Efficient separation of C2 hydrocarbons in a permanently porous hydrogen-bonded organic framework†
Tae-Ung
Yoon‡
a,
Seung Bin
Baek‡
 *b,
Dongwook
Kim
c,
Eun-Jung
Kim
a,
Wang-Geun
Lee
*b,
Dongwook
Kim
c,
Eun-Jung
Kim
a,
Wang-Geun
Lee
 b,
Bhupendra Kumar
Singh
b,
Myoung Soo
Lah
b,
Bhupendra Kumar
Singh
b,
Myoung Soo
Lah
 c,
Youn-Sang
Bae
*a and
Kwang S.
Kim
c,
Youn-Sang
Bae
*a and
Kwang S.
Kim
 *b
*b
aDepartment of Chemical and Biomolecular Engineering, and Graduate School of Integrated Engineering, Yonsei University, 50 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea. E-mail: mowbae@yonsei.ac.kr
bCenter for Superfunctional Materials and Department of Chemistry, Ulsan National Institute of Science and Technology (UNIST), 50 UNIST-gil, Ulsan 44919, Korea. E-mail: sepbirth@gmail.com; kimks@unist.ac.kr
cDepartment of Chemistry, Ulsan National Institute of Science and Technology (UNIST), 50 UNIST-gil, Ulsan 44919, Korea
First published on 31st July 2018
Abstract
A highly robust porous hydrogen-bonded organic framework (HOF) constructed by 4,4′,4′′-benzene-1,3,5-triyl-tris(benzoic acid) not only achieves the highest uptakes of ethylene and ethane among the HOF materials, but also exhibits unusual adsorption selectivity of C2H6 over other C2 gases. Besides, it exhibits the second highest acetylene uptake among all the reported HOF materials.
Hydrogen-bonded organic frameworks (HOFs), also referred to as supramolecular organic frameworks (SOFs), are promising porous organic crystalline materials that are self-assembled through multiple hydrogen bonding interactions and other non-covalent interactions like π–π stacking.1 Over the past few years, HOFs have attracted increasing interests due to their potential applications in several areas including hydrocarbon separation,2 CO2 separation,3 proton conduction,4 molecular capture5 and sensing.6 In addition, compared with other porous materials, they show inherent advantages such as solution processibility, easy purification, and reproducibility by simple recrystallization. However, the multiple non-covalent interactions generally do not guarantee the stability of a porous framework, thus it is difficult to maintain a permanent porosity upon solvent removal. Consequently, we still have witnessed a few permanently porous HOFs to date.
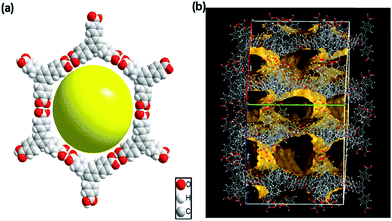
Carboxylic acids play an important role in directing various supramolecular structures and C3-symmetric planar carboxylic acids are of interest because they can form hexagonal networks with voids through the formation of carboxylic acid dimers.1,7 In fact, Hisaki and coworkers demonstrated that hexagonal networks prepared with various C3-symmetric planar carboxylic acids can be used as structural motifs for porous HOFs.8 Similarly, Zentner and coworkers recently reported a microporous HOF of C3-symmetric 4,4′,4′′-benzene-1,3,5-triyl-tris(benzoic acid) (H3BTB) via a hexagonal (6,3) honeycomb structural motif (Fig. 1a) in ethanol and propanols.9 The π–π stacking and eight-fold interpenetration of two-dimensional (2D) hexagonal sheets in this HOF-BTB generates one-dimensional (1D) solvent channel in the framework (Fig. 1b). Meanwhile, H3BTB is also known to form nonporous DMF solvate crystals (H3BTB–DMF) in N,N-dimethylformamide (DMF) where the carboxylic acids are masked by DMF molecules, prohibiting the formation of carboxylic acid dimers.10
Herein, we report single-component adsorption properties of light hydrocarbons (CH4, C2H2, C2H4, and C2H6) in a robust microporous hydrogen-bonded organic framework HOF-BTB prepared from the H3BTB–DMF crystals in methanol. We also report efficient selective separations of C2/CH4 binary gas mixtures in HOF-BTB. In our effort to find new self-assembled H3BTB structures, we found that H3BTB–DMF crystals readily recrystallize in methanol to produce clear, transparent crystals suitable for single crystal X-ray diffraction. H3BTB is practically insoluble in methanol so that it is inefficient to attempt direct crystallization of H3BTB in methanol. Instead, our finding shows that soaking H3BTB–DMF crystals in methanol at 50 °C facilitates recrystallization of H3BTB–DMF crystals and the resulting crystals were found to exhibit essentially the same structure as the previously reported HOF-BTB by Zentner.9 The bulk purity of HOF-BTB crystals is examined by powder X-ray diffraction (PXRD). An observed PXRD pattern of as-prepared HOF-BTB crystals is in good agreement to the simulated PXRD pattern obtained from the reported single crystal structure (Fig. 2a). The crystals of HOF-BTB were treated under high vacuum at 120 °C for 15 hours to generate an activated sample. The PXRD pattern of activated HOF-BTB crystals is identical to that of as-prepared HOF-BTB crystals, which means that the HOF-BTB crystals are very stable even after the removal of solvent molecules. Furthermore, we also found that the immersion of the activated crystals in water for 20 days has no effect on structural stability of the activated HOF-BTB crystals (Fig. S1, ESI†). Thermogravimetric analysis (TGA) curve indicates that HOF-BTB can be stable over 300 °C, resulted from the strong hydrogen bonding interactions as well as the consecutive strong π–π stacking interactions. In addition, the TGA curve of the activated HOF-BTB sample reveals that no residual solvent molecules remain in the activated HOF-BTB (Fig. 2b).
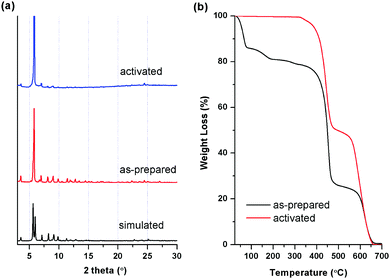 | ||
| Fig. 2 (a) Powder X-ray diffraction patterns and (b) TGA curves of as-prepared and activated HOF-BTB crystals. | ||
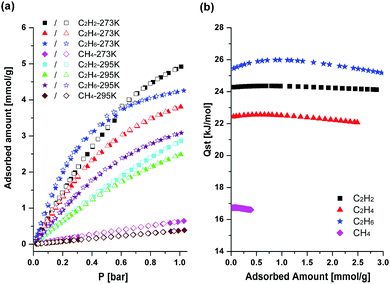
The high stability and the large solvent-accessible void space of HOF-BTB allowed us to investigate its permanent porosity. The N2 adsorption isotherm of the activated sample obtained at 77 K shows rapid N2 uptake at relative low pressures (P/P0 < 0.1), which is typical for a Type I isotherm curve and is also typical characteristic for microporous solids (Fig. S2a, ESI†). The total uptake of N2 at relative pressure P/P0 = 1 is 256 cm3 g−1. The BET and Langmuir surface areas are estimated to be 955 and 1128 m2 g−1, respectively. The mean pore diameter and total pore volume are determined to be 16.6 Å and 0.40 cm3 g−1, respectively. In addition, we calculated micropore size distribution using nonlocal density functional theory (NLDFT) method (Fig. S2b, ESI†). HOF-BTB has a very narrow micropore size distribution of 1.1 to 1.5 nm diameter and a distribution peak at 1.2 nm. The high surface area and total pore volume of HOF-BTB indicates that HOF-BTB possesses a highly porous structure. In order to evaluate the adsorption properties for small hydrocarbons, we performed the adsorption experiments for CH4, C2H2, C2H4, and C2H6 at 273 K and 295 K, respectively. Fig. 3a shows their adsorption isotherms at both temperatures. All adsorption isotherms are fully reversible in HOF-BTB, indicating pure physisorption processes and easy regeneration of HOF-BTB after gas adsorption. At 273 K, HOF-BTB is the most effective adsorbent for C2H2, taking up C2H2 up to 110.3 cm3 g−1 (4.92 mmol g−1) at 1 bar. The uptake capacities of HOF-BTB for C2H4 and C2H6 are also fairly high at 273 K and 1 bar, with the values of 85.3 cm3 g−1 (3.80 mmol g−1) and 95.4 cm3 g−1 (4.25 mmol g−1), respectively. Meanwhile, at 295 K and 1 bar, HOF-BTB shows a higher C2H6 uptake (69.2 cm3 g−1, 3.09 mmol g−1) than C2H2 (64.3 cm3 g−1, 2.87 mmol g−1) and C2H4 (55.7 cm3 g−1, 2.48 mmol g−1) uptakes. Among the C2 hydrocarbons, C2H4 has the lowest adsorption capacities at both temperatures. Interestingly, at 273 K, HOF-BTB adsorbs more of C2H6 than the others at lower pressures. As the pressure increases, the adsorption amount of C2H2 exceeds the adsorption amount of C2H6. At 295 K, on the other hand, the adsorption amount of C2H6 exceeds the adsorption amount of C2H2 and C2H4 in the entire pressure range. This is remarkable because selective adsorption of C2H6 over C2H2 and C2H4 is rarely observed in porous materials including MOFs and zeolites.11 The higher affinity for C2H6, the largest among C2 hydrocarbons, might suggest that the molecular sizes (C2H2, 3.32 × 3.34 × 5.70 Å; C2H4, 3.28 × 4.18 × 4.84 Å; C2H6, 3.81 × 4.08 × 4.82 Å)2e and/or kinetic diameters (C2H2, 3.3 Å; C2H4, 4.2 Å; C2H6, 4.4 Å)12 of the C2 hydrocarbons are less important in the adsorption of C2 hydrocarbons. It might be likely that van der Waals interactions between C2 hydrocarbons and HOF-BTB are more crucial factor for C2 hydrocarbons adsorption than the molecular sizes of the gases at lower pressures. The van der Waals interaction is generally stronger as the molecular size increases, so it can be expected that the largest molecule C2H6 is the most efficient to interact with HOF-BTB. It should be noted that HOF-BTB achieves the highest C2H4 and C2H6 uptakes among all the HOF materials reported to date (Table 1). Compared with covalent organic frameworks (COFs), C2H6 uptake of HOF-BTB is comparable to most reported COFs. ZnP-CTF-50013a and T-COF13b outperform the C2H6 uptake capacity of HOF-BTB (Table S1, ESI†). Furthermore, C2H4 uptake capacity of HOF-BTB surpasses those of the reported COFs such as DBA-3D-COF-113c and MCOF-1.13d It also exhibits the second highest C2H2 uptake among the HOF materials reported to date. Only HOF-5a exceeds the C2H2 uptake amount of HOF-BTB. In contrast to the high C2 hydrocarbons uptakes, much less amount of CH4, with the values of 14.5 and 8.7 cm3 g−1, is adsorbed in HOF-BTB at 273 K and 295 K, respectively. The loading dependent isosteric heats (Qst) of adsorption of C2 hydrocarbons are larger than that of CH4, which indicates that the stronger interactions between the C2 hydrocarbons and the pore surfaces of HOF-BTB than that of CH4 and HOF-BTB (Fig. 3b). For all the hydrocarbons, the values of Qst remain relatively constant through the whole loading ranges. High affinity for C2H6 is also evident in the Qst value. As shown in Fig. 3b, the Qst value of C2H6 is higher than those of C2H2 and C2H4. Compared with other HOF materials, zero-coverage isosteric heats of adsorption for C2H2 and C2H4 are smaller than those of reported HOFs (Table 1). These lower values of Qst suggest HOF-BTB would be a promising candidate for energy-saving separation of C2 hydrocarbons/CH4 mixtures.
| HOFs | SABET (m2 g−1) | C2H2 (cm3 g−1) | C2H4 (cm3 g−1) | C2H6 (cm3 g−1) | Q st (kJ mol−1) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 273 K | 295 K | 273 K | 295 K | 273 K | 295 K | C2H2 | C2H4 | C2H6 | |||
| SOF-1a | 474 | 61 | 50 | — | — | — | — | 36.2 | — | — | 2f |
| HOF-1a | 359 | 63 | 57 | 8.3 | 3.9 | — | — | 58.1 | 31.9 | — | 2e |
| HOF-3a | 165 | 58 | 47 | — | — | — | — | 18 | — | — | 2c |
| HOF-4a | 312 | — | — | 17.3 | 11.1 | 5.1 | 3.6 | — | 44 | 14 | 2d |
| HOF-5a | 1101 | 182 | 102 | — | — | — | — | 27.6 | — | — | 3c |
| HOF-BTB | 955 | 110.3 | 64.3 | 85.3 | 55.7 | 95.4 | 69.2 | 24.3 | 22.6 | 25.4 | This work |
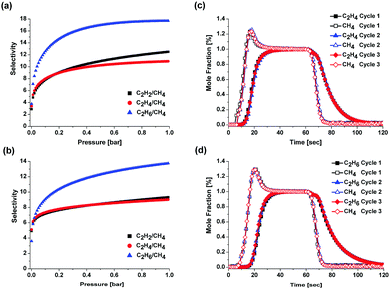
To analyse the binary gas mixture separation abilities, the pure single-component isotherms were fitted with the Langmuir–Freundlich isotherm model (Fig. S3–S6, ESI†).14 Then, ideal adsorbed solution theory (IAST)15 was used to predict the separation selectivity of binary gas mixtures (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) at 273 K and 295 K (Tables S2 and S3, ESI†). The IAST predicts that HOF-BTB would allow selective separations of C2 hydrocarbons over CH4. At both temperatures, C2H6/CH4 gas mixture shows the highest selectivity values among the C2 hydrocarbon/CH4 gas mixtures. The observed C2H6/CH4 selectivity at 295 K (14) is comparable with the IAST-predicted C2H6/CH4 selectivities for N-COF (18), P-COF (12) and T-COF (10) at 298 K.13b The IAST also predicts the fairly good separation selectivities for C2H2/CH4 and C2H4/CH4 binary gas mixtures (Fig. 4a and b). Although the IAST can predict the mixture behaviours in terms of adsorption equilibrium, it cannot reflect the mixture behaviours in terms of adsorption kinetics, which cannot be ignored in real adsorption separation processes. Hence, we performed dynamic breakthrough experiments to evaluate the potential of HOF-BTB for the adsorptive separation of C2H6/CH4 and C2H4/CH4 mixtures under dynamic flow conditions.16 Unfortunately, we could not perform the dynamic breakthrough experiment for C2H2/CH4 mixture due to the University's safety regulations. Fig. 4c and d show the experimental breakthrough curves of C2H4/CH4 and C2H6/CH4 mixtures on a column packed with HOF-BTB pellets. In the case of C2H6/CH4 separation, CH4 elutes first from the column, whereas C2H6 is strongly retained. This indicates that under mixture flow condition, HOF-BTB primarily adsorbs C2H6 rather than CH4. Similarly, the breakthrough curves for C2H4/CH4 mixture exhibit selective C2H4 adsorption over CH4. For all cases, breakthrough curves of weaker adsorbate CH4 show “roll-up” behaviours, which are normally explained as a result of the competitive adsorption between two adsorbate species. Although the weaker adsorbate molecules initially occupy the adsorption sites in the column, some of the adsorbed molecules are replaced by stronger adsorbate molecules.17 These experimental breakthrough results indicate that HOF-BTB can effectively separate C2H4/CH4 and C2H6/CH4 mixtures under dynamic flow conditions. Easy regeneration of the used adsorbent is an essential consideration for practical operation of an adsorptive separation process. After performing a breakthrough experiment, we regenerated the column by simply purging it for 10 min under a He flow of 40 ml min−1 without heating the column. As displayed in Fig. 4c and d, essentially identical breakthrough curves were produced during the three consecutive cycles for both gas mixtures. This is remarkable because the regenerations were performed under mild conditions. This mild regeneration condition could be attributed to the relatively small Qst values for C2 hydrocarbons.
50) at 273 K and 295 K (Tables S2 and S3, ESI†). The IAST predicts that HOF-BTB would allow selective separations of C2 hydrocarbons over CH4. At both temperatures, C2H6/CH4 gas mixture shows the highest selectivity values among the C2 hydrocarbon/CH4 gas mixtures. The observed C2H6/CH4 selectivity at 295 K (14) is comparable with the IAST-predicted C2H6/CH4 selectivities for N-COF (18), P-COF (12) and T-COF (10) at 298 K.13b The IAST also predicts the fairly good separation selectivities for C2H2/CH4 and C2H4/CH4 binary gas mixtures (Fig. 4a and b). Although the IAST can predict the mixture behaviours in terms of adsorption equilibrium, it cannot reflect the mixture behaviours in terms of adsorption kinetics, which cannot be ignored in real adsorption separation processes. Hence, we performed dynamic breakthrough experiments to evaluate the potential of HOF-BTB for the adsorptive separation of C2H6/CH4 and C2H4/CH4 mixtures under dynamic flow conditions.16 Unfortunately, we could not perform the dynamic breakthrough experiment for C2H2/CH4 mixture due to the University's safety regulations. Fig. 4c and d show the experimental breakthrough curves of C2H4/CH4 and C2H6/CH4 mixtures on a column packed with HOF-BTB pellets. In the case of C2H6/CH4 separation, CH4 elutes first from the column, whereas C2H6 is strongly retained. This indicates that under mixture flow condition, HOF-BTB primarily adsorbs C2H6 rather than CH4. Similarly, the breakthrough curves for C2H4/CH4 mixture exhibit selective C2H4 adsorption over CH4. For all cases, breakthrough curves of weaker adsorbate CH4 show “roll-up” behaviours, which are normally explained as a result of the competitive adsorption between two adsorbate species. Although the weaker adsorbate molecules initially occupy the adsorption sites in the column, some of the adsorbed molecules are replaced by stronger adsorbate molecules.17 These experimental breakthrough results indicate that HOF-BTB can effectively separate C2H4/CH4 and C2H6/CH4 mixtures under dynamic flow conditions. Easy regeneration of the used adsorbent is an essential consideration for practical operation of an adsorptive separation process. After performing a breakthrough experiment, we regenerated the column by simply purging it for 10 min under a He flow of 40 ml min−1 without heating the column. As displayed in Fig. 4c and d, essentially identical breakthrough curves were produced during the three consecutive cycles for both gas mixtures. This is remarkable because the regenerations were performed under mild conditions. This mild regeneration condition could be attributed to the relatively small Qst values for C2 hydrocarbons.
In summary, we have reported a highly robust microporous hydrogen-bonded organic framework HOF-BTB, which shows the higher adsorption capacities for C2 hydrocarbons at 273 K and 295 K. The adsorption amounts of C2H4 and C2H6 reach the highest values among all the reported HOFs, while C2H2 uptake amount reaches the second highest values among all the HOFs. Breakthrough experiments reveal that HOF-BTB can selectively separate C2H4 and C2H6 from CH4 under dynamic mixture flow conditions.
This work was supported by NRF (National Honor Scientist Program: 2010-0020414). This work was also supported by the National Research Foundation of Korea under Grant (NRF-2016R1A2B4014256). This work was also supported by “Next Generation Carbon Upcycling Project” (Project No. 2017M1A2A2043449) through the National Research Foundation (NRF) funded by the Ministry of Science and ICT, Republic of Korea (2017M1A2A2043449). We acknowledge the Pohang Accelerator Laboratory (PAL) for the use of the synchrotron 2D (SMC) beamline (2017-1st-2D-004 and 2017-2nd-2D-007). Experiments at Pohang Light Source (PLS) were supported in part by Ministry of Science ICT and Future Planning of Korea (MSIP) and Pohang University of Science and Technology (POSTECH).
Conflicts of interest
There are no conflicts to declare.Notes and references
- Y.-F. Han, Y.-X. Yuan and H.-B. Wang, Molecules, 2017, 22, 266 CrossRef PubMed.
- (a) A. Pulido, L. Chen, T. Kaczorowski, D. Holden, M. A. Little, S. Y. Chong, B. J. Slater, D. P. McMahon, B. Bonillo, C. J. Stackhouse, A. Stephenson, C. M. Kane, R. Clowes, T. Hasell, A. I. Cooper and G. M. Day, Nature, 2017, 543, 657 CrossRef PubMed; (b) F. Hu, C. Liu, M. Wu, J. Pang, F. Jiang, D. Yuan and M. Hong, Angew. Chem., Int. Ed., 2017, 56, 2101 CrossRef PubMed; (c) P. Li, Y. He, Y. Zhao, L. Weng, H. Wang, R. Krishna, H. Wu, W. Zhou, M. O’Keeffe, Y. Han and B. Chen, Angew. Chem., Int. Ed., 2015, 54, 574 Search PubMed; (d) P. Li, Y. He, H. D. Arman, R. Krishna, H. Wang, L. Weng and B. Chen, Chem. Commun., 2014, 50, 13081 RSC; (e) Y. He, S. Xiang and B. Chen, J. Am. Chem. Soc., 2011, 133, 14570 CrossRef PubMed; (f) W. Yang, A. Greenaway, X. Lin, R. Matsuda, A. J. Blake, C. Wilson, W. Lewis, P. Hubberstey, S. Kitagawa, N. R. Champness and M. Schröder, J. Am. Chem. Soc., 2010, 132, 14457 CrossRef PubMed.
- (a) R. S. Patil, D. Banerjee, C. Zhang, P. K. Thallapally and J. L. Atwood, Angew. Chem., Int. Ed., 2016, 55, 4523 CrossRef PubMed; (b) S. Nandi, D. Chakraborty and R. Vaidhyanathan, Chem. Commun., 2016, 52, 7249 RSC; (c) H. Wang, B. Li, H. Wu, T.-L. Hu, Z. Yao, W. Zhou, S. Xiang and B. Chen, J. Am. Chem. Soc., 2015, 137, 9963 CrossRef PubMed; (d) W. Yang, B. Li, H. Wang, O. Alduhaish, K. Alfooty, M. A. Zayed, P. Li, H. D. Arman and B. Chen, Cryst. Growth Des., 2015, 15, 2000 CrossRef; (e) J. Lü, C. Perez-Krap, M. Suyetin, N. H. Alsmail, Y. Yan, S. Yang, W. Lewis, E. Bichoutskaia, C. C. Tang, A. J. Blake, R. Cao and M. Schröder, J. Am. Chem. Soc., 2014, 136, 12828 CrossRef PubMed; (f) X.-Z. Luo, X.-J. Jia, J.-H. Deng, J.-L. Zhong, H.-J. Liu, K.-J. Wang and D.-C. Zhong, J. Am. Chem. Soc., 2013, 135, 11684 CrossRef PubMed; (g) M. Mastalerz and I. M. Oppel, Angew. Chem., Int. Ed., 2012, 51, 5252 CrossRef PubMed.
- (a) A. Karmakar, R. Illathvalappil, B. Anothumakkool, A. Sen, P. Samanta, A. V. Desai, S. Kurungot and S. K. Ghosh, Angew. Chem., Int. Ed., 2016, 55, 10667 CrossRef PubMed; (b) W. Yang, F. Yang, T.-L. Hu, S. C. King, H. Wang, H. Wu, W. Zhou, J.-R. Li, H. D. Arman and B. Chen, Cryst. Growth Des., 2016, 16, 5831 CrossRef.
- (a) W. Yan, X. Yu, T. Yan, D. Wu, E. Ning, Y. Qi, Y.-F. Han and Q. Li, Chem. Commun., 2017, 53, 3677 RSC; (b) T.-H. Chen, I. Popov, W. Kaveevivitchai, Y.-C. Chuang, Y.-S. Chen, O. Daugulis, A. J. Jacobson and O. Š. Miljanić, Nat. Commun., 2014, 5, 5131 CrossRef PubMed.
- (a) H. Wang, H. Wu, J. Kan, G. Chang, Z. Yao, B. Li, W. Zhou, S. Xiang, J. C.-G. Zhao and B. Chen, J. Mater. Chem. A, 2017, 5, 8292 RSC; (b) Z. Sun, Y. Li, L. Chen, X. Jing and Z. Xie, Cryst. Growth Des., 2015, 15, 542 CrossRef; (c) H. Zhou, Q. Ye, X. Wu, J. Song, C. M. Cho, Y. Zong, B. Z. Tang, T. S. Andy Hor, E. K. Lee Yeow and J. Xu, J. Mater. Chem. C, 2015, 3, 11874 RSC.
- O. Ivasenko and D. F. Perepichka, Chem. Soc. Rev., 2011, 40, 191 RSC.
- (a) I. Hisaki, S. Nakagawa, N. Ikenaka, Y. Imamura, M. Katouda, M. Tashiro, H. Tsuchida, T. Ogoshi, H. Sato, N. Tohnai and M. Miyata, J. Am. Chem. Soc., 2016, 138, 6671 CrossRef PubMed; (b) I. Hisaki, S. Nakagawa, N. Tohnai and M. Miyata, Angew. Chem., Int. Ed., 2015, 54, 3008 CrossRef PubMed.
- C. A. Zentner, H. W. H. Lai, J. T. Greenfield, R. A. Wiscons, M. Zeller, C. F. Campana, O. Talu, S. A. FitzGerald and J. L. C. Rowsell, Chem. Commun., 2015, 51, 11642 RSC.
- (a) S. B. Baek, D. Moon, R. Graf, W. J. Cho, S. W. Park, T.-U. Yoon, S. J. Cho, I.-C. Hwang, Y.-S. Bae, H. W. Spiess, H. C. Lee and K. S. Kim, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 14156 CrossRef PubMed; (b) E. Weber, M. Hecker, E. Koepp, W. Orlia, M. Czugler and I. Csöregh, J. Chem. Soc., Perkin Trans. 2, 1988, 1251 RSC.
- (a) P.-Q. Liao, W.-X. Zhang, J.-P. Zhang and X.-M. Chen, Nat. Commun., 2015, 6, 8697 CrossRef PubMed; (b) Y. Chen, H. Wu, D. Lv, R. Shi, Y. Chen, Q. Xia and Z. Li, Ind. Eng. Chem. Res., 2018, 57, 4063 CrossRef.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- (a) H. Ma, H. Ren, S. Meng, F. Sun and G. Zhu, Sci. Rep., 2013, 3, 2611 CrossRef PubMed; (b) J. Dong, Y. Wang, G. Liu, Y. Cheng and D. Zhao, CrystEngComm, 2017, 19, 4899 RSC; (c) L. A. Baldwin, J. W. Crowe, D. A. Pyles and P. L. McGrier, J. Am. Chem. Soc., 2016, 138, 15134 CrossRef PubMed; (d) H. Ma, H. Ren, S. Meng, Z. Yan, H. Zhao, F. Sun and G. Zhu, Chem. Commun., 2013, 49, 9773 RSC.
- J. A. Mason, K. Sumida, Z. R. Herm, R. Krishna and J. R. Long, Energy Environ. Sci., 2011, 4, 3030 RSC.
- A. L. Myers and J. M. Prausnitz, AIChE J., 1965, 11, 121 CrossRef.
- S.-J. Lee, K. C. Kim, T.-U. Yoon, M.-B. Kim and Y.-S. Bae, Microporous Mesoporous Mater., 2016, 236, 284–291 CrossRef.
- R. T. Yang, Adsorbents: Fundamentals and Applications, John Wiley & Sons, 2003 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of experimental procedures, supplementary tables and figures. See DOI: 10.1039/c8cc04139c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |