Alkaline electrolyte: toward high-quality CdTe films with the assistance of strong complexing agent and organic base†
Yuxuan
Zhang
 ,
Weibing
Wu
,
Weibing
Wu
 *,
Yueyi
Liu
,
Weijie
Yang
,
Wenwen
Chen
and
Jizuo
Zhao
*,
Yueyi
Liu
,
Weijie
Yang
,
Wenwen
Chen
and
Jizuo
Zhao
School of Materials Science and Engineering, University of Jinan, Jinan, 250022, P.R. China. E-mail: mse_wuwb@ujn.edu.cn
First published on 23rd November 2017
Abstract
CdTe films were electrochemically deposited from an alkaline solution with the assistance of nitrilotriacetic acid (NTA) and tetramethylammonium hydroxide (TMAH). CdTe film prepared at the complexing ratio of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was well-crystallized and highly (111)-oriented; after annealing, it was free of voids with good interface contact. The deposition is demonstrated to occur via UPD mechanism. TMAH prevented the introduction of alkaline metal ions into CdTe films. This provides a novel approach for depositing CdTe films at the lower temperature.
1 was well-crystallized and highly (111)-oriented; after annealing, it was free of voids with good interface contact. The deposition is demonstrated to occur via UPD mechanism. TMAH prevented the introduction of alkaline metal ions into CdTe films. This provides a novel approach for depositing CdTe films at the lower temperature.
Cadmium telluride is a competitive material for thin film solar cells due to its ideal direct band gap energy (1.45 eV). High-efficiency CdTe solar cells have been typically prepared by high-temperature processes.1 Moreover, the low-temperature techniques including sputtering,2 physical vapor deposition (PVD),3 low-temperature vapor transport deposition (VTD),4 and electrochemical deposition,5–7 are potentially highly efficient through the post-treatment;6,8 they are also significant for low-cost fabrication particularly on a large-area substrate. As a matured industrial technique, the electrochemical method has long been used in CdTe films, but is restricted in the commonly used acidic solution due to the extremely low TeO2 solubility and the possible corrosion of the window layer (CdS
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O) and the high-resistance layer (i-ZnO).5,9 Alkaline solution, due to the large TeO2 solubility and process compatibility, is thus expected to be the appropriate choice.5,10–12
O) and the high-resistance layer (i-ZnO).5,9 Alkaline solution, due to the large TeO2 solubility and process compatibility, is thus expected to be the appropriate choice.5,10–12
According to the electrochemical kinetics, a high-quality CdTe film follows the underpotential deposition (UPD) mechanism, which involves the reduction of elemental Te0 and then the transformation into CdTe at the underpotential for Cd0.13 Since the reduction of TeO32− in alkaline solutions requires more negative potential than HTeO2+ and even Cd(II) in acidic solution,13 a complexing agent is required to negatively shift the reduction potential of Cd(II). However, NH3 in ammoniacal alkaline electrolyte was too weak to stabilize Cd(II) to realize the UPD deposition, usually leading to Cd0 along with CdTe.5,14 Moreover, the higher temperature required for the UPD deposition to provide the necessary activation energy caused rapid evaporation of NH3.15 Recently, NTA has been used as the strong complexing agent, but films of high structure quality were hard to obtain at the lower NTA/Cd complexing ratio.2,6,16 Worse, alkali metal ions would be incorporated into CdTe film while tuning the pH with inorganic base, which is detrimental to the device performance.1,17,18
Herein, a novel alkaline electrolyte was developed for high-quality CdTe film. A NTA/Cd2+ complexing ratio of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, inconceivable previously,7,16 was used to realize the UPD deposition. TMAH, an etching agent for Si cells,19 was used to replace inorganic base to avoid incorporating alkaline ions into CdTe films. From this alkaline electrolyte, the obtained CdTe films were well-crystallized and highly (111)-oriented and after annealing transformed into a dense structure of large randomly oriented grains with good CdS/CdTe interface. The electrochemical mechanisms were also obtained.
1, inconceivable previously,7,16 was used to realize the UPD deposition. TMAH, an etching agent for Si cells,19 was used to replace inorganic base to avoid incorporating alkaline ions into CdTe films. From this alkaline electrolyte, the obtained CdTe films were well-crystallized and highly (111)-oriented and after annealing transformed into a dense structure of large randomly oriented grains with good CdS/CdTe interface. The electrochemical mechanisms were also obtained.
Alkaline electrolyte solutions of 0.1 M CdCl2, 2 mM TeO2 and varied amounts of NTA were prepared to electrochemically deposit CdTe films. The pH was tuned to 9.0 with the organic base TMAH. Prior to the electrochemical deposition, CdS film of about 100 nm was deposited on FTO substrate via chemical bath deposition (CBD) method. Electrodeposition was performed on CdS films prepared with a standard three-electrode system at 80 °C under stirring.20 The counter electrode and reference electrode were Pt plate and Hg/HgO in 4 M KOH, respectively. Prior to the deposition, CdS films were annealed at 415 °C in air for 15 min. The as-prepared CdTe films were annealed at 430 °C in N2.
All electrochemical characteristics of all electrolyte solutions were measured on an electrochemical workstation (CHI1140, CH Instruments). In detail, the CdS/FTO substrates were used as the working electrode, and to ensure accuracy, the CdS/FTO substrates were cut into the narrow shapes and sealed to expose a small area about 3 mm × 3 mm. The linear potential scanning was performed at a rate of 30 mV s−1 in the solutions with the varied NTA/Cd2+ complexing ratios of 0, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 14
1, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 20
1 and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at the temperature identical to that used for deposition (80 °C). The structural, compositional, and optical characteristics were determined on X-ray diffraction spectroscope (XRD, D8-Advance Bruker), energy dispersion spectroscope (EDS), scanning electron microscope (Quanta FEG 250 FEI) and ultraviolet-visible spectrometer (UV-3600, Shimadzu).
1 at the temperature identical to that used for deposition (80 °C). The structural, compositional, and optical characteristics were determined on X-ray diffraction spectroscope (XRD, D8-Advance Bruker), energy dispersion spectroscope (EDS), scanning electron microscope (Quanta FEG 250 FEI) and ultraviolet-visible spectrometer (UV-3600, Shimadzu).
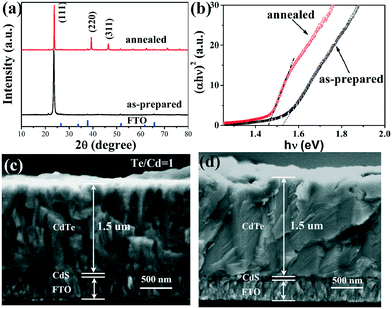
Fig. 1 shows the characteristics of CdTe films prepared at −0.50 V in the solution with NTA/Cd2+ ratio of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The narrow and intensive (111) peak in the XRD pattern of the as-prepared CdTe film indicates the good crystallinity and orientation, which is in good agreement with the columnar grains in SEM image and comparable to that from the acidic solution.21 In the SEM images of the as-prepared and annealed CdTe films, it is observed that the thickness of the CdTe film deposited for 1 h at −0.50 V is about 1.5 μm, and there is a thin CdS layer about 100 nm. The EDS result (Fig. S1, ESI†) indicates that the as-prepared film has the typical stoichiometry of Te/Cd = 1
1. The narrow and intensive (111) peak in the XRD pattern of the as-prepared CdTe film indicates the good crystallinity and orientation, which is in good agreement with the columnar grains in SEM image and comparable to that from the acidic solution.21 In the SEM images of the as-prepared and annealed CdTe films, it is observed that the thickness of the CdTe film deposited for 1 h at −0.50 V is about 1.5 μm, and there is a thin CdS layer about 100 nm. The EDS result (Fig. S1, ESI†) indicates that the as-prepared film has the typical stoichiometry of Te/Cd = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for CdTe film. After annealing, peaks at 16.65° and 23.22° appear in the XRD pattern, which are indexed to (220) and (311) facets of the zinc blende phase CdTe. The film is dense with fewer voids within the film and at the CdS/CdTe interface. The large grains provide boundaries across the entire thickness, which serve as the transport paths for the photon-generated carriers in solar cells.6,12 Accordingly, the band-gap energy transforms from 1.53 eV for the as-prepared CdTe film into the theoretical value (1.45 eV) for the annealed CdTe film (Fig. 1b). To the best of our knowledge, this is the first report on high-quality CdTe films obtained from the alkaline solution.
1 for CdTe film. After annealing, peaks at 16.65° and 23.22° appear in the XRD pattern, which are indexed to (220) and (311) facets of the zinc blende phase CdTe. The film is dense with fewer voids within the film and at the CdS/CdTe interface. The large grains provide boundaries across the entire thickness, which serve as the transport paths for the photon-generated carriers in solar cells.6,12 Accordingly, the band-gap energy transforms from 1.53 eV for the as-prepared CdTe film into the theoretical value (1.45 eV) for the annealed CdTe film (Fig. 1b). To the best of our knowledge, this is the first report on high-quality CdTe films obtained from the alkaline solution.
In combination with the previous results in the ammonical alkaline electrolytes and the NTA solutions of lower complexing ratio,2,5,7,14 the present results suggest that both the complexing type and ratio have deep influence on the film composition and structure. To clarify the effects of NTA, Fig. 2 shows the structural and compositional results of two additional CdTe films at the ratios 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 20
1 and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It should be noted that for the ratio of 3
1. It should be noted that for the ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the deposition must be conducted at the potential more negative than −0.70 V, otherwise the deposition would be quickly terminated due to the excessive Cd in film, which would be discussed later. Distinctly different from the film at the complexing ratio of 14
1, the deposition must be conducted at the potential more negative than −0.70 V, otherwise the deposition would be quickly terminated due to the excessive Cd in film, which would be discussed later. Distinctly different from the film at the complexing ratio of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the two films appear dense (Fig. 2a and d), but are actually poorly crystallized (Fig. 2c and f). After annealing, numerous voids resulted in both films due to the poor crystallinity of the primary CdTe film, while their morphologies are much different in the SEM images (Fig. 2b and e). The film at the complexing ratio of 3
1, the two films appear dense (Fig. 2a and d), but are actually poorly crystallized (Fig. 2c and f). After annealing, numerous voids resulted in both films due to the poor crystallinity of the primary CdTe film, while their morphologies are much different in the SEM images (Fig. 2b and e). The film at the complexing ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 consists of small crystal grains and voids, while the large grains and voids are observed in the film at the ratio of 20
1 consists of small crystal grains and voids, while the large grains and voids are observed in the film at the ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The voids should be attributed to recrystallization and stress relief.2,22 The EDS results of the as-prepared CdTe films reveal the structure dependence on the complexing ratio. Increasing the complexing ratio from 3
1. The voids should be attributed to recrystallization and stress relief.2,22 The EDS results of the as-prepared CdTe films reveal the structure dependence on the complexing ratio. Increasing the complexing ratio from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 14
1 to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 20
1 and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 leads to the rise in Te/Cd atomic ratio in film from 0.94 to 1.0 and 1.01, respectively. This indicates that the film prepared at the low complexing ratio of 3
1 leads to the rise in Te/Cd atomic ratio in film from 0.94 to 1.0 and 1.01, respectively. This indicates that the film prepared at the low complexing ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 has excess of Cd, while that prepared at the much higher complexing ratio of 20
1 has excess of Cd, while that prepared at the much higher complexing ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 has excess of Te. The increase in Te/Cd atomic ratio in the as-prepared CdTe film with the complexing ratio should be attributed to the different complexing form of Cd(II) in the varied solutions. Cd(II) in the solution with the higher complexing ratio should exist in the more stable form, which makes the reduction of Cd(II) difficult and as a result produces a film with the higher Te/Cd ratio, which would be further elucidated in the later discussion.
1 has excess of Te. The increase in Te/Cd atomic ratio in the as-prepared CdTe film with the complexing ratio should be attributed to the different complexing form of Cd(II) in the varied solutions. Cd(II) in the solution with the higher complexing ratio should exist in the more stable form, which makes the reduction of Cd(II) difficult and as a result produces a film with the higher Te/Cd ratio, which would be further elucidated in the later discussion.
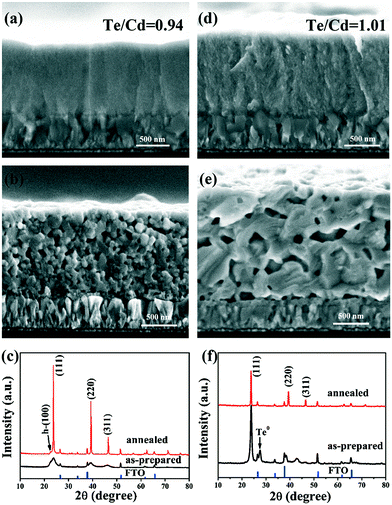 | ||
Fig. 2 Cross-section SEM images and XRD patterns of the as-prepared and annealed CdTe films at various NTA/Cd ratio (a–c) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at −0.75 V, and (d–f) 20 1 at −0.75 V, and (d–f) 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and at −0.50 V. 1 and at −0.50 V. | ||
It should be noted that the composition deviation from the normal stoichiometry of CdTe film either in excess Cd or Te always inhibits the grain growth during the deposition, leading to poor crystallinity of the CdTe film at low deposition temperature. However, the composition of the as-prepared CdTe films has distinctly different effect on the annealed films. The excessive Cd limits the grain growth not only during the deposition but also during annealing, while the excessive Te limits the growth only during the deposition, but not much during the annealing. We attribute this phenomenon to grain growth by the assistance of the VCd defects formed during annealing. It is known that the excessive Te promotes the formation of VCd during the annealing, which could facilitate Cd diffusion and then promote grain growth.23
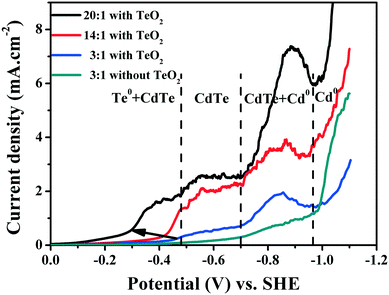
To clarify the action mechanisms of the complexing ratio on the deposition, Fig. 3 shows the cyclic voltammogram (C–V) plots recorded for CdS/FTO substrates at varied complexing ratios at pH = 9.0. In solution without TeO2, the C–V plot displays two onset currents at −0.70 V and −0.97 V, corresponding to the reduction of the dissoluble oxygen and the NTA–Cd(II) complex, respectively,5 both of which are constant at the complexing ratio in all plots. The constant reduction potential of the NTA–Cd(II) complex at varied complexing ratios suggests that the NTA concentration is enough to stabilize Cd(II) at the complexing ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It should be noted that the reduction of the dissoluble oxygen near the reaction interface produces OH− ions, which would improve the pH at the located reaction interface according to the reaction O2 + 2H2O + 4e− → 4OH−. The improved pH at the located reaction interface, on one hand, dissolves Te in the as-deposited CdTe, leading to the Cd excess and poor crystallinity. On the other hand, the improved pH also leads to difficulty in the reduction of TeO32− due to the serious pH dependence of the TeO32− reduction. This, to a certain degree, accounts for the large current decrease at the very beginning of the deposition (Fig. S2, ESI†). Therefore, to obtain a CdTe film with good crystallinity the deposition potential should be no more negative than −0.70 V lest the reduction of the dissoluble oxygen near the reaction interface disturbs the crystal growth, leading to the formation of excess Cd.
1. It should be noted that the reduction of the dissoluble oxygen near the reaction interface produces OH− ions, which would improve the pH at the located reaction interface according to the reaction O2 + 2H2O + 4e− → 4OH−. The improved pH at the located reaction interface, on one hand, dissolves Te in the as-deposited CdTe, leading to the Cd excess and poor crystallinity. On the other hand, the improved pH also leads to difficulty in the reduction of TeO32− due to the serious pH dependence of the TeO32− reduction. This, to a certain degree, accounts for the large current decrease at the very beginning of the deposition (Fig. S2, ESI†). Therefore, to obtain a CdTe film with good crystallinity the deposition potential should be no more negative than −0.70 V lest the reduction of the dissoluble oxygen near the reaction interface disturbs the crystal growth, leading to the formation of excess Cd.
As 2 mM TeO2 is added at the complexing ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a new onset current appears at −0.48 V and exists at varied complexing ratios. Increasing the complexing ratio to 14
1, a new onset current appears at −0.48 V and exists at varied complexing ratios. Increasing the complexing ratio to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 produces another onset current at −0.40 V, which, however, shifts positively to −0.30 V at the ratio of 20
1 produces another onset current at −0.40 V, which, however, shifts positively to −0.30 V at the ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The XRD result indicates that the film deposited at −0.48 V primarily consists of CdTe, while at the more positive potentials excess Te0 is generated. Accordingly, the relationship between the deposition potential and film composition in the entire potential range is divided into four regions (Fig. 3). Excessive Te0 at the potential that is more positive than −0.48 V in the solution with the ratio of 20
1. The XRD result indicates that the film deposited at −0.48 V primarily consists of CdTe, while at the more positive potentials excess Te0 is generated. Accordingly, the relationship between the deposition potential and film composition in the entire potential range is divided into four regions (Fig. 3). Excessive Te0 at the potential that is more positive than −0.48 V in the solution with the ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 suggests that the increasing complexing ratio facilitates TeO2 reduction, which is very similar to the case in the acidic solution, in which the increasing Cd2+ concentration suppresses Te reduction.15 Te0 production due to the reduction of the adsorbing TeO32− on the reaction interface would be suppressed by the complete adsorption of Cd2+ ions released by the ion equilibrium of the Cd(II)–NTA complex. The increased complexing ratio would promote the formation of the more stable NTA–Cd(II) complex, which reduces the free Cd2+ ions and supports the production of Te0. Thus, the deposition of CdTe film should be conducted under the potential more negative than −0.48 V to promote the production of CdTe and reduce the formation of excess Te0.
1 suggests that the increasing complexing ratio facilitates TeO2 reduction, which is very similar to the case in the acidic solution, in which the increasing Cd2+ concentration suppresses Te reduction.15 Te0 production due to the reduction of the adsorbing TeO32− on the reaction interface would be suppressed by the complete adsorption of Cd2+ ions released by the ion equilibrium of the Cd(II)–NTA complex. The increased complexing ratio would promote the formation of the more stable NTA–Cd(II) complex, which reduces the free Cd2+ ions and supports the production of Te0. Thus, the deposition of CdTe film should be conducted under the potential more negative than −0.48 V to promote the production of CdTe and reduce the formation of excess Te0.
According to the above discussion, the formation potential of CdTe at the complexing ratio of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 lies between −0.48 V and −0.70 V. Since the formation potential of CdTe is greatly positive as compared to that of the reduction of Cd0 (−0.97 V), the deposition of CdTe film surely follows the UPD mechanisms as can be summarized in the following reactions:
1 lies between −0.48 V and −0.70 V. Since the formation potential of CdTe is greatly positive as compared to that of the reduction of Cd0 (−0.97 V), the deposition of CdTe film surely follows the UPD mechanisms as can be summarized in the following reactions:
| TeO32− + 3H2O + 4e− → Te0 + 6OH− | (1) |
| Cd(NTA)n2+ ↔ Cd2+ + nNTA | (2) |
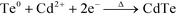 | (3) |
The UPD mechanisms are very similar to the acidic solution except for the different TeO2 form in reaction (1) and the ionic equilibrium (2). Accordingly, the composition and structure of the as-prepared CdTe film depend on the relative rates of the above three reactions at varied complexing ratios. The higher complexing ratio would result in slower reaction rates of (2) and (3), leading to the formation of excess Te in the film.
The UPD mechanisms suggest that the concentration of NTA is critical for the deposition rate and the film composition. The strong complexing ability of NTA together with the large complexing ratio to Cd(II) ensures that the reduction potential of Cd(II) (−0.97 V) is far from that of the onset formation potential of CdTe (−0.48 V). In addition, the high concentration of NTA shifts the deposition potential positively and is far from the reduction potential of the dissoluble oxygen, which favors the production of the stoichiometric film of high crystallinity. In addition, it should be noted that besides the change in peaks of the CV plots with the complexing ratio, the current density also increases gradually with the increase in complexing ratio. We speculate that the increased complexing ratio stabilizes the Cd2+, increases the adsorption of TeO32− on the deposition substrate, and thus increases the current density.
In our present study, TMAH, widely used as the etching reagent in Si solar cells19 was used to replace NaOH for adjusting the solution pH, which successfully avoids the incorporation of the detrimental alkaline metal ions into CdTe films, and makes it viable for assembling solar cells based on the CdTe films from this alkaline solution system. Because of the larger solubility of TeO2 in the alkaline solution, the present solution retains a large space for the rapid deposition of CdTe film. Moreover, an alkaline process for CdTe film prevents the etching of the traditional acid solution to CdS layer during electrodeposition; hence, the CdS/CdTe interface quality is significantly improved. Thus, the present study provides an alternative approach for preparing high-quality CdTe films under a low-temperature process.
Conclusions
CdTe films were electrodeposited from the alkaline electrolyte containing NTA and organic base. High concentration of NTA having strong complexing ability ensured the UPD deposition of CdTe film. TMAH prevents the introduction of alkaline metal ions into CdTe film. The as-prepared CdTe film at the complexing ratio of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 had typical stoichiometry and was well-crystallized and highly (111)-oriented. After annealing, the structure became randomly oriented without voids within the film and at the CdS/CdTe interface. The alkaline electrochemical approach is predicted to have potential in CdTe solar cells.
1 had typical stoichiometry and was well-crystallized and highly (111)-oriented. After annealing, the structure became randomly oriented without voids within the film and at the CdS/CdTe interface. The alkaline electrochemical approach is predicted to have potential in CdTe solar cells.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Nature Science foundation of China (grant no. 50802035) and Shandong Provincial Natural Science Foundation, China (ZR2010EZ003).Notes and references
- R. Treharne, J. D. Major, L. J. Phillips and K. Durose, Nature, 2014, 511, 334–337 CrossRef PubMed.
- H. Li, X. X. Liu, B. Yang and P. J. Wang, RSC Adv., 2014, 4, 5046–5054 RSC.
- C. Heisler, M. Brückner, F. Lind, C. Kraft, U. Reislöhner, C. Ronning and W. Wesch, J. Appl. Phys., 2013, 113, 224504 CrossRef.
- T. W. Kim, H. L. Park, J. Y. Lee, H. J. Lee and T. W. Kim, Appl. Phys. Lett., 1994, 2597, 2597–2599 CrossRef.
- K. Murase, H. Uchida, T. Hirato and Y. Awakura, J. Electrochem. Soc., 1999, 146, 531–536 CrossRef CAS.
- I. M. Dharmadasa, P. A. Bingham, O. K. Echendu, H. I. Salim, T. Druffel, R. Dharmadasa, G. U. Sumanasekera, R. R. Dharmasena, M. B. Dergacheva, K. A. Mit, K. A. Urazov, L. Bowen, M. Walls and A. Abbas, Coatings, 2014, 4, 380–415 CrossRef.
- B. Shan, W. Wu, K. Feng and H. Nan, Mater. Lett., 2016, 166, 85–88 CrossRef CAS.
- A. A. Ojo and I. M. Dharmadasa, Sol. Energy, 2016, 136, 10–14 CrossRef CAS.
- R. Yang, D. Wang, L. Wan and D. Wang, RSC Adv., 2014, 4, 22162–22171 RSC.
- Q. Li, W. Fu, Y. Mu, W. Zhang, P. Lv, L. Zhou, H. Yang, K. Chi and L. Yang, CrystEngComm, 2014, 16, 5227–5233 RSC.
- K. Arai, K. Murase, T. Hirato and Y. Awakura, J. Electrochem. Soc., 2006, 153, C121–C126 CrossRef CAS.
- K. Arai, K. Murase, T. Hirato and Y. Awakura, Electrochim. Acta, 2006, 51, 4987–4993 CrossRef CAS.
- D. Lincot, Thin Solid Films, 2005, 487, 40–48 CrossRef CAS.
- J. Wang, Q. Li, Y. Mu, S. Li, L. Yang, P. Lv, S. Su, T. Liu, W. Fu and H. Yang, J. Alloys Compd., 2015, 636, 97–101 CrossRef CAS.
- S. Soundeswaran, O. S. Kumar and R. Dhanasekaran, Mater. Lett., 2004, 58, 2381–2385 CrossRef CAS.
- X. Wang, H. Zhu, Y. Xu, H. Wang, Y. Tao, S. Hark, X. Xiao and Q. Li, ACS Nano, 2010, 4, 3302–3308 CrossRef CAS PubMed.
- L. Kranz, J. Perrenoud, F. Pianezzi, C. Gretener, P. Rossbach and S. Buecheler, Sol. Energy Mater. Sol. Cells, 2012, 105, 213–219 CrossRef CAS.
- B. L. Williams, J. D. Major, L. Bowen, W. Keuning, M. Creatore and K. Durose, Adv. Energy Mater., 2015, 5, 1500554 CrossRef.
- H. Savin, P. Repo, G. V. Gastrow, P. Ortega, E. Calle, M. Garín and R. Alcubilla, Nat. Nanotechnol., 2015, 1–6 Search PubMed.
- K. Feng, W. Wu, B. Shan and H. Nan, Mater. Res. Express, 2016, 3, 106404 CrossRef.
- M. Miyake, K. Murase, T. Hirato and Y. Awakura, J. Electroanal. Chem., 2004, 562, 247–253 CrossRef CAS.
- M. Takahashi, K. Uosaki, H. Kita and Y. Suzuki, J. Appl. Phys., 1985, 58, 4292–4295 CrossRef CAS.
- S.-H. Wei and S. B. Zhang, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 155211 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ce01816a |
| This journal is © The Royal Society of Chemistry 2018 |