Gold-catalyzed [5+2] cycloaddition of quinolinium zwitterions and allenamides as an efficient route to fused 1,4-diazepines†
Nirupam
De
 a,
Choong Eui
Song
a,
Choong Eui
Song
 b,
Do Hyun
Ryu
b,
Do Hyun
Ryu
 b and
Eun Jeong
Yoo
b and
Eun Jeong
Yoo
 *ac
*ac
aDepartment of Applied Chemistry, Kyung Hee University, Yongin, 17104, Korea
bDepartment of Chemistry, Sungkyunkwan University, Suwon, 16419, Korea
cGlobal Center for Pharmaceutical Ingredient Materials, Kyung Hee University, Yongin, 17104, Korea. E-mail: ejyoo@khu.ac.kr; Fax: +82-31-201-2340; Tel: +82-31-201-3833
First published on 21st May 2018
Abstract
Herein, we demonstrate a new catalytic cycloaddition of quinolinium zwitterions involving a gold-bound allylic cation intermediate. This ligand-free higher-order cycloaddition efficiently affords a variety of fused 1,4-diazepine derivatives in a stereospecific manner at room temperature.
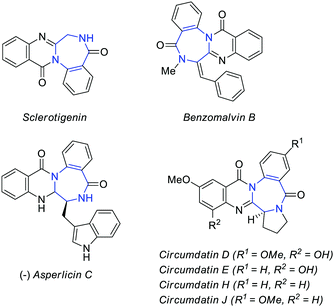
Polycyclic heterocycles are one of the most common skeletons found in natural products, biologically active compounds, and functional materials. Among these polycycles, fused 1,4-diazepine derivatives are of particular significance in pharmaceutical chemistry and can be found in Alprazolam, Flumazenil, and related drugs,1 as well as in bioactive natural products such as sclerotigenin, benzomalvins (A–C), asperlicins, and circumdatin analogues (A–G)2–5 (Fig. 1).
Higher-order cycloaddition is one of the most powerful and widely used synthetic methods for the construction of medium-sized heterocyclic skeletons.6 Although the application of dipolar cycloaddition to the synthesis of five- and six-membered heterocycles is well documented, examples of higher-order cycloadditions employing well-established 1,n-dipoles are still rare. One of the few examples includes the utilization of [4+3] cycloadditions of 1,3-dipoles to access seven-membered heterocycles.7 In comparison to the above-mentioned case, [5+2] cycloadditions have been underexplored because of the absence of practical 1,5-dipoles, e.g., most catalytic [5+2] cycloadditions employ vinylcyclopropanes and their derivatives as five-carbon synthons.8 Although we have previously reported the synthesis of seven- and eight-membered heterocycles using a unique isolable pyridinium zwitterion as a 1,5-dipole,9 the development of the respective cycloadditions is still in its infancy.
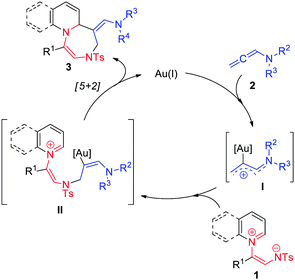
Activated allene substrates,10 especially allenamides, have been proved to be versatile and useful substrates in organic synthesis and have recently been used as two-carbon synthons in gold(I)-catalyzed cycloadditions. Since the first suggestion of the involvement of Au-bound allylic cation intermediates in these reactions, formal [2+2], [4+2], and [2+2+2] cycloadditions of allenamides with different types of 2π-dipolarophiles, such as indoles (Scheme 1a), olefins (1b), dienes (1c), and carbonyl compounds (1d) to afford four- or six-membered cyclic compounds have been established.10–12 Interestingly, in contrast to 2π-dipolarophiles, 1,n-dipoles have never been explored as allenamide partners in gold-catalyzed cycloadditions.
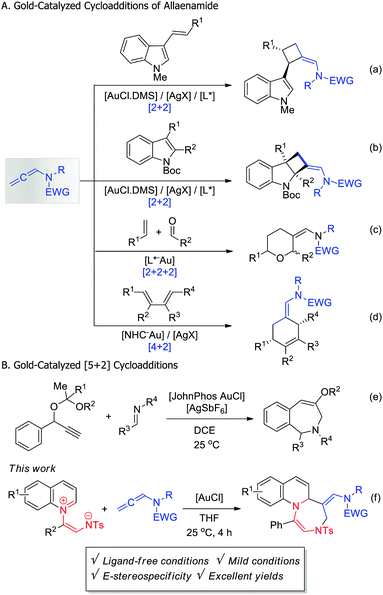 | ||
| Scheme 1 [A] Au-Catalyzed [m+2] cycloadditions of allenamides. [B] Au-catalyzed [5+2] cycloadditions. | ||
Herein, we describe a new gold(I)-catalyzed [5+2] cycloaddition of quinoline-based 1,5-dipoles and allenamides that furnishes fused 1,4-diazepine derivatives. Compared to the first and only example of gold(I)-catalyzed [5+2] cycloaddition of phenylpropargyl acetals (Scheme 1e),13 the developed cycloaddition is a very simple, mild, and efficient route to fused N-heterocyclic compounds.
Inspired by the well-studied Au-bound allylic cation intermediate I generated by the nucleophilic addition of the Au catalyst to allenamides (2),10d,e,11a we envisioned that the above-mentioned intermediate might be nucleophilically attacked by a zwitterionic 1,5-dipole (1) and could thus undergo [5+2] cycloaddition. As illustrated in Scheme 2, the gold catalyst is thought to initially activate the allenamide (2) to afford a Au-bound allylic cation (I), which is nucleophilically attacked by the nitrogen of 1 to generate a tethered intermediate II. Subsequently, the intramolecular cyclization of II affords seven-membered 1,4-diazepines (3) and leads to catalyst regeneration.
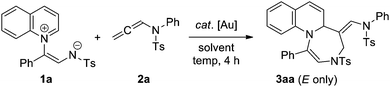
To test this hypothesis, we reacted quinolinium zwitterion 1a, which is more reactive than the previously reported pyridinium zwitterion, with allenamide 2a under various conditions (Table 1). To our delight, in the presence of AuCl3, the desired seven-membered ring 3aa was obtained in 62% yield in dichloromethane (DCM) at 40 °C (entry 1). Notably, this reaction stereospecifically furnished E-cycloadduct 3aa, as confirmed by 2D NMR and NOE experiments.14 Another Au(III) catalyst, dichloro(2-pyridinecarboxylato)gold, provided a better result under identical reaction conditions (entry 2). Although phosphine-chelated PPh3AuCl was completely ineffective in the absence of an Ag additive (entry 3), the sole use of AuCl afforded the best results even at room temperature (entry 4). Subsequently, diverse solvents were screened to optimize the reaction conditions (entries 5–10). The best results were observed with the relatively non-polar THF, which allowed for the desired products to be exclusively obtained within 4 h (entry 6). The role of the Au catalyst was confirmed by performing a control experiment under catalyst-free conditions (entry 11), which afforded intramolecular cyclized product 1a at high temperatures.14 Additionally, the [5+2] cycloaddition of the above-mentioned pyridinium zwitterion was unsuccessful because of the occurrence of a competing cascade [5+2]/[2+2] cycloaddition,14 which was very similar to that observed for the reaction of the same pyridinium zwitterion with benzyne.9c
| Entry | Au catalyst | Solvent | Temp. (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: quinolinium zwitterion 1a (0.1 mmol), allenamide 2a (1.3 equiv.), gold catalyst (5 mol%), and solvent (2.0 mL) for 4 h. b NMR yield of 3aa using CH2Br2 as an internal standard. c AuPy-cat: dichloro(2-pyridinecarboxylato)gold. d 60% product (3aa) was formed when AgSbF6 (5 mol%) was additionally added. e Isolated yield. f 2.5 mol% AuCl was used. | ||||
| 1 | AuCl3 | DCM | 40 | 62 |
| 2 | AuPy-catc | DCM | 40 | 86 |
| 3 | PPh3AuCld | DCM | 25 | 0 |
| 4 | AuCl | DCM | 25 | 88 |
| 5 | AuCl | DCE | 25 | 90 |
| 6 | AuCl | THF | 25 | 99 (96)e |
| 7 | AuClf | THF | 25 | 87 |
| 8 | AuCl | Toluene | 25 | 24 |
| 9 | AuCl | 1,4-Dioxane | 25 | 92 |
| 10 | AuCl | THF | 25 | 87 |
| 11 | — | THF | 25 → 80 | 0 |
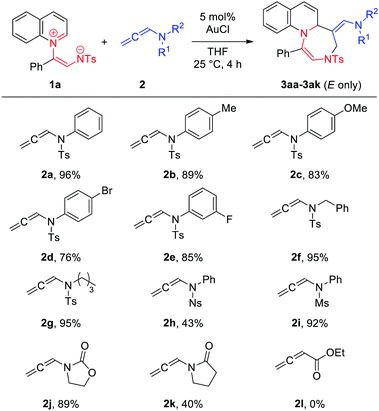
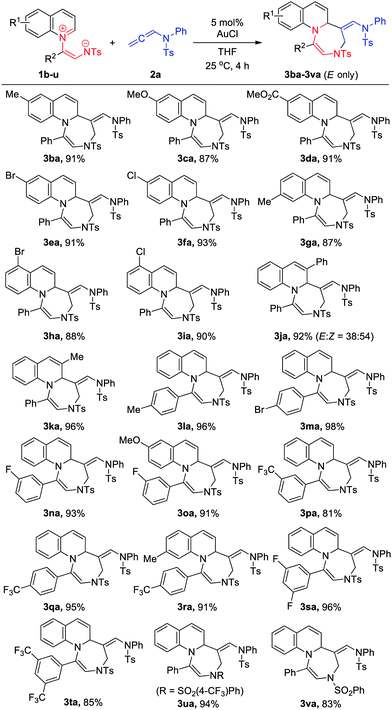
With the optimized conditions in hand, we then explored the scope of [5+2] cycloadditions using different allenamide substrates (Table 2). A wide range of allenamides furnished the desired cycloadducts in good to excellent yields irrespective of the electronic nature of the N-substituent in 2a–2e. Both N-benzyl and N-alkyl allenamide variants (2f and 2g, respectively) were tolerated. Compared to the reactions of N-tosyl- or N-mesyl-substituted allenamides, that of N-nosyl-substituted allenamide 2h was rather sluggish. Interestingly, although oxazolidone-based allenamide 2j was found to efficiently undergo [5+2] cycloaddition, the corresponding pyrrolidone derivative 2k was less effective under the optimized reaction conditions. Notably, no reaction was observed for allenoate analogue 2l. Moreover, 1,1- and 1,3- disubstituted allenamides were found to be ineffective in this protocol.
Next, higher-order cycloadditions between quinolinium zwitterions 1 and allenamide 2a were tested to confirm the generality and stereoselectivity of this method (Table 3). As a result, it was found that, except for 1j, 1,5-dipoles 1 bearing both electron-donating and electron-withdrawing substituents on the quinoline backbone (1b-1k) were well tolerated and exclusively provided (E)-cycloadducts. The proximal 3-phenyl group of the quinoline backbone adjacent to the reaction site was believed to significantly impact product stereoselectivity, whereas no considerable change was observed for the corresponding methyl analogues 1k. Quinoline zwitterions bearing various aryl substituents on the enamide moiety of 1,5-dipoles (1l-1t) also provided the desired adducts in excellent yields and stereospecificities.
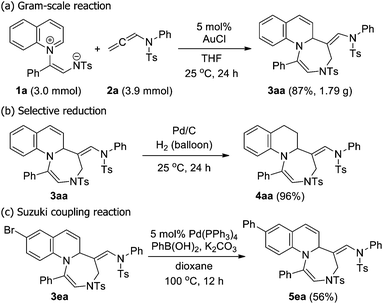
To further demonstrate the synthetic utility of the developed method, a gram-scale [5+2] cycloaddition was carried out (Scheme 3), and the E-cycloadduct was exclusively isolated in 87% yield (1.79 g), which indicated that the reaction efficiency and stereospecificity were not affected by the scale-up. Additionally, the obtained product underwent smooth reduction or cross-coupling to furnish the corresponding cyclic compounds (4aa and 5ea, respectively) in acceptable yields. However, the attempted exocyclic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleavage via ozonolysis was unsuccessful, thereby resulting in the decomposition of the obtained 1,4-diazepine.
C bond cleavage via ozonolysis was unsuccessful, thereby resulting in the decomposition of the obtained 1,4-diazepine.
In conclusion, in this study, we developed a new ligand-free gold-catalyzed [5+2] cycloaddition of quinolinium zwitterions and allenamides that provides stereospecific access to fused 1,4-diazepine derivatives in good to excellent yields. A wide range of substrates were tolerated for both reagents, and a gram-scale feasibility test proved the high potential and versatility of this protocol. The development of an enantioselective [5+2] cycloaddition is currently underway in our laboratory.
This research was supported by the National Research Foundation of Korea (NRF) (NRF-2016R1A2B4015351, NRF- 2016R1A4A1011451) funded by the Korea government and the GRRC program of Gyeonggi province (GRRCKyungHee2018-A01).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) G. A. Archer and L. H. Sternbach, Chem. Rev., 1968, 68, 747 CrossRef; (b) L. H. Sternbach, Angew. Chem., Int. Ed. Engl., 1971, 10, 34 CrossRef PubMed; (c) H. Mohler and T. Okada, Science, 1977, 198, 849 CrossRef PubMed; (d) G. E. Pakes, R. N. Brogden, R. C. Heel, T. M. Speight and G. S. Avery, Drugs, 1981, 22, 81 CrossRef PubMed; (e) D. Berezhnoy, Y. Nyfeler, A. Gonthier, H. Schwob, M. Goeldner and E. Sigel, J. Biol. Chem., 2004, 279, 3160 CrossRef PubMed; (f) C. Mombereau, K. Kaupmann, H. van der Putten and J. F. Cryan, Eur. J. Pharmacol., 2004, 497, 119 CrossRef PubMed.
- (a) B. K. Joshi, J. B. Gloer, D. T. Wicklow and P. F. Dowd, J. Nat. Prod., 1999, 62, 650 CrossRef PubMed; (b) T. O. Larsen, K. Frydenvang and J. C. Frisvad, Biochem. Syst. Ecol., 2000, 28, 881 CrossRef.
- H. H. Sun, C. J. Barrow, D. M. Sedlock, A. M. Gillum and R. Cooper, J. Antibiot., 1994, 47, 515 CrossRef PubMed.
- (a) M. A. Goetz, M. Lopez, R. L. Monaghan, R. S. L. Chang, V. J. Lotti and T. B. Chen, J. Antibiot., 1985, 38, 1633 CrossRef PubMed; (b) L. Rahbæk, J. Breinholt, J. C. Frisvad and C. Christophersen, J. Org. Chem., 1999, 64, 1689 CrossRef; (c) L. Rahbæk and J. Breinholt, J. Nat. Prod., 1999, 62, 904 CrossRef PubMed; (d) F. He, B. M. Foxman and B. B. Snider, J. Am. Chem. Soc., 1998, 120, 6417 CrossRef.
- J.-R. Dai, B. K. Carté, P. J. Sidebottom, A. L. S. Yew, S.-B. Ng, Y. Huang and M. S. Butler, J. Nat. Prod., 2001, 64, 125 CrossRef.
- (a) F. López and J. L. Mascareñas, Beilstein J. Org. Chem., 2011, 7, 1075 CrossRef PubMed; (b) D. Garayalde and C. Nevado, ACS Catal., 2012, 2, 1462 CrossRef; (c) F. López and J. L. Mascareñas, Chem. Soc. Rev., 2014, 43, 2904 RSC; (d) N. De and E. J. Yoo, ACS Catal., 2018, 8, 48 CrossRef.
- For [4+3] cycloaddition review see: (a) A. Hosomi and Y. Tominaga, in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon, Oxford, 1991, vol. 5, p. 593 Search PubMed; (b) M. Harmata and P. Rashatasakhon, Tetrahedron, 2003, 59, 2371 CrossRef; (c) B. Niess and H. M. R. Hoffmann, Angew. Chem., Int. Ed., 2005, 44, 26 CrossRef PubMed; (d) M. Harmata, Chem. Commun., 2010, 46, 8886 RSC ; For gold-catalyzed [4+3] cycloaddition see: ; (e) S. K. Pawar, R. L. Sahani and R.-S. Liu, Chem. Eur. J., 2015, 21, 10843 CrossRef PubMed.
- For [5+2] cycloaddition see: (a) P. A. Wender, G. G. Gamber and T. J. Williams, in Modern Rhodium-Catalyzed Organic Reactions, ed. P. A. Evans, WILEY-VCH, Weinheim, Germany, 2005, Chapter 13 Search PubMed; (b) H. Kusama, Y. Suzuki, J. Takaya and N. Iwasawa, Org. Lett., 2006, 8, 895 CrossRef PubMed; (c) H. Pellissier, Adv. Synth. Catal., 2011, 353, 189 CrossRef; (d) K. E. O. Ylijoki and J. M. Stryker, Chem. Rev., 2013, 113, 2244 CrossRef PubMed; (e) A. Seoane, N. Casanova, N. Quiñones, J. L. Mascareñas and M. Gulías, J. Am. Chem. Soc., 2014, 136, 834 CrossRef PubMed; (f) J.-J. Feng, T.-Y. Lin, H.-H. Wu and J. Zhang, J. Am. Chem. Soc., 2015, 137, 3787 CrossRef PubMed.
- (a) D. J. Lee, H. S. Han, J. Shin and E. J. Yoo, J. Am. Chem. Soc., 2014, 136, 11606 CrossRef PubMed; (b) D. J. Lee, D. Ko and E. J. Yoo, Angew. Chem., Int. Ed., 2015, 54, 13715 CrossRef PubMed; (c) J. Shin, J. Lee, D. Ko, N. De and E. J. Yoo, Org. Lett., 2017, 19, 2901 CrossRef PubMed.
- For gold-catalyzed [2+2] cycloaddition of allenamides see: (a) X.-X. Li, L.-L. Zhu, W. Zhou and Z. Chen, Org. Lett., 2012, 14, 436 CrossRef PubMed; (b) S. Suárez-Pantiga, C. Hernández-Díaz, E. Rubio and J. M. González, Angew. Chem., Int. Ed., 2012, 51, 11552 CrossRef PubMed; (c) S. Suárez-Pantiga, C. Hernández-Díaz, M. Piedrafita, E. Rubio and J. M. González, Adv. Synth. Catal., 2012, 354, 1651 CrossRef; (d) H. Faustino, P. Bernal, L. Castedo, F. López and J. L. Mascareñas, Adv. Synth. Catal., 2012, 354, 1658 CrossRef; (e) M. Jia, M. Monari, Q.-Q. Yang and M. Bandini, Chem. Commun., 2015, 51, 2320 RSC; (f) H. Hu, Y. Wang, D. Qian, Z.-M. Zhang, L. Liu and J. Zhang, Org. Chem. Front., 2016, 3, 759 RSC.
- For gold-catalyzed [4+2] cycloaddition of allenamides see: (a) H. Faustino, F. López, L. Castedo and J. L. Mascareñas, Chem. Sci., 2011, 2, 633 RSC; (b) J. Francos, F. Grande-Carmona, H. Faustino, J. Iglesias-Sigüenza, E. Díez, I. Alonso, R. Fernández, J. M. Lassaletta, F. López and J. L. Mascareñas, J. Am. Chem. Soc., 2012, 134, 14322 CrossRef PubMed; (c) Y. Wang, P. Zhang, Y. Liu, F. Xia and J. Zhang, Chem. Sci., 2016, 6, 5564 RSC ; For gold-catalyzed [3+2] cycloaddition of allenamides see: ; (d) R. R. Singh, S. K. Pawar, M.-J. Huang and R.-S. Liu, Chem. Commun., 2016, 52, 11434 RSC.
- For gold-catalyzed [2+2+2] cycloaddition of allenamides see: (a) I. Varela, H. Faustino, E. Díez, J. Iglesias-Sigüenza, F. Grande-Carmona, R. Fernández, J. M. Lassaletta, J. L. Mascarenãs and F. López, ACS Catal., 2017, 7, 2397 CrossRef; (b) H. Faustino, I. Alonso, J. L. Mascareñas and F. López, Angew. Chem., Int. Ed., 2013, 52, 6526 CrossRef PubMed.
- N. Iqbal and A. Fiksdahl, J. Org. Chem., 2013, 78, 7885 CrossRef PubMed.
- See the ESI,† for details.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8cc02570c |
| This journal is © The Royal Society of Chemistry 2018 |