvents. Unlike the zwitterions, salt 2a can also be formed mechanochemically by grinding the starting materials together with the addition of a few drops of tetrahydrofuran.To investigate the formation of polymorphs or solvates of zwitterions 1 and 2, various solvent mixtures were used during synthesis, and crystallisation temperature and reagent ratios were also varied (see ESI† for full details). Combinations of methanol, ethanol, water, dimethylformamide and dimethyl sulfoxide with each other, as well as with 11 other common laboratory solvents, were investigated. Synthesis of zwitterion 1 always gave the same crystalline product, with no observed polymorphs or solvates, despite the fact that approximately 70 solvent combinations were tried, and crystallisation at both room temperature and in the refrigerator (4 °C) was attempted. The combination of ADC and isonicotinamide, on the other hand, results either in the formation of zwitterion 2, which crystallises as a hydrate, or in the formation of a salt of the two components, 2a, formed via simple charge transfer. In our previous work12 it was observed that such salts are the kinetic product of the reaction between ADC and the pyridyl derivative, which forms more quickly and at lower temperatures than the zwitterion itself. It is therefore simple to exclude the unwanted salt by-product by removing crystals that form initially (i.e. after 24 hours) and then leaving the filtrate to form further crystals. No other polymorphs or solvates of zwitterion 2 were observed. The synthesis of zwitterion 3 is similar to that of zwitterion 1 and 2 and was reported in a previous publication.11
Crystal structures of 1, 2 and 2a
Crystallographic data are summarised in Table 1, and hydrogen bond geometries are given in Table 2.| Structure | 1 | 2 | 2a | 1·melamine | 2·melamine | 3·melamine |
|---|---|---|---|---|---|---|
| Chemical formula | C10H8N2O5 | C10H10N2O6 | C8H7N2O3 | C13H18N8O7 | C13H16N8O6 | C13H14N8O5 |
| Formula weight/g mol−1 | 236.18 | 254.20 | 179.16 | 398.35 | 380.34 | 362.32 |
| Crystal system | Monoclinic | Triclinic | Triclinic | Triclinic | Triclinic | Monoclinic |
| Space group | P21/c |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21/c |
| a/Å | 8.8986(2) | 7.2047(1) | 3.671(3) | 5.7312(5) | 8.6886(3) | 17.184(2) |
| b/Å | 7.9933(2) | 7.5455(1) | 7.594(5) | 12.3326(1) | 8.9638(3) | 7.328(5) |
| c/Å | 14.175(3) | 10.8058(2) | 13.772(1) | 12.8974(1) | 11.2137(4) | 13.045(9) |
| α/° | 90.00 | 91.800(2) | 95.732(8) | 71.530(2) | 94.473(2) | 90.00 |
| β/° | 97.628(3) | 100.509(2) | 93.218(8) | 86.574(3) | 103.931(2) | 111.257(1) |
| γ/° | 90.00 | 115.757(2) | 97.153(8) | 89.673(3) | 106.6570(1) | 90.00 |
| Calculated density/g cm−3 | 1.570 | 1.636 | 1.574 | 1.533 | 1.575 | 1.572 |
| Volume/Å3 | 999.3(3) | 516.12(1) | 378.1(5) | 863.01(1) | 801.88(5) | 1531(2) |
| Z | 4 | 2 | 2 | 2 | 2 | 4 |
| Temperature/K | 100(2) | 100(2) | 100(2) | 100(2) | 100(2) | 100(2) |
| Independent reflections | 2350 | 2301 | 1758 | 4460 | 4114 | 3395 |
| R int | 0.0546 | 0.0113 | 0.0518 | 0.0383 | 0.0271 | 0.0854 |
| R 1 [I > 2σ(I)] | 0.0445 | 0.0317 | 0.0424 | 0.0719 | 0.0452 | 0.0560 |
| Structure | D–H⋯A | D–H/Å | H⋯A/Å | D⋯A/Å | D–H⋯A/° | Symmetry code |
|---|---|---|---|---|---|---|
| 1 | O2–H2⋯O8 | 1.04 (3) | 1.44 (3) | 2.476 (2) | 179 (3) | x, y + 1, z |
| N17–H17B⋯O7 | 0.88 | 2.08 | 2.950 (2) | 169.4 | x − 1, y, z | |
| 2 | O1–H1⋯O7 | 0.93 (2) | 1.63 (2) | 2.559 (1) | 175 (2) | x, y + 1, z |
| N17–H17A⋯O18 | 0.88 | 2.11 | 2.959 (1) | 162.0 | −x + 2, −y + 2, −z + 1 | |
| O18–H18A⋯O16 | 0.88 (2) | 1.93 (2) | 2.810 (1) | 171 (2) | x, y − 1, z | |
| O18–H18B⋯O8 | 0.88 (2) | 2.02 (2) | 2.879 (1) | 165 (2) | ||
| 2a | N1–H1⋯O11 | 1.06 (2) | 1.51 (2) | 2.563 (2) | 174 (2) | −x + 2, −y, −z + 1 |
| N9–H9A⋯O8 | 0.88 | 2.04 | 2.916 (2) | 177.7 | −x + 2, −y, −z + 1 | |
| N9–H9B⋯O10 | 0.88 | 1.98 | 2.817 (2) | 159.1 | x + 1, y − 1, z |
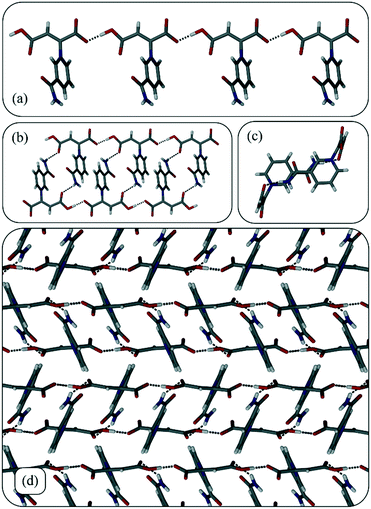
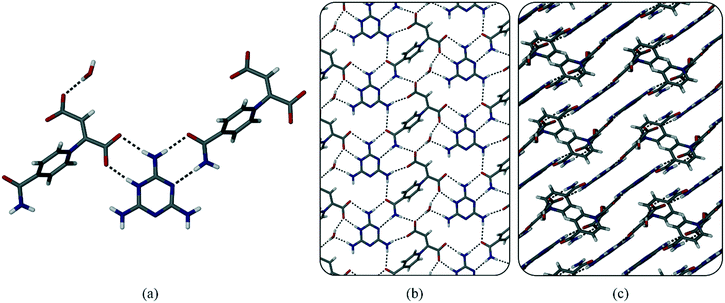
Zwitterion 1 crystallises in the monoclinic space group P21/c and has one zwitterion in the asymmetric unit. The zwitterions form chains via O–H···O hydrogen bonds between the carboxylate/carboxylic acid backbones (Fig. 1a). Two such chains then interdigitate to form ladders via hydrogen bonds between an amide hydrogen atom on one chain and one of the oxygen atoms of the carboxylic acid moiety in the second chain (Fig. 1b & c). Ladders pack next to each other to form a layered structure (Fig. 1d).
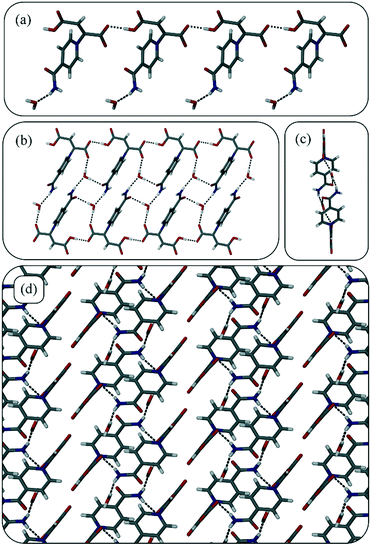
Zwitterion 2 crystallises in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The asymmetric unit contains one zwitterion and one molecule of water that is hydrogen bonded through its oxygen atom to the amide functional group of the zwitterion. Once again the zwitterions form chains via hydrogen bonds between the carboxylic acid backbones (Fig. 2a), and chains are linked to form ladders via hydrogen bonds between the amide groups, the included water molecules, and the second oxygen atom of the carboxylate moieties. Ladders are hydrogen bonded to one another in a slipped stack via an amide–carboxylate oxygen interaction to give the three-dimensional structure (Fig. 2d).
. The asymmetric unit contains one zwitterion and one molecule of water that is hydrogen bonded through its oxygen atom to the amide functional group of the zwitterion. Once again the zwitterions form chains via hydrogen bonds between the carboxylic acid backbones (Fig. 2a), and chains are linked to form ladders via hydrogen bonds between the amide groups, the included water molecules, and the second oxygen atom of the carboxylate moieties. Ladders are hydrogen bonded to one another in a slipped stack via an amide–carboxylate oxygen interaction to give the three-dimensional structure (Fig. 2d).
Salt 2a also crystallises in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . There is one 4-carbamoylpyridinium ion and half an ADC ion in the asymmetric unit, with an N+–H···−O charge-assisted hydrogen bond between the positively charged pyridinium nitrogen atom and the negatively charged carboxylate group (Fig. 3a). Each ADC dianion is thus hydrogen bonded to two 4-carbamoylpyridinium cations. These units are further hydrogen bonded via the familiar 8-atom amide-amide dimeric unit (Fig. 3b). Hydrogen-bonded chains are joined into layers through a hydrogen bond between the second amine hydrogen atom and the carboxylate oxygen atom on another chain. These hydrogen-bonded layers stack on top of one another, with no close intermolecular contacts between layers.
. There is one 4-carbamoylpyridinium ion and half an ADC ion in the asymmetric unit, with an N+–H···−O charge-assisted hydrogen bond between the positively charged pyridinium nitrogen atom and the negatively charged carboxylate group (Fig. 3a). Each ADC dianion is thus hydrogen bonded to two 4-carbamoylpyridinium cations. These units are further hydrogen bonded via the familiar 8-atom amide-amide dimeric unit (Fig. 3b). Hydrogen-bonded chains are joined into layers through a hydrogen bond between the second amine hydrogen atom and the carboxylate oxygen atom on another chain. These hydrogen-bonded layers stack on top of one another, with no close intermolecular contacts between layers.
Synthesis of multi-component crystals
Many co-crystallisations with a variety of co-formers were attempted with zwitterions 1, 2 and 3 (for details see the ESI†). The co-formers were chosen based on known supramolecular synthons that they could potentially form with amide and carboxylic acid groups. Unfortunately, the insoluble nature of the zwitterions (to our knowledge they only dissolve in water at 100 °C) made these crystallisations extremely difficult practically, especially since most organic co-formers are very soluble. This discord is also one of the reasons why most of our co-crystallisation attempts resulted in crystallisation of the two molecular components separately. Nevertheless, using liquid-assisted grinding as a quick screening tool, melamine was identified as a potentially successful co-former. The salts 1·melamine, 2·melamine, and 3·melamine can easily be formed by grinding a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of the zwitterion and melamine together with a few drops of solvent. Crystals were obtained by recrystallisation of the mechanochemically-produced powder, or by dissolution of a 1
1 molar ratio of the zwitterion and melamine together with a few drops of solvent. Crystals were obtained by recrystallisation of the mechanochemically-produced powder, or by dissolution of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of the starting materials in water. In all three salts, proton transfer occurs from the carboxylic acid group of the zwitterion to one of the nitrogen atoms in the melamine ring. It is important to note that these salts dissolve more easily than the zwitterions on their own.
1 molar ratio of the starting materials in water. In all three salts, proton transfer occurs from the carboxylic acid group of the zwitterion to one of the nitrogen atoms in the melamine ring. It is important to note that these salts dissolve more easily than the zwitterions on their own.
Crystal structures of the melaminium salts
Crystallographic data for the salts are summarised in Table 1, and hydrogen bond geometries are given in Table 3. In all three salts, the carboxylic acid on the zwitterion has lost a proton to give the respective anions, 1−, 2− and 3−.| Structure | D–H⋯A | D–H/Å | H⋯A/Å | D⋯A/Å | D–H⋯A/° | Symmetry code |
|---|---|---|---|---|---|---|
| 1·melamine | N27–H27B⋯O30 | 0.88 | 1.94 | 2.767 (3) | 156.1 | x − 1, y, z |
| N28–H28B⋯O29 | 0.88 | 1.97 | 2.765 (3) | 150.5 | x − 1, y + 1, z | |
| N20–H1⋯O2 | 0.84 (3) | 1.93 (3) | 2.733 (3) | 158 (3) | ||
| 2·melamine | N18–H18⋯O8 | 0.88 (2) | 1.90 (2) | 2.7692 (2) | 172.9 (2) | x, y − 1, z − 1 |
| O27–H27B⋯O8 | 0.88 (3) | 1.92 (3) | 2.7464 (2) | 156 (2) | −x + 1, −y + 2, −z + 1 | |
| O27–H27A⋯O2 | 0.89 (3) | 1.90 (3) | 2.7305 (2) | 154 (3) | x, y − 1, z − 1 | |
| 3·melamine | N17–H17⋯O7 | 0.88 (3) | 1.93 (3) | 2.814 (4) | 173 (3) | −x + 2, y − 1/2, −z + 3/2 |
| O26–H26A⋯O1 | 1.07 (4) | 1.68 (4) | 2.736 (4) | 171 (6) | ||
| N23–H23A⋯O2 | 0.88 (3) | 1.85 (4) | 2.731 (4) | 175 (3) | ||
| N25–H25A⋯O8 | 0.91 (4) | 1.86 (4) | 2.774 (4) | 176 (4) | −x + 2, y − 1/2, −z + 3/2 |
The salt 1·melamine, contains the anion 1−, the melaminium cation and water in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mole ratio, and crystallises in the triclinic space group P
2 mole ratio, and crystallises in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The backbone of the anion is disordered over two positions in an approximately 50
. The backbone of the anion is disordered over two positions in an approximately 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
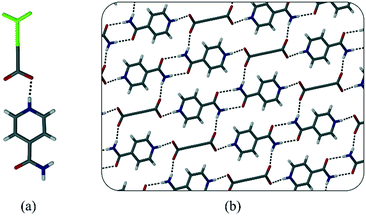
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio, such that the oxygen atoms are on the same positions for both conformations (i.e. the backbone is flipped by 180°). The structure has a multitude of hydrogen bonds (Fig. 4) due to the many hydrogen-bond donor and acceptor atoms present in melaminium. Most notable is the hydrogen bond between the amide group of 1−, and melaminium – an interaction that seems to play an important role in the formation of these salts (Fig. 4a).
50 ratio, such that the oxygen atoms are on the same positions for both conformations (i.e. the backbone is flipped by 180°). The structure has a multitude of hydrogen bonds (Fig. 4) due to the many hydrogen-bond donor and acceptor atoms present in melaminium. Most notable is the hydrogen bond between the amide group of 1−, and melaminium – an interaction that seems to play an important role in the formation of these salts (Fig. 4a).
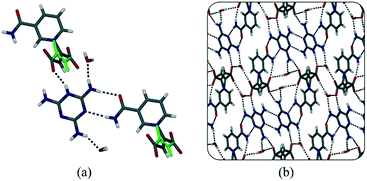 | ||
| Fig. 4 (a) Hydrogen bonding in 1·melamine. The disorder in the zwitterion backbones is shown in green. (b) Packing diagram of 1·melamine viewed down the a-axis. | ||
There is also a charge-assisted hydrogen bond (CAHB) between the zwitterion carboxylate group and the melaminium ion (Fig. 4a). The significance of this interaction has been noted previously.16 Water molecules occur in pairs and are located in pockets, where they are hydrogen bonded to both the anion and the cation. There is no discrete mass loss in the TGA that corresponds to the 9.07% water present (see ESI†), indicating that the water molecules start to slowly be removed from the structure during heating, but are only completely lost during decomposition.
The salt 2·melamine contains the anion 2−, melaminium, and water in a mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This salt also crystallises in the triclinic space group, P
1. This salt also crystallises in the triclinic space group, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . There are numerous hydrogen bonds present (Fig. 5a and b) – note the familiar R22(8) hydrogen-bonded unit between the amide group and the melaminium ion, as well as the charge-assisted hydrogen bond that was also observed in 1·melamine. The geometry of the carboxylate–pyridinium hydrogen bond is quite different here: due to the R22(8) motif formed with melaminium (Fig. 5a), the melaminium and the anion backbone are in the same plane. This results in hydrogen-bonded sheets that are not directly bonded to one another (Fig. 5c). This is in contrast to the three-dimensional hydrogen bonding network seen in 1·melamine. In 2·melamine the water molecules are once again involved in extensive hydrogen bonding, and it seems unlikely that they could be removed without the crystals decomposing. However, a 5.1% mass loss is observed in the TGA (see ESI†), which corresponds well with the 4.8% mass loss expected for one water molecule. There is also a broad endotherm corresponding to this mass loss in the DSC. However, the mass loss is directly followed by decomposition of the crystals, and dehydrated crystals could not be isolated.
. There are numerous hydrogen bonds present (Fig. 5a and b) – note the familiar R22(8) hydrogen-bonded unit between the amide group and the melaminium ion, as well as the charge-assisted hydrogen bond that was also observed in 1·melamine. The geometry of the carboxylate–pyridinium hydrogen bond is quite different here: due to the R22(8) motif formed with melaminium (Fig. 5a), the melaminium and the anion backbone are in the same plane. This results in hydrogen-bonded sheets that are not directly bonded to one another (Fig. 5c). This is in contrast to the three-dimensional hydrogen bonding network seen in 1·melamine. In 2·melamine the water molecules are once again involved in extensive hydrogen bonding, and it seems unlikely that they could be removed without the crystals decomposing. However, a 5.1% mass loss is observed in the TGA (see ESI†), which corresponds well with the 4.8% mass loss expected for one water molecule. There is also a broad endotherm corresponding to this mass loss in the DSC. However, the mass loss is directly followed by decomposition of the crystals, and dehydrated crystals could not be isolated.
The salt 3·melamine contains the 3− anion, melaminium, and water in a mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 6a). This salt crystallises in the monoclinic space group P21/c. As with the other two salts, there are numerous hydrogen bonds present, the most notable synthon being that between the carboxylate group of the anion backbone and the melaminium ion. As with 2·melamine, this interaction consists of an R22(8) motif of CAHBs between the carboxylate group and melaminium (Fig. 6a). The packing diagram for this salt (Fig. 6b) shows an extensive network of hydrogen bonds throughout the structure.
1 (Fig. 6a). This salt crystallises in the monoclinic space group P21/c. As with the other two salts, there are numerous hydrogen bonds present, the most notable synthon being that between the carboxylate group of the anion backbone and the melaminium ion. As with 2·melamine, this interaction consists of an R22(8) motif of CAHBs between the carboxylate group and melaminium (Fig. 6a). The packing diagram for this salt (Fig. 6b) shows an extensive network of hydrogen bonds throughout the structure.
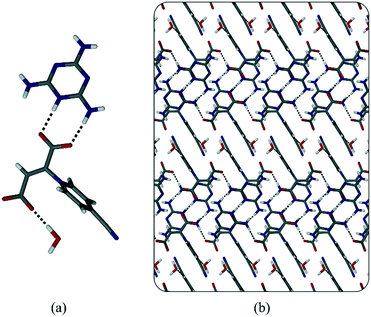 | ||
| Fig. 6 (a) Asymmetric unit of 3·melamine showing the hydrogen bonds present. (b) Packing diagram of 3·melamine showing the layered structure (viewed down the b-axis). | ||
Mechanochemistry
The use of mechanochemical methods (specifically grinding in a mortar and pestle) to prepare bulk samples of either zwitterions 1 and 2, or the melaminium salts, was investigated. This was attempted using both neat- and liquid-assisted grinding (LAG). For the latter, drops of methanol, ethanol, water and tetrahydrofuran were tested. Both of these methods were unsuccessful with regard to zwitterion formation, perhaps because more energy is needed to form the covalent bonds in the zwitterion, which is the thermodynamic product of the reaction between ADC and pyridine derivatives.12 As mentioned above, grinding of the components used to synthesise zwitterion 2 resulted in formation of the kinetic product, salt 2a. Mechanochemical methods were however very successful in forming the melaminium salts of 1–3 – both neat and liquid-assisted grinding (using a few drops of methanol or water) were successful, however LAG resulted in a faster conversion to the salt than neat grinding.Further mechanochemical experiments, carried out with molecules having similar hydrogen bonding potential to melamine (2-aminopyridine, 2-aminopyrimidine and 2,6-diaminopyridine), were also attempted. Combination of zwitterions 1–3 with 2-aminopyrimidine left the starting materials unchanged (as assessed by PXRD). However, attempted co-crystallisation of zwitterions 1, 2, and 3 with 2-aminopyridine (AP) and 2,6-diaminopyridine (DAP), using LAG with a few drops of methanol, seemed to be successful as powder patterns were obtained that did not match either of the starting materials in each case. It is therefore possible that salts 1·AP, 2·AP, 3·AP, 1·DAP, 2·DAP and 3·DAP were formed. 1H NMR analysis of the powders obtained from LAG clearly shows the presence of both starting materials, supporting the hypothesis of salt formation. FTIR analysis of the powders shows a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequency at 1550–1635 cm−1, indicating the presence of a carboxylate group instead of a carboxylic acid. This confirms that the powders contain a salt of the two starting materials in each case. As of yet, recrystallisation of these six salts has been unsuccessful, as has direct crystallisation from solution.
O stretching frequency at 1550–1635 cm−1, indicating the presence of a carboxylate group instead of a carboxylic acid. This confirms that the powders contain a salt of the two starting materials in each case. As of yet, recrystallisation of these six salts has been unsuccessful, as has direct crystallisation from solution.
Cambridge structural database analysis
For two different molecules in a solution to recognise each other and form heteromeric molecular solids, the interactions between the different molecules must be more favourable than interactions between the molecules themselves.10 Therefore, in order to successfully form multi-component crystals, there needs to be an understanding of the hierarchy of intermolecular interactions. In purely organic molecules, hydrogen bonds are usually the strongest interactions present, and so they are usually all that needs to be taken into account.17 For example, nitrogen-containing heterocycles and carboxylic acids form very strong hydrogen bonds to each other; this interaction is frequently observed in the Cambridge Structural Database (CSD),10 and can therefore confidently be used in the design of co-crystals. From the CSD it is also clear that the carboxylic acid–amide interaction is more common than the amide–amide and carboxylic acid–carboxylic acid interactions.18 Combining carboxylic acids and amides is thus also a viable strategy for multi-component crystal formation.Furthermore, the possibility of proton transfer between molecules also needs to be taken into account. Unexpected proton transfer may result in unexpected intermolecular interactions, as CAHBs are likely to form preferentially, as they are stronger than other hydrogen bonds. Here pKa values can be very useful for predicting whether such a proton transfer will occur, i.e. if a salt or co-crystal will form.19 Unfortunately, the pKa values for zwitterions 1–3 are not known, and could also not be measured as they are very difficult to dissolve. The formation of salts with zwitterions 1–3 was unexpected: the original aim of this study was in fact to form co-crystals via the pyridinium moiety substituents.
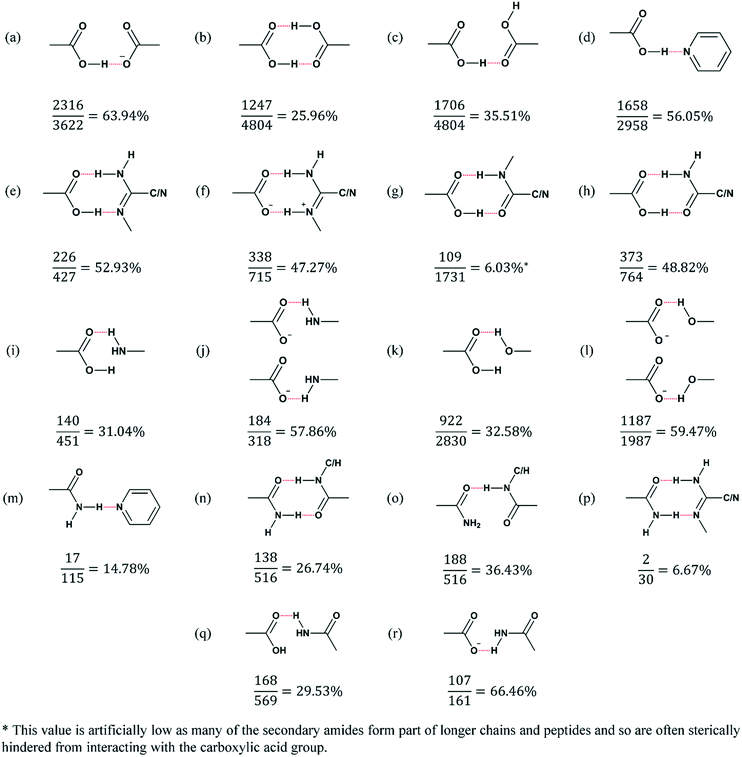
One of the factors that may be significant in determining whether this class of zwitterions forms multi-component crystals at all is the likelihood that the zwitterions will hydrogen bond to one another, instead of interacting with the available co-formers. Based on the many co-crystallisation attempts that led to recrystallization of the pure zwitterions, we can assume that this is in fact happening. However, a search of the CSD,20–23 using ConQuest,23 confirms that this is likely to be an explanation for the observed low occurrence of multi-component crystals in this system (Fig. 7).
The principal intermolecular interaction between the zwitterions is a carboxylic acid–carboxylate charge-assisted hydrogen bond. Our objective was to test whether this interaction is more abundant in the CSD, and thus presumably more favourable, than the interaction a zwitterion would have with a certain co-former. The first step was to search the CSD for all structures containing both a carboxylic acid and a carboxylate group (whether on separate molecules or on the same molecule). Only organic molecules, for which the 3D coordinates of all atoms have been determined, were taken into account. A subsequent search within this subset allowed for the calculation of the number of times that an intermolecular CAHB forms between these two groups. This was followed by a search for other common interactions that the zwitterions could have with the co-formers that were used (Fig. 7).
When both carboxylic acid and carboxylate functional groups are present in a crystal (as with the zwitterions, and 3622 other structures in the CSD), they form a CAHB 63.94% of the time. However, visual inspection of the structures not containing this interaction made it clear that in many cases the interaction could not form because both functional groups were located on the same molecule, and were interacting with each other intramolecularly. When these structures are removed from the search, the propensity for the CAHB rises to 76.79%. When this result is compared to the propensity for other interactions to form (Fig. 7), it is clear that the type of interaction formed between these zwitterions is more robust than all of the other interactions investigated, which would explain why it is dominant in these structures. Another demonstration of the strength of the homomeric zwitterion–zwitterion interaction is the length of the hydrogen bonds between the zwitterions, compared to those observed in the melaminium salts. The carboxylate–carboxylic acid CAHB formed between the zwitterions is approximately 2.5 Å in all cases (Table 2), while the hydrogen bonds in the salts are longer and range from 2.73 to 2.81 Å (Table 3). This does not, however, explain why the zwitterions did not form co-crystals by hydrogen bonding through the amide group, whilst still maintaining the strong charge-assisted hydrogen bonds between zwitterion backbones in the structure. We have observed this lack of co-crystal formation despite the presence of strong hydrogen bonding groups in other systems studied by our group.24
It is interesting to note that the synthon observed in the melaminium salts reported here is only 47.27% abundant (Fig. 7f). This indicates that a CSD search cannot confidently be used to rule out the possibility of multi-component crystals forming, but rather as a tool to try and understand experimental results. Of course, the values reported here are not accurate predictors of which interactions will form – the CSD is inherently biased, as not all materials diffract well enough to give diffraction data that results in a crystal structure. There could also be other factors inhibiting the interactions that were searched for, such as steric constraints. Furthermore, even though strong intermolecular interactions, such as the hydrogen bond, play an important role in determining crystal packing, it is not the only determining factor, many weaker interactions such as π⋯π interactions and van der Waals interactions could also have a significant effect. Nonetheless, the information does give a good indication of the robustness of various hydrogen bonding motifs, and therefore of the likelihood of molecules to interact.
Conclusion
Two new zwitterions, as well as a related salt, have successfully been synthesised and structurally characterised. These two zwitterions, as well as a related cyano-functionalised zwitterion, were investigated for their ability to form multi-component crystals. Three new hydrated salts with melamine were obtained. The salts can be obtained from solution and via mechanochemistry – the latter proving to be a very fast and convenient technique for salt-formation screening purposes. The three salts have been fully characterised and described, with emphasis on the intermolecular interactions that contribute to their formation. All three salts display charge-assisted hydrogen bonds between the carboxylate group of the anion and the positively charged nitrogen atom in the melaminium cation. The extra amide functionality in 1 and 2 also plays an important role, as this group is utilised in hydrogen bonds to melaminium.This work highlights the importance of mechanochemistry as a screening tool. LAG is fast and simple and was used in conjunction with PXRD, NMR and FTIR to identify six other salts for which crystal structures could not be obtained.
When salt and co-crystal formation was attempted with zwitterions 1–3, a variety of co-formers were used, yet only three salts were crystallised per zwitterion. Could it be that the zwitterions interact so strongly with each other, that this inhibits the incorporation of other co-formers? A search of the Cambridge Structural Database was carried out to compare the likelihood of the formation of various hydrogen bonding motifs. The carboxylic acid–carboxylate CAHB, which is observed between the zwitterions, occurs in 63.94% of structures that contain both of these functional groups. Compared to this, other interactions that the zwitterions could form with the co-formers used were found to be less likely. Experimentally we observe that the zwitterion–zwitterion CAHB is not only abundant, but very strong. Hydrogen bond lengths in the zwitterions are around 2.5 Å, while the hydrogen bond lengths observed in the salts range from 2.73 to 2.81 Å. However, this does not explain why co-formers were not included between hydrogen-bonded chains of zwitterions.
Whilst an analysis of the occurrence of intermolecular interactions in known materials should be carefully considered when predicting the outcome of new supramolecular syntheses, the results presented here make it clear that attempts to form multi-component crystals should not be abandoned on the basis of this information.
Experimental
All PXRD, TGA, DSC, NMR, and FTIR data are available as ESI.†Synthesis and crystallisation
Zwitterion 1, (Z)-2-(3-carbamoylpyridin-1-ium-1-yl)-3-carboxy-acrylate, was made by combining acetylenedicarboxylic acid (ADC, 0.020 g, 0.175 mmol) with nicotinamide (0.021 g, 0.172 mmol) in a mixture of acetonitrile (2 ml) and water (0.5 ml). The mixture was stirred at 100 °C until the components had completely dissolved. The vial was capped and left on a shelf to crystallise at room temperature. Small, colourless plate-like crystals formed after 24 hours. If the solution was stirred for any longer, the zwitterion started to precipitate out as a fine white powder.Zwitterion 2, (Z)-2-(4-carbamoylpyridin-1-ium-1-yl)-3-carboxyacrylate, was made by combining acetylenedicarboxylic acid (ADC, 0.020 g, 0.175 mmol) with isonicotinamide (0.021 g, 0.172 mmol) in a mixture of water (3 ml) and THF (3 ml). The mixture was stirred for 5 minutes at 100 °C. The vial was capped and left on a shelf to crystallise at room temperature. Colourless plate-like crystals formed within 3 days.
The salt 2a was obtained by stirring a mixture of acetylenedicarboxylic acid (ADC, 0.020 g, 0.175 mmol) and isonicotinamide (0.021 g, 0.172 mmol) at 100 °C for 5 minutes in a mixture of 0.5 ml diethyl ether and 6 ml water. Colourless, needle-like crystals formed within a day. The salt could also be synthesised mechanochemically by grinding the reagents together for 10 minutes in a mortar and pestle with a few drops of THF.
1·melamine was made by combining zwitterion 1 (0.010 g, 0.042 mmol) with melamine (0.005 g, 0.040 mmol) in 3 ml water and stirring them together at 100 °C until the components had completely dissolved. The vial was then capped and left on a shelf to crystallise at room temperature. Small, colourless plate-like crystals formed after 2 weeks. A powder of this salt could also be obtained by grinding the two components together for 10 minutes in a mortar and pestle (neat or with a few drops of water).
2·melamine was made by combining zwitterion 2 (0.010 g, 0.042 mmol) with melamine (0.005 g, 0.040 mmol) and grinding them together for 10 minutes in a few drops of MeOH. The powder was then recrystallised from D2O and a drop of pyridine. Colourless plate-like crystals were obtained within 2 days.
3·melamine was made by combining zwitterion 3 (0.010 g, 0.042 mmol) with melamine (0.006 g, 0.048 mmol) and stirring these together for 30 minutes in 7 ml water at 100 °C. The vial was capped and left to crystallise on a shelf at room temperature. Colourless plate-like crystals formed after 2 months. This salt can also be obtained by grinding the two components together for 10 minutes in a mortar and pestle with a few drops of MeOH.
Methods of characterisation
All products obtained were crystalline. Powder X-ray diffraction (PXRD) was used to confirm purity of the bulk material. Where single crystal data were not obtainable, nuclear magnetic resonance (NMR) spectroscopy was used for identification. The thermal properties of all materials were investigated using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). FTIR was used to confirm that the material was a salt, rather than a co-crystal. Details are given in the ESI.†Single-crystal X-ray Diffraction (SCXRD) for all compounds, except 3·melamine, was performed using a Bruker SMART Apex II diffractometer. The diffractometer makes use of MoKα radiation of wavelength 0.71073 Å produced using a microfocus sealed tube and a 0.5 mm MonoCap collimator. Frames were detected by a Bruker APEXII CCD area-detector. An Oxford Cryosystems cryostat (Cryostream Plus 700 Controller) was used to keep the single crystals at 100 K throughout all data collections.
Single-crystal X-ray Diffraction (SCXRD) of 3·melamine was performed using a Bruker DUO Apex II CCD area-detector diffractometer. This dual source diffractometer was operated using MoKα radiation of wavelength 0.71073 Å produced by an Incoatec IμS microsource coupled with a multilayer mirror optics monochromator. An Oxford Cryosystems Cryostat (700 Series Cryostream Plus) was used to keep the single crystal at 100 K throughout the data collection.
Data were collected, and reduced using the Bruker software package SAINT25 in the Apex3 software. SADABS26,27 was used for the absorption correction and correcting for other systematic errors. Finally, the structures were solved (SHELXS-13)28 using direct methods and refined (SHELXL-16)29 through the graphical user interface XSeed.30,31 Hydrogen atoms on sp2-hybridised carbon atoms were placed in calculated positions using riding models, while O–H and pyridinium N–H hydrogen atoms were placed on maxima in the electron density difference maps (with the exception of 3·melamine where all N–H hydrogen atoms were placed on maxima in the electron density). All images were created using POV-ray.32
CSD search
To carry out a search of the CSD (version 5.38, May 2017),20–23 the ConQuest software, version 1.19 (Build RC1, 2016), was used. Filters were applied in order to search for only organic molecules for which the 3D coordinates of all atoms have been determined.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the Wilhelm Frank Scholarship Fund, the National Research Foundation of South Africa and Stellenbosch University is gratefully acknowledged. NMR was carried out on instruments managed by the Central Analytical Facility at Stellenbosch University.Notes and references
- D. J. Berry and J. W. Steed, Adv. Drug Delivery Rev., 2017, 117, 3–24 CrossRef CAS PubMed.
- G. Kuminek, F. Cao, A. Bahia de Oliveira da Rocha, S. Gonçalves Cardoso and N. Rodríguez-Hornedo, Adv. Drug Delivery Rev., 2016, 101, 143–166 CrossRef CAS PubMed.
- M. F. Arafa, S. A. El-Gizawy, M. A. Osman and G. M. El Maghraby, J. Drug Delivery Sci. Technol., 2017, 38, 9–17 CrossRef CAS.
- F. Grifasi, M. R. Chierotti, K. Gaglioti, R. Gobetto, L. Maini, D. Braga, E. Dichiarante and M. Curzi, Cryst. Growth Des., 2015, 15, 1939–1948 CAS.
- N. K. Duggirala, M. L. Perry, Ö. Almarsson and M. J. Zaworotko, Chem. Commun., 2016, 52, 640–655 RSC.
- S. Hiendrawan, B. Veriansyah, E. Widjojokusumo, S. N. Soewandhi, S. Wikarsa and R. R. Tjandrawinata, Int. J. Pharm., 2016, 497, 106–113 CrossRef CAS PubMed.
- E. Grothe, H. Meekes, E. Vlieg, J. H. ter Horst and R. de Gelder, Cryst. Growth Des., 2016, 16, 3237–3243 CAS.
- G. R. Desiraju, CrystEngComm, 2003, 5, 466–467 RSC.
- J. D. Dunitz, CrystEngComm, 2003, 5, 506 RSC.
- C. B. Aakeröy and D. J. Salmon, CrystEngComm, 2005, 72, 439–448 RSC.
- L. Loots, D. A. Haynes and T. le Roex, New J. Chem., 2014, 38, 2778–2786 RSC.
- L. Loots, J. P. O'Connor, T. le Roex and D. A. Haynes, Cryst. Growth Des., 2015, 15, 5849–5857 CAS.
- J. Lombard, L. Loots, D. A. Haynes and T. le Roex, Manuscript in preparation, 2017 Search PubMed.
- J. Lombard, D. A. Haynes and T. le Roex, Cryst. Growth Des., 2017, 17, 6625–6633 CAS.
- F. H. Allen, W. D. S. Motherwell, P. R. Raithby, G. P. Shields and R. Taylor, New J. Chem., 1999, 23, 25–34 RSC.
- Y. Pang, P. Xing, X. Geng, Y. Zhu, F. Liu and L. Wang, RSC Adv., 2015, 5, 40912–40923 RSC.
- G. R. Desiraju, Acc. Chem. Res., 2002, 35, 565–573 CrossRef CAS PubMed.
- L. Leiserowitz and F. Nader, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1977, 33, 2719–2733 CrossRef.
- A. J. Cruz-Cabeza, CrystEngComm, 2012, 14, 6362–6365 RSC.
- C. F. Macrae, P. R. Edgington, P. McCabe, E. Pidcock, G. P. Shields, R. Taylor, M. Towler and J. van de Streek, J. Appl. Crystallogr., 2006, 39, 453–457 CrossRef CAS.
- C. F. Macrae, I. J. Bruno, J. A. Chisholm, P. R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. van de Streek and P. A. Wood, J. Appl. Crystallogr., 2008, 41, 466–470 CrossRef CAS.
- R. Taylor and C. F. Macrae, Acta Crystallogr., Sect. B: Struct. Sci., 2001, 57, 815–827 CAS.
- I. J. Bruno, J. C. Cole, P. R. Edgington, M. Kessler, C. F. Macrae, P. McCabe, J. Pearson and R. Taylor, Acta Crystallogr. Sect. B Struct. Sci., 2002, 58, 389–397 CrossRef.
- H. Wahl, D. A. Haynes and T. le Roex, S. Afr. J. Chem., 2016, 69, 35–43 CrossRef CAS.
- SAINT Data Collection Software, version V7.99A, Bruker AXS Inc., Madison, WI, 2012 Search PubMed.
- SADABS, version 2012/1, Bruker AXS Inc., Madison, WI, 2012 Search PubMed.
- R. H. Blessing, Acta Crystallogr., Sect. A: Found. Crystallogr., 1995, 51, 33–38 CrossRef.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 CrossRef PubMed.
- L. J. Barbour, J. Supramol. Chem., 2001, 1, 189–191 CrossRef CAS.
- J. L. Atwood and L. J. Barbour, Cryst. Growth Des., 2003, 3, 3–8 CAS.
- POV-RayTM for Windows, version 3.6, Persistence of Vision Raytracer Pty. Ltd., Williamstown, Australia, 2004 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details on synthetic procedures; details of co-formers used; instrumental characterisation and analysis for all compounds (PXRD, TGA, DSC); NMR and FTIR spectra for the materials suspected to be salts. CCDC 1584660–1584665. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ce01953j |
| This journal is © The Royal Society of Chemistry 2018 |