Reductive cyclisations of amidines involving aminal radicals†
Huan-Ming
Huang
 ,
Ralph W.
Adams
,
Ralph W.
Adams
 and
David J.
Procter
and
David J.
Procter
 *
*
School of Chemistry, Oxford Road, University of Manchester, Manchester, M13 9PL, UK. E-mail: david.j.procter@manchester.ac.uk
First published on 15th August 2018
Abstract
Amidines bearing simple alkenes undergo aminal radical cyclisation upon treatment with SmI2. The mild, reductive electron transfer process delivers medicinally-relevant, polycyclic quinazolinone derivatives in good to excellent yield and typically with complete diastereocontrol.
Nitrogen-containing heterocycles are ubiquitous components in the molecular architectures of natural products, materials and drug candidates.1 As a feature in biologically active alkaloids,2 the quinazolinone ring system is a significant member of the family and its presence in nature has inspired the search for synthetic quinazolinones with medicinal potential (Scheme 1A).3 Although various approaches to these polycyclic scaffolds have been described,4 expedient, stereoselective synthetic strategies to quinazolinones that operate under mild conditions on simple, readily accessible substrates, are of high value.
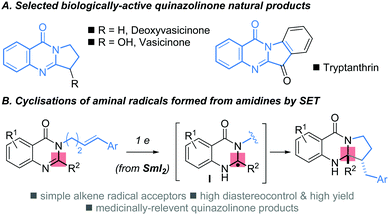 | ||
| Scheme 1 (A) Selected biologically-active quinazolinone natural products (B) this work: the cyclisation of aminal radicals, formed from amidines by SET, provides efficient access to quinazolinones. | ||
Radical cyclisations have emerged as an important tool for the efficient generation of complex polycyclic products.5 However, few radical cyclisation strategies have been developed for the synthesis of quinazolinone analogues.6 Of these, Malacria, Courillon and Fensterbank have described an elegant radical cyclisation approach using tributyltin hydride and applied the method to a synthesis of Luotonin A.6a–f Weaver and Bowman have also reported a radical cyclisation approach to quinazolinones using tributyltin hydride.6g Recently, Chiba reported an elegant oxidative radical rearrangement that constructs quinazolinones.6h,i Finally, quinazolinone scaffolds have been accessed using radical processes involving Ag(I)6j and Cu(I),6k visible-light photoredox catalysis,6l and DTBP in a metal-free process.6m Despite these reports,6 there remains a need for a straightforward method that constructs polycyclic quinazolinones under mild conditions.
We recently reported the first radical reduction, cyclisations and cyclisation cascades involving radical anions generated from urea-type carbonyls by single electron transfer (SET).7 During the study, we found that the aminal radical anion intermediates could be generated and trapped by tethered alkenes to form heterocyclic products.7a Related aminal radicals were formed and trapped, in an intermolecular sense, by Beaudry in his highly effective cross-coupling of amidines with electron-deficient alkenes.8 Both processes are mediated by the reductive SET reagent, samarium iodide (SmI2, Kagan's reagent).9 This highly versatile, commercially available or readily prepared reagent often proves to be the only viable mediator of challenging radical cyclisations and cyclisation cascades designed to deliver high value products not easily accessible by alternative means.10 Crucially, in Beaudry's study, only two intramolecular examples of amidine–alkene coupling were described and in all examples, both inter- and intramolecular, alkenes bore strongly electron-withdrawing groups.8a We recognised that the intramolecular SmI2-mediated coupling of amidines with simple unactivated alkene radical acceptors could provide expedient access to important quinazolinones. Herein, we describe the first general study of aminal radical cyclisations triggered by SET reduction of amidines using SmI2 (Scheme 1B). The radical cyclisations deliver quinazolinones in good yield and typically with complete diastereocontrol.
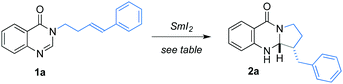
We began our studies by optimising the cyclisation of 1a; efficiently synthesised in one step from commercial 4-hydroxyquinazoline. Pleasingly, the desired cyclisation product 2a was obtained in 50% yield upon treatment with SmI2 (Table 1, entry 1). The fact that SmI2, in the absence of additives that increase the reducing power of the reagent, can reduce 1a highlights the reactive nature of the N-acyl amidine functional group relative to, for example, amides,11a acids,11b esters,11c and nitriles.11d Drawing on the observations of Beaudry, NH4Cl proved to be an effective proton source in the reductive coupling, and its use gave 2a in 57% yield (entry 2). Using the more established proton sources, H2O and t-BuOH, resulted in the formation of 2a in 61% and 60% yield, respectively (entries 3 and 4). When the amount of H2O was reduced, the yield of 3a did not improve (entries 5 and 6). The use of LiBr as an additive in combination with H2O12 gave 2a in a lower 26% yield (entry 7). The key to further improvement in the yield of 2a proved to lie in the rate of addition of the SET reagent; slow addition of SmI2 gave 2a in 81% isolated yield (entry 8). It is likely that slow addition prevents the over-reduction of aminal radical I that would compete with radical cyclisation. The combination of SmI2 and H2O was clearly too reducing for the amidine substrate (even with slow addition of SmI2) and thus Beaudry's NH4Cl additive was used in further studies.
| Entry | SmI2 (equiv.) | NH4Cl (equiv.) | H2O (equiv.) | Yieldb [%] | |
|---|---|---|---|---|---|
| 1a | 2a | ||||
| a Reaction conditions: 1a (0.1 mmol, in THF) under N2, was added proton source followed by SmI2 (0.1 M in THF). The reaction was quenched after 2 h. b Yield was determined by 1H NMR spectroscopy using 2,3,5,6-tetrachloronitrobenzene as internal standard. c 10 equiv. t-BuOH was added. d 100 equiv. LiBr was added. e The SmI2 solution was added over 1 h by syringe pump. f Isolated yield. | |||||
| 1 | 3 | — | — | — | 50 |
| 2 | 3 | 3 | — | — | 57 |
| 3 | 3 | — | 100 | — | 61 |
| 4c | 3 | — | — | — | 60 |
| 5 | 3 | — | 3 | — | 55 |
| 6 | 3 | — | 20 | — | 58 |
| 7d | 3 | — | 100 | — | 26 |
| 8 | 3 | 3 | — | — | 84 (81) |
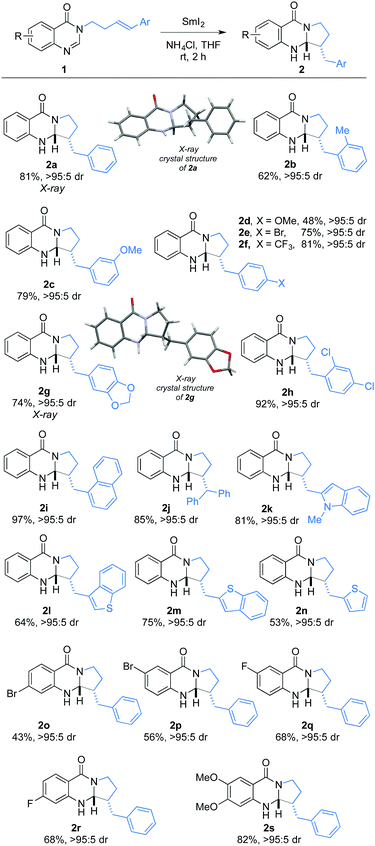
Using the optimised conditions, we have explored the generality of the amidine–alkene radical cyclisation (Scheme 2). In all cases, the desired quinazolinone products of cyclisation were obtained with complete diastereocontrol (>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr) and in good to excellent yield. Various functional groups on the alkenyl aryl ring were found to be compatible with the reductive conditions, including methoxy (2c, 2d), bromo (2e), chloro (2h), trifluoromethyl (2f), and acetal (2g). Furthermore, the presence of medicinally-relevant heteroaromatic rings including indole (2k), benzothienyl (2l and 2m), and thienyl (2n) did not impede radical cyclisation. Finally, various functional groups on the benzenoid ring of the 4-quinazolinone motif, including bromo (2o and 2p), fluoro (2q and 2r) and methoxy (2s), were also tolerated in the radical cyclisation. The relative configuration of the quinazolinone products was assigned after X-ray crystallographic analysis of 2a and 2g.13
5 dr) and in good to excellent yield. Various functional groups on the alkenyl aryl ring were found to be compatible with the reductive conditions, including methoxy (2c, 2d), bromo (2e), chloro (2h), trifluoromethyl (2f), and acetal (2g). Furthermore, the presence of medicinally-relevant heteroaromatic rings including indole (2k), benzothienyl (2l and 2m), and thienyl (2n) did not impede radical cyclisation. Finally, various functional groups on the benzenoid ring of the 4-quinazolinone motif, including bromo (2o and 2p), fluoro (2q and 2r) and methoxy (2s), were also tolerated in the radical cyclisation. The relative configuration of the quinazolinone products was assigned after X-ray crystallographic analysis of 2a and 2g.13
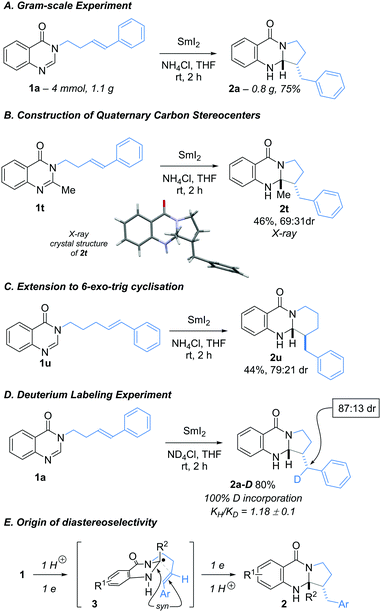
The radical cyclisation could be carried out on gram-scale with no loss of efficiency: Using the optimised conditions, 1a (4.0 mmol, 1.1 g) was converted to 2a (3.0 mmol, 0.83 g) in 75% yield (Scheme 3A). In addition, radical cyclisation of 2-methyl quinazolin-4-one 1t gave 2t containing a quaternary carbon center in 46% isolated yield as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereoisomers (Scheme 3B). The structure of the major diastereoisomer of 2t was confirmed by X-ray crystallographic analysis.13 Substrate 1u underwent a challenging 6-exo-trig cyclisation to give 2u in 44% isolated yield with moderate diastereocontrol. In this case, the anti diastereoisomeric product predominated, as determined by NOE and also supported by the comparison of measured and calculated coupling constants (Scheme 3C).14 A deuterium labeling experiment was also performed using SmI2–ND4Cl. As expected, the labelled cyclisation product 2a-D was obtained, thus confirming that the cyclisation is terminated by protonation of a benzylic organosamarium (100% D incorporation, 83
1 mixture of diastereoisomers (Scheme 3B). The structure of the major diastereoisomer of 2t was confirmed by X-ray crystallographic analysis.13 Substrate 1u underwent a challenging 6-exo-trig cyclisation to give 2u in 44% isolated yield with moderate diastereocontrol. In this case, the anti diastereoisomeric product predominated, as determined by NOE and also supported by the comparison of measured and calculated coupling constants (Scheme 3C).14 A deuterium labeling experiment was also performed using SmI2–ND4Cl. As expected, the labelled cyclisation product 2a-D was obtained, thus confirming that the cyclisation is terminated by protonation of a benzylic organosamarium (100% D incorporation, 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 dr at the labelled benzylic position).15 The KIE measured for this reductive cyclisation suggests that proton transfer is not involved in the rate-determining step (Scheme 3D). Finally, a likely transition structure for the 5-exo-trig radical cyclisation that explains the origin of the syn-diastereoselectivity is shown in Scheme 3E. In contrast, to the cyclisations of ketyl radical anions that often proceed to give anti-products,16 the cyclisation of neutral aminal radical I, formed by protonation of a radical anion after SET or by protonation of the amidine prior to SET, favours cyclisation via syn-transition structure 3.
17 dr at the labelled benzylic position).15 The KIE measured for this reductive cyclisation suggests that proton transfer is not involved in the rate-determining step (Scheme 3D). Finally, a likely transition structure for the 5-exo-trig radical cyclisation that explains the origin of the syn-diastereoselectivity is shown in Scheme 3E. In contrast, to the cyclisations of ketyl radical anions that often proceed to give anti-products,16 the cyclisation of neutral aminal radical I, formed by protonation of a radical anion after SET or by protonation of the amidine prior to SET, favours cyclisation via syn-transition structure 3.
In summary, reductive amidine–alkene radical cyclisations, involving the intramolecular addition of aminal radicals to simple alkenes, deliver polycyclic quinazolinones. The radical process is mediated by single electron transfer from commercially available SmI2, operates under mild conditions on readily-available substrates, proceeds with complete diastereocontrol, and delivers a range of medicinally-relevant, quinazolinone derivatives in good to excellent yield.
We thank the EPSRC (EPSRC Established Career Fellowship to D. J. P.; EP/M005062/1), EPSRC Core Capability Grant (EP/K039547/1), the Leverhulme Trust (Research Fellowship to D. J. P.; RF-2013-286), and the University of Manchester (President's Scholarship to H. H.) for funding.
Conflicts of interest
There are no conflicts to declare.Notes and references
- For reviews on nitrogen-containing heterocycles, see: (a) J. P. Michael, Nat. Prod. Rep., 2002, 19, 719 RSC; (b) P. A. Evans and B. Holmes, Tetrahedron, 1991, 47, 9131 CrossRef; (c) Y. Yamamoto, Chem. Soc. Rev., 2014, 43, 1575 RSC; (d) B. H. Lipshutz, Chem. Rev., 1986, 86, 795 CrossRef.
- Selected reviews, see: (a) S. B. Mhaske and N. P. Argade, Tetrahedron, 2006, 62, 9787 CrossRef; (b) D. J. Connolly, D. Cusack, T. P. O’Sullivan and P. J. Guiry, Tetrahedron, 2005, 61, 10153 CrossRef; (c) I. Khan, A. Ibrar, N. Abbas and A. Saeed, Eur. J. Med. Chem., 2014, 76, 193 CrossRef PubMed; (d) I. Khan, A. Ibrar, W. Ahmed and A. Saeed, Eur. J. Med. Chem., 2015, 90, 124 CrossRef PubMed; (e) L. He, H. Li, J. Chen and X.-F. Wu, RSC Adv., 2014, 4, 12065 RSC; (f) U. A. Kshirsagar, Org. Biomol. Chem., 2015, 13, 9336 RSC; (g) P. C. Sharma, G. Kaur, R. Pahwa, A. Sharma and H. Rajak, Curr. Med. Chem., 2011, 18, 4786 CrossRef PubMed; (h) R. Rohokale and U. A. Kshirsagar, Synthesis, 2016, 1253 Search PubMed.
- (a) Z.-Z. Ma, Y. Hano, T. Nomura and Y.-J. Chen, Heterocycles, 1997, 46, 541 CrossRef; (b) A. Astulla, K. Zaima, Y. Matsuno, Y. Hirasawa, W. Ekasari, A. Widyawaruyanti, N. C. Zaini and H. Morita, J. Nat. Med., 2008, 62, 470–472 CrossRef PubMed; (c) A. Al-Shamma, S. Drake, D. L. Flynn, L. A. Mitscher, Y. H. Park, G. S. R. Rao, A. Simpson, J. K. Swayze, T. Veysoglu and S. T.-S. Wu, J. Nat. Prod., 1981, 44, 745–747 CrossRef PubMed.
- Selected elegant examples of total synthesis of related natural produts, see: (a) S. B. Mhaske and N. P. Argade, J. Org. Chem., 2001, 66, 9038 CrossRef PubMed; (b) J.-F. Liu, P. Ye, K. Sprague, K. Sargent, D. Yohannes, C. M. Baldino, C. J. Wilson and S.-C. Ng, Org. Lett., 2005, 7, 3363 CrossRef PubMed; (c) S. P. Chavan and R. Sivappa, Tetrahedron Lett., 2004, 45, 997 CrossRef.
- Selected reviews and book, see: (a) M. P. Plesniak, H.-M. Huang and D. J. Procter, Nat. Rev. Chem., 2017, 1, 0077 CrossRef; (b) J. Xuan and A. Studer, Chem. Soc. Rev., 2017, 46, 4329 RSC; (c) B. M. Loertscher and S. L. Castle, in Comprehensive Organic Synthesis II, Elsevier, 2014, pp. 742–809 Search PubMed; (d) K. C. Majumdar, P. K. Basu and S. K. Chattopadhyay, Tetrahedron, 2007, 63, 793 CrossRef; (e) A. J. Clark, Chem. Soc. Rev., 2002, 31, 1 RSC; (f) A. G. Fallis and I. M. Brinza, Tetrahedron, 1997, 53, 17543 CrossRef; (g) B. B. Snider, Chem. Rev., 1996, 96, 339 CrossRef PubMed; (h) S. Kim, Pure Appl. Chem., 1996, 68, 623 Search PubMed.
- Selected examples of the synthesis of quinazolinone scaffolds invloving radical cyclisation, see (a) A. Servais, M. Azzouz, D. Lopes, C. Courillon and M. Malacria, Angew. Chem., Int. Ed., 2007, 46, 576 CrossRef PubMed; (b) A. Beaume, C. Courillon, E. Derat and M. Malacria, Chem. – Eur. J., 2008, 14, 1238 CrossRef PubMed; (c) M.-H. Larraufie, C. Ollivier, L. Fensterbank, M. Malacria and E. Lacôte, Angew. Chem., Int. Ed., 2010, 49, 2178 CrossRef PubMed; (d) M.-H. Larraufie, C. Courillon, C. Ollivier, E. Lacôte, M. Malacria and L. Fensterbank, J. Am. Chem. Soc., 2010, 132, 4381 CrossRef PubMed; (e) M.-H. Larraufie, G. Maestri, M. Malacria, C. Ollivier, L. Fensterbank and E. Lacôte, Synthesis, 2012, 1279 Search PubMed; (f) M.-H. Larraufie, M. Malacria, C. Courillon, C. Ollivier, L. Fensterbank and E. Lacôte, Tetrahedron, 2013, 69, 7699 CrossRef; (g) W. R. Bowman, M. R. J. Elsegood, T. Stein and G. W. Weaver, Org. Biomol. Chem., 2007, 5, 103 RSC; (h) Y. F. Wang, F. L. Zhang and S. Chiba, Org. Lett., 2013, 15, 2842 CrossRef PubMed; (i) F.-L. Zhang, Y.-F. Wang and S. Chiba, Org. Biomol. Chem., 2013, 11, 6003 RSC; (j) J. Zheng, Y. Zhang, D. Wang and S. Cui, Org. Lett., 2016, 18, 1768 CrossRef PubMed; (k) J. Zheng, Z. Deng, Y. Zhang and S. Cui, Adv. Synth. Catal., 2016, 358, 746 CrossRef; (l) P. Qian, Y. Deng, H. Mei, J. Han, J. Zhou and Y. Pan, Org. Lett., 2017, 19, 4798 CrossRef PubMed; (m) X.-K. Liu, P. Qian, Y. Wang and Y. Pan, Org. Chem. Front., 2017, 4, 2370 RSC; (n) Q. Li, Y. Huang, T. Chen, Y. Zhou, Q. Xu, S. F. Yin and L. B. Han, Org. Lett., 2014, 16, 3672 CrossRef PubMed; (o) Y. Bao, Y. Yan, K. Xu, J. Su, Z. Zha and Z. Wang, J. Org. Chem., 2015, 80, 4736 CrossRef PubMed; (p) T. Yang, W. Wang, D. Wei, T. Zhang, B. Han and W. Yu, Org. Chem. Front., 2017, 4, 421 RSC; (q) A. V. A. Gholap, S. Maity, C. Schulzke, D. Maiti and A. R. Kapdi, Org. Biomol. Chem., 2017, 15, 7140 RSC; (r) Á. Gutiérrez-Bonet, C. Remeur, J. K. Matsui and G. A. Molander, J. Am. Chem. Soc., 2017, 139, 12251 CrossRef PubMed; (s) P. S. Mahajan and S. B. Mhaske, Org. Lett., 2018, 20, 2092 CrossRef PubMed.
- (a) H.-M. Huang, J. J. W. McDouall and D. J. Procter, Angew. Chem., Int. Ed., 2018, 57, 4995 CrossRef PubMed; (b) H.-M. Huang and D. J. Procter, Eur. J. Org. Chem., 2018 DOI:10.1002/ejoc.201800794.
- (a) D. A. Schiedler, Y. Lu and C. M. Beaudry, Org. Lett., 2014, 16, 1160 CrossRef PubMed; (b) D. A. Schiedler, J. K. Vellucci, Y. Lu and C. M. Beaudry, Tetrahedron, 2015, 71, 1448 CrossRef . Aminal radicals could also be generated under Bu3SnH or (TMS)3SiH conditions, and have been applied in the carbon-carbon bond forming reaction, see: ; (c) D. A. Schiedler, J. K. Vellucci and C. M. Beaudry, Org. Lett., 2012, 14, 6092 CrossRef PubMed.
- Selected leading reviews on SmI2-mediated radical cyclisations and cyclisation cascades, see: (a) S. Shi and M. Szostak, Molecules, 2017, 22, 2018 CrossRef PubMed; (b) X. Just-Baringo and D. J. Procter, Acc. Chem. Res., 2015, 48, 1263 CrossRef PubMed; (c) M. Szostak, N. J. Fazakerley, D. Parmar and D. J. Procter, Chem. Rev., 2014, 114, 5959 CrossRef PubMed; (d) C. Beemelmanns and H.-U. Reissig, Chem. Soc. Rev., 2011, 40, 2199 RSC; (e) C. Beemelmanns and H.-U. Reissig, Pure Appl. Chem., 2011, 83, 507 Search PubMed; (f) K. C. Nicolaou, S. P. Ellery and J. S. Chen, Angew. Chem., Int. Ed., 2009, 48, 7140 CrossRef PubMed; (g) D. J. Procter, R. A. Flowers, II and T. Skrydstrup, Organic Synthesis using Samarium Diiodide: A Practical Guide, RSC Publishing, Cambridge, 2009 Search PubMed; (h) R. Flowers, II, Synlett, 2008, 1427 CrossRef; (i) D. J. Edmonds, D. Johnston and D. J. Procter, Chem. Rev., 2004, 104, 3371 CrossRef PubMed; (j) G. A. Molander and C. R. Harris, Chem. Rev., 1996, 96, 307 CrossRef PubMed; (k) G. A. Molander, Chem. Rev., 1992, 92, 29 CrossRef.
- For selected recent examples of Sm(II)-mediated radical cyclisations, see: (a) C. Morrill, C. Jensen, X. Just-Baringo, G. Grogan, N. J. Turner and D. J. Procter, Angew. Chem., Int. Ed., 2018, 57, 3692 CrossRef PubMed; (b) N. Kern, M. P. Plesniak, J. J. W. McDouall and D. J. Procter, Nat. Chem., 2017, 9, 1198 CrossRef PubMed; (c) H.-M. Huang and D. J. Procter, Angew. Chem., Int. Ed., 2017, 56, 14262 CrossRef PubMed; (d) H.-M. Huang and D. J. Procter, J. Am. Chem. Soc., 2017, 139, 1661 CrossRef PubMed; (e) H.-M. Huang, P. Bonilla and D. J. Procter, Org. Biomol. Chem., 2017, 15, 4159 RSC; (f) H.-M. Huang and D. J. Procter, J. Am. Chem. Soc., 2016, 138, 7770 CrossRef PubMed; (g) S. Shi, R. Lalancette, R. Szostak and M. Szostak, Chem. – Eur. J., 2016, 22, 11949 CrossRef PubMed; (h) S. Shi and M. Szostak, Org. Lett., 2015, 17, 5144 CrossRef PubMed; (i) Z. Li, M. Nakashige and W. J. Chain, J. Am. Chem. Soc., 2011, 133, 6553 CrossRef PubMed; (j) J. Y. Cha, J. T. S. Yeoman and S. E. Reisman, J. Am. Chem. Soc., 2011, 133, 14964 CrossRef PubMed; (k) C. Beemelmanns and H.-U. Reissig, Angew. Chem., Int. Ed., 2010, 49, 8021 CrossRef PubMed; (l) M. D. Helm, M. Da Silva, D. Sucunza, T. J. K. Findley and D. J. Procter, Angew. Chem., Int. Ed., 2009, 48, 9315 CrossRef PubMed; (m) S. E. Reisman, J. M. Ready, M. M. Weiss, A. Hasuoka, M. Hirata, K. Tamaki, T. V. Ovaska, C. J. Smith and J. L. Wood, J. Am. Chem. Soc., 2008, 130, 2087 CrossRef PubMed.
- (a) M. Szostak, M. Spain, A. J. Eberhart and D. J. Procter, J. Am. Chem. Soc., 2014, 136, 2268 CrossRef PubMed; (b) M. Szostak, M. Spain and D. J. Procter, Org. Lett., 2012, 14, 840 CrossRef PubMed; (c) M. Szostak, M. Spain and D. J. Procter, Chem. Commun., 2011, 47, 10254 RSC; (d) M. Szostak, B. Sautier, M. Spain and D. J. Procter, Org. Lett., 2014, 16, 1092 CrossRef PubMed.
- SmI2−H2O−LiBr has recently been used to promote radical cyclisations and cyclisation cascades. For example, see: (a) C. N. Rao, D. Lentz and H.-U. Reissig, Angew. Chem., Int. Ed., 2015, 54, 2750 CrossRef PubMed; (b) C. N. Rao, C. Bentz and H.-U. Reissig, Chem. – Eur. J., 2015, 21, 15951 CrossRef PubMed . And ref. 10e–g.
- See ESI† for X-ray structures and CCDC numbers (CCDC 1846530 for 2a, 1846531 for 2g and 1846532 for 2t).
- See ESI† for NOE experiments and a comparison of measured and calculated J values.
- X. Just-Baringo, J. Clark, M. J. Gutmann and D. J. Procter, Angew. Chem., Int. Ed., 2016, 55, 12499 CrossRef PubMed.
- For a review, see: (a) A. L. J. Beckwith, Tetrahedron, 1981, 37, 3073 CrossRef . For selected examples, see: ; (b) G. A. Molander and C. Kenny, J. Am. Chem. Soc., 1989, 111, 8236 CrossRef; (c) G. A. Molander and J. A. McKie, J. Org. Chem., 1995, 60, 872 CrossRef; (d) D. Johnston, C. M. McCusker and D. J. Procter, Tetrahedron Lett., 1999, 40, 4913 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental procedures, characterization details, 1H and 13C NMR spectra of compounds, X-ray crystallographic data for 2a, 2g and 2t, nOe study and analysis of coupling constants for 2u. CCDC 1846530–1846532. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8cc05178j |
| This journal is © The Royal Society of Chemistry 2018 |