Low-voltage-driven soft actuators
Onnuri
Kim
 ,
Seung Jae
Kim
and
Moon Jeong
Park
,
Seung Jae
Kim
and
Moon Jeong
Park
 *
*
Department of Chemistry, Pohang University of Science and Technology (POSTECH), Pohang, 790-784, Korea. E-mail: moonpark@postech.ac.kr
First published on 27th March 2018
Abstract
Soft actuators based on electroactive polymers (EAPs) are the core constituents of future soft robots owing to their fascinating properties such as lightweight, compactness, easy fabrication into various forms, and low cost. Ionic EAP actuators are particularly attractive owing to the low driving voltages (<3 V) as compared to those of electronic EAP actuators (usually kilovolts). This paper presents a brief overview of the recent progress in a range of EAP actuators by focusing on low voltage operation, in addition to the challenges and future strategies for their wide applicability in artificial muscles and various innovative soft robot technologies.
Introduction
There is a growing interest in future soft robotics, which are represented by highly compliant materials, flexibility, and good adaptability.1–3 While the robots in the past were developed for repetitive labor, future robot technologies aim to enhance human's strength and ability with artificial intelligence;4,5 for example, wearable exoskeletons. As one of the categories of wearable exoskeletons, wearable robotic orthoses have been in the spotlight in recent years for redefining our life in the ‘homo-hundred’ era.6,7The challenge lies in making these soft robots portable and truly wearable so that patients can have a social life. The innovation of actuators will be the key to featuring these robots, offering lightweight actuators with minimized volumes of other parts such as motors, gears, and battery accessories. However, actuator technology is still in the nascent stage.
Polymer actuators that show mechanical deformation in response to a given physical stimulus (e.g., electricity,8,9 light,10,11 temperature,12–14 and pressure15) and a chemical stimulus (e.g., solvent vapor16 and pH change17) are promising candidates for realizing such robots. Among the various types of polymer actuators, those based on electroactive polymers (EAPs) are the long-standing actuator technologies. EAP actuators are classified into electrical EAP actuators and ionic EAP actuators, where their actuation mechanisms differ from electrostatic forces between two electrodes under several kilovolts (which compress the polymer thickness and expand the area) to ion flow inside the polymer layer under a few volts (which causes asymmetric volume changes near each electrode).18 Ionic EAP actuators are especially attractive owing to their striking characteristics of low driving voltages towards artificial muscles and biomimetic applications. Simple mechanical compliances and scalability are also great prospects for ionic EAP actuators.
For over 20 years, various types of EAPs that can be operated under low voltage conditions have been developed, including conjugated polymers,19–22 ionic polymer–metal composites (IPMCs),23–25 bucky gel polymers,26–29 and interpenetrating polymer networks (IPNs).30–33 Although several EAP materials and their properties have been known for many decades, they have found very limited applications and have not replaced traditional actuators (electromagnetic, pneumatic, hydraulic, and piezoelectric) because of poor actuation performance.
Conjugated polymer actuators show fast actuation speed; however, they require contact with water (or organic solvents) to achieve durable and large mechanical deformation, limiting their practical applications.34,35 IPMC actuators and bucky gel actuators, which are so-called dry actuators based on commercially available fluorinated polymers and ionic liquids, have thus attracted immense interest.36–38 There have been considerable efforts to obtain high levels of strain for such actuators; unfortunately, slow switching speed39–41 and back relaxation36,42 remain a challenge. Another long-standing problem of most low-voltage EAP actuators is the low generated force (a few tens of millinewton),43 which is far below the value of Newtonian force that the current biomechanics require.44 Some of these problems have begun to be addressed recently based on paradigm shifts in material designs, leading to unprecedented electromechanical properties.45,46
In this review article, we describe the recent progress in advancing EAP actuator technologies that have been made in the last 20 years with a focus on low driving voltages and operation in air. Along with discussion on the major problems solved, the challenges encountered and future strategies towards practically viable actuators will be addressed. Furthermore, we will contemplate the benefits of using phase-separated block copolymers and single-ion conducting polymers to achieve improved bending strain and rapid switching motion. Based on the discussion on the above mentioned aspects, we will describe the prospects of obtaining future soft actuators with low activation voltage, rapid response times, large generated force, and various actuation motions.
Conjugated polymer actuators
The first conjugated polymer actuators operable under ambient conditions were reported by Mattes et al. in 2002 by the introduction of ionic liquid electrolytes.47 This was a significant innovation beyond aqueous or organic electrolytes to improve the electrochemical stability and cycle life. This also stimulated the transformation of an actuator structure from traditional bi-layer to tri-layer by sandwiching a porous separator filled with ionic liquid electrolytes between conjugated polymer electrodes.48,49Fig. 1 schematically illustrates the structure and actuation mechanism of tri-layer conjugated polymer actuators. The commonly employed conjugated polymers in such actuators are polypyrrole (PPy),49–51 polyaniline (PANI),52 and poly(3,4-ethylenedioxythiophene):polystyrenesulfonate (PEDOT:PSS).43,53–55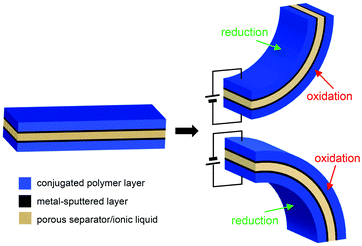 | ||
| Fig. 1 Schematic illustrations of the tri-layer structure and actuation mechanism for the conjugated polymer actuators. | ||
While the actuators comprising ionic liquids were operable in air at low driving voltages (<5 V), the poor charge transport properties between the conjugated polymer electrodes and polymer separator posed a hindrance, requiring additional metal sputtering on both sides of the separator.56 Furthermore, the low electronic conductivity of conjugated polymers in the de-doped state (reduced state) became an inherent drawback of conjugated polymer actuators.57
This prompted extensive research efforts into the modification of conjugated polymers with nanocarbon materials to attain a synergy between Faradaic doping/de-doping of conjugated polymers and electrical charging/discharging of nanocarbons.43,51,53 Examples include PPy/graphene,51 PEDOT:PSS/multi-walled carbon nanotube (MWCNT),43 and PEDOT:PSS/co-doped reduced graphene oxide (rGO).53 By controlling the intermolecular interactions (π–π interaction and charge–charge interaction) and thus the morphology of the composite electrodes, thereby optimizing the electrical conductivity and mechanical strength, a reversible large bending strain of a few percent could be achieved at low driving voltages (<3 V) with excellent cycle stability up to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.43,53
000 cycles.43,53
The introduction of additional interfacial polymeric layers between the conjugated polymer electrode and polymer layer has also been attempted by Oh et al. to eliminate metal sputtering.54 By facilitating ion hopping across the electrolyte/electrode interfaces, a low-voltage actuation performance, i.e., a peak-to-peak strain of 0.05% at ±0.5 V and 0.1 Hz, was achieved.
In contrast to the great interest in electrode materials, only a small number of studies have been made to develop/modify the electrolyte layers. This is because concurrent optimization of ion migration in the electrolyte layers and the electrochemical doping processes of the conjugated electrodes is difficult. Examples of electrolyte studies include the use of porous bacterial cellulose by Oh et al.55 and IPN based on polyethylene oxide (PEO) by Vidal et al.30,31 The main aim of such approaches is to yield good adhesive properties between electrodes and electrolytes to find applications in flexible micro-electromechanical systems.
IPMC actuators
Along with the research interest in conjugated polymer actuators, IPMC actuators have been widely investigated to develop durable electromechanical transducers.23,24 IPMC actuators are composed of an ionic polymer sandwiched between metal electrodes. If the redox reactions in the electrodes are the major actuation mechanism of conjugated polymer actuators, ion migration across the polymer layer under an applied voltage drives the bending motion of IPMC actuators.Pioneered by Leo et al.,23 IPMC actuators based on Nafion™ containing ionic liquids were successfully operated in air; however, the actuation performance was considerably lower at high frequencies compared to those of the actuators in contact with water. The key strategies for achieving high-performance IPMC actuators are the use of ionic liquids and/or the control of charge distribution in polymer electrolytes for better ion migration dynamics and fast electromechanical response.24
Zhang et al. reported a series of systematic studies on IPMC actuators comprising ionic liquid-containing Nafion™ by varying the type of ionic liquid.58 The binding energy and diffusion coefficients of the ionic liquid cation and anion were controlled to tune the charging time and bending strain of the actuators. The effects of type of polymer on the actuation performance were also explored by employing a few commercially available polymers, highlighting the importance of electromechanical coupling between the ionic liquid and polymer matrix to eliminate the back relaxation behaviour.39 Jho et al. investigated IPMC actuators by modifying commercially available poly(vinylidene fluoride) (PVdF) with grafted cationic or anionic moieties, showing precise control over the actuation performance with attached ionic side chains.40
Likewise, more extensive research on polymer layers has been conducted for IPMC actuators than for conjugated polymer actuators; however, they are still limited to commercially available polymers.23,24,39,40,58Fig. 2 shows the chemical structures of various polymers that have been employed for IPMC actuators by several research groups.
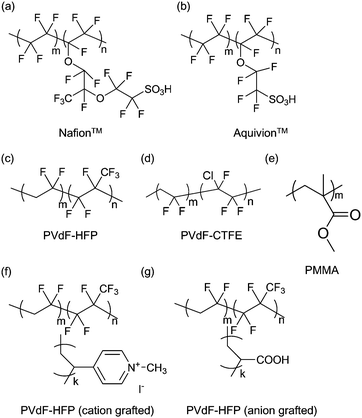 | ||
| Fig. 2 Chemical structures of various polymers that have been employed for IPMC actuators: (a) Nafion™, (b) Aquivion™, (c) PVdF-HFP, (d) PVdF-CTFE, (e) PMMA, (f) cation-grafted PVdF-HFP, and (g) anion-grafted PVdF-HFP. (f) and (g) are reproduced from ref. 40 with the permission of American Chemical Society. | ||
There are some notable in-depth studies on ion distribution across a polymer electrolyte layer in IPMC actuators. Zhang et al. mapped the ion migration profile near each electrode surface under device operation by employing time-of-flight secondary ion mass spectrometry.59 The excess ion accumulation/depletion at both the cathode and anode was observed to be closely related to the strain generated in the actuators. Ion dynamics in IPMC actuators was further analysed by Elabd et al. by in situ attenuated total reflectance-surface-enhanced infrared absorption spectroscopy (ATR-SEIRAS), as shown in Fig. 3.60 It has been revealed that the ionic liquid cation is the dominant charge carrier; however, aggregates of cation/anion move together. This is a very important fundamental underlying the actuation mechanisms of polymer actuators comprising ionic liquids.
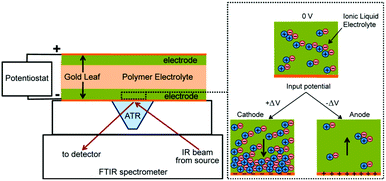 | ||
| Fig. 3 In situ ion dynamics measurements in IPMC actuators comprising ionic liquid-embedded Nafion™ by ATR-SEIRAS. The enlarged image on the right shows ion detection at the gold/electrode interface during the measurements under a given dc voltage. Figure is adapted from ref. 60 with the permission of American Chemical Society. | ||
Bucky gel actuators
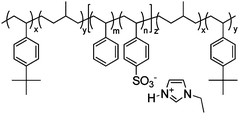
Another category of dry-state polymer actuators is the bucky gel actuator, pioneered by Aida et al.26–29,61 Using a simple layer-by-layer casting method, tri-layer actuators comprising SWCNTs/PVdF-HFP/SWCNTs were fabricated with ionic liquids embedded in both the SWCNT electrodes and PVdF-HFP layer. The SWCNT electrodes were flexible, enabling the development of the so-called ‘fully plastic’ actuators. Fig. 4 shows the bimorph configuration of the bucky gel actuators.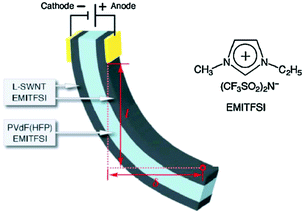 | ||
| Fig. 4 Schematic bimorph configuration of the actuator strip and chemical structure of the ionic liquid used. Figure is reproduced from ref. 28 with the permission of Wiley-VCH. | ||
For the very first bucky gel actuators, the obtained bending strain was 0.9% and the generated stress was 0.1 MPa under ±3.5 V at 0.01 Hz—the highest properties among those reported for low-voltage actuators at that time. However, the actuation performance deteriorated with increasing frequency, which remains a problem for bucky gel actuators (and IPMC actuators).
Successive work of the authors led to the development of high-performance bucky gel actuators by the introduction of super-growth (SC)-SWCNTs.28 The actuators demonstrated an order of magnitude greater bending displacement (2.28% s−1, 3.26 MPa s−1) than that of the first bucky gel actuator in quick response to ±2.5 V. Durable actuation was demonstrated for more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times in air at ±1.0 V and 1 Hz. Most importantly, when the applied frequency was increased from 1 to 10 Hz, the displacement decreased only by ca. 20%.
000 times in air at ±1.0 V and 1 Hz. Most importantly, when the applied frequency was increased from 1 to 10 Hz, the displacement decreased only by ca. 20%.
This drew the attention of many researchers in the era of bucky gel actuators on the importance of electrode materials. Asaka et al.62,63 employed carbide-derived carbon, SWCNTs, MWCNTs and carboxyl-functionalized MWCNTs for actuator electrodes and demonstrated the role of porosity and surface area of the active materials in enhancing the actuator performance. Xie et al.64 employed the as-grown SWCNT films having a unique hierarchical structure and high electrical and mechanical properties, which resulted in orders-of-magnitude improvements in actuator displacement, including a superfast actuation response (19 ms) and a large stress rate (1080 MPa s−1). Electrochemical kinetic models indicated that steric repulsion and charge injection in the electrodes are important for improving the electromechanical properties of actuators.
Chen et al. further employed a hierarchically architectured electrode based on vertically aligned nickel oxide nanowalls, rGO, and MWCNTs.65 The large specific surface area of the electrode and fast ion diffusion channels at the interface enabled large deformation in quick switching response (8.31% s−1, 12.16 MPa s−1) and good durability up to 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles in air. Fig. 5 depicts the actuation mechanism of the hierarchically nanostructured electrodes that improves the performance of bucky gel actuators.
000 cycles in air. Fig. 5 depicts the actuation mechanism of the hierarchically nanostructured electrodes that improves the performance of bucky gel actuators.
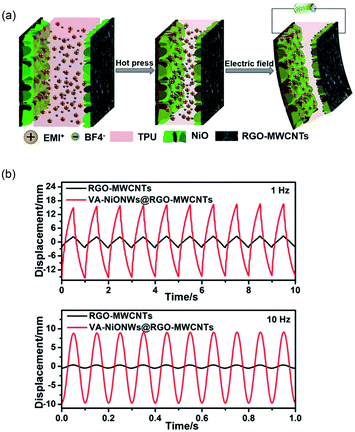 | ||
| Fig. 5 (a) Schematic illustration of the structure and assembly procedure of the actuators based on a hierarchically architectured electrode. (b) Comparison of the bending performance of the actuators with and without vertically aligned NiO nanowires with an applied voltage of 2.5 V with frequencies of 1 Hz and 10 Hz. Figure is reproduced from ref. 65 with the permission of Royal Society of Chemistry. | ||
Next, we explore the effects of a polymer layer on the bending displacement and switching speed of bucky gel actuators. The most important ingredient enabling double-layer charging in actuators is an ionic liquid. Therefore, one can expect the actuation performance to be primarily associated with charge migration dynamics, concentration polarization derived from dissimilar ion diffusivities, and van der Waals volumes of the cations and anions of the ionic liquids.
In this regard, the relationship between the type of ionic liquid and actuation performance has attracted immense interest over the past decades.61,66,67 A diverse combination of cations and anions in ionic liquids was chosen by several research groups (Asaka et al. and Terasawa et al.) using commercially available polymers.66,67 Nevertheless, the literature does not show clear correlation between the inherent properties of ionic liquids and actuator performance, and conclusions were rather case-by-case. This is because the ionic conductivity and ion transference number of ionic liquid-embedded polymers are not always consistent with the values observed in neat ionic liquids owing to the interactions of ions with the polymer matrix. In this respect, the model fits to the experimental data based on ion diffusion coefficients and the cation/anion transference number in the polymer layers, developed by Watanabe et al., are noteworthy.29
EAP actuators based on nanostructured block copolymers
While there have been advances in improving the dry polymer actuator technologies, the design and synthesis of new polymers have been significantly lacking. In 2012, the first study on introducing new synthetic polymers to bucky gel actuators and IPMC actuators was reported by Watanabe et al.29,68 and Long et al.,41,69–72 respectively. The focuses of the research were particularly directed to the design of block copolymer electrolytes to achieve nanoscale ionic channels. This was based on the prediction that block copolymer electrolytes can contribute to faster and greater ion migration at low activation potentials along organized ion diffusion pathways. Fig. 6 schematically depicts the commonly projected electromechanical actuation mechanism of the actuators comprising self-assembled block copolymer electrolytes.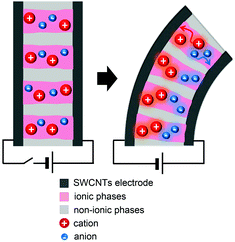 | ||
| Fig. 6 Schematic illustration of the electromechanical actuation mechanism for the actuators comprising self-assembled block copolymer electrolytes under an applied voltage. | ||
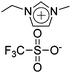
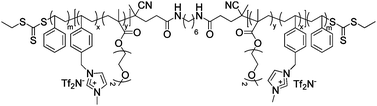
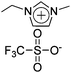
Long et al. had synthesized pentablock copolymers based on sulfonated midblock69 and triblock copolymers using imidazolium-tethered midblocks.41 With selective incorporation of ionic liquids into the midblocks, microphase-separated ionic domains could be formed in the polymer layers. The resultant IPMC actuators exhibited large bending curvatures, surpassing that of a Nafion™-containing actuator, at an applied voltage of 4 V. The authors had also synthesized zwitterion-tethered triblock copolymers, wherein additional loadings of ionic liquids offered fine-tuning of the actuation performance.70 Nevertheless, the issue of slow actuation response (a few tens of seconds) was unresolved.
Subsequent studies by the authors demonstrated more advanced IPMC actuators by synthesizing triblock copolymers based on imidazolium-tethered midblocks combined with short ether moieties.71 A direct comparison with actuators comprising random copolymer analogues revealed that the formation of phase-separated morphologies in polymer electrolyte layers is the key to improving the actuation performance. However, slow actuation response and back relaxation remained a challenge.
Koo et al. showed positive prospects of nanocomposite polymer electrolytes in IPMC actuators.72 By using the same midblock sulfonated pentablock copolymers as those used by Long et al., the intercalation of surface-sulfonated montmorillonite into the microdomains of block copolymers not only enhanced the bending strain, but also eliminated the back relaxation behaviour. This was ascribed to the improved stress/strain rate and the creation of highly connected ion conduction pathways.
In terms of lowering the driving voltages and achieving fast switching speed for EAP actuators, the most prominent results were reported by Park et al.73,74 By synthesizing end-block sulfonated diblock and triblock copolymers, very well-defined ionic channels with optimized viscoelastic properties were implemented with embedded ionic liquids, which yielded much better actuation properties than any previously reported, i.e., a large generated strain (up to 4%) without any evidence of back relaxation. In particular, the authors made noteworthy progress in the era of low-voltage driven actuators by demonstrating millimetre-scale displacements with fast switching time (<1 s) under sub-1V conditions over 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cycles in air (Fig. 7).73
500 cycles in air (Fig. 7).73
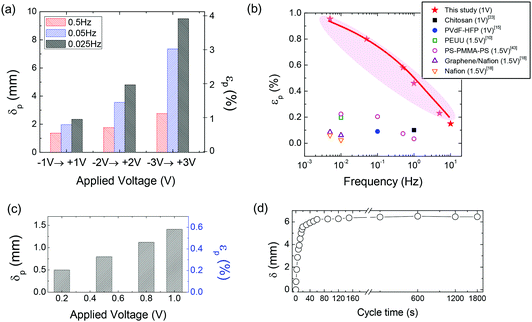 | ||
| Fig. 7 (a) Displacement (δ)/bending strain (ε)–frequency–voltage dependency of the actuator comprising a sulfonated block copolymer and ionic liquid with ±3 V. (b) Strain–frequency–voltage dependency of the actuator, compared with those reported in literature with a focus on low-voltage operation. (c) Actuation performance obtained under sub-1 V conditions measured at the tip position of the actuator strip every 1 s with step changes in voltage from 0 to 0.2, 0 to 0.5 and 0 to 0.8 V. (d) Time-dependent displacement of the actuator at 3 V, demonstrating the absence of back relaxation. Figure is adapted from ref. 73 with the permission of the Nature publishing group. | ||
Table 1 summarizes the performance of low-voltage EAP actuators comprising self-assembled polymers developed so far.
| Block copolymers | Ionic liquids | Actuation performance | Ref. |
|---|---|---|---|
|
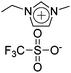
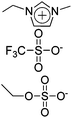
|
IPMC actuator | 69 |
| – operation voltage: 4 V | |||
| – actuation curvature: 0.26 mm−1 (at 20 s) | |||
| – pros: high mechanical properties (modulus ∼ 700 MPa) | |||
| – cons: back relaxation | |||
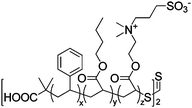
|
|
IPMC actuator | 70 |
| – operation voltage: 4 V | |||
| – actuation curvature ∼ 0.11 mm−1 (at 2 s), modulus ∼ 100 MPa | |||
| – pros: fast switching time (∼1 s) | |||
| – cons: back relaxation | |||
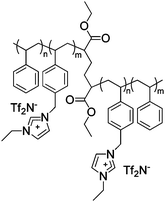
|
|
IPMC actuator | 41 |
| – operation voltage: 4 V | |||
| – actuation curvature ∼ 0.4 mm−1 (at 80 s), modulus ∼ 100 MPa | |||
| – pros: absence of back relaxation, modulus control | |||
| – cons: slow switching speed | |||
|
|
IPMC actuator | 71 |
| – operation voltage: 4 V | |||
| – actuation curvature ∼ 0.6 mm−1 (at 1 s) | |||
| – pros: fast response, morphology control | |||
| – cons: back relaxation | |||
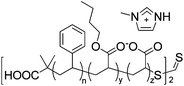
|
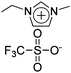
|
IPMC actuator | 75 |
| – operation voltage: 4 V | |||
| – actuation curvature ∼ 0.1 mm−1 (at 10 s), modulus ∼ 100 MPa | |||
| – pros: morphology control | |||
| – cons: back relaxation, slow response | |||
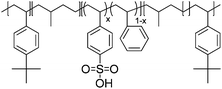
|
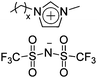
|
IPMC actuator | 72 |
| – operation voltage: 2–5 V | |||
| – interconnected ion channels with inorganic fillers | |||
| – actuation strain ∼ 1.5% (at 3 V, 120 s) | |||
| – blocking force: 0.29 gf (at 3 V) | |||
| – pros: absence of back relaxation | |||
| – cons: slow switching response | |||
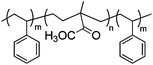
|
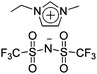
|
Bucky gel actuator | 68 |
| – operation voltage: 0.5–3.5 V | |||
| – actuation strain ∼ 0.8% (at ±3 V, 100 s) | |||
| – pros: absence of back relaxation, morphology control | |||
| – cons: slow switching response | |||
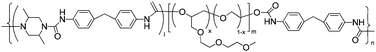
|
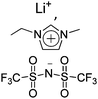
|
Bucky gel actuator | 29 |
| – operation voltage: ±1.5 V | |||
| – actuation strain ∼ 0.2% (at ±1.5 V, 50 s) | |||
| – pros: absence of back relaxation | |||
| – cons: slow switching response | |||
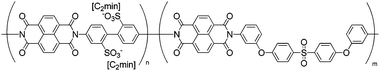
|
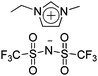
|
Bucky gel actuator | 46 |
| – operation voltage: 0.5–3 V | |||
| – actuation strain ∼ 0.3% (at ±1.5 V, 20 s) | |||
| – blocking force: 68 mgf (stress ∼ 67 MPa at 3 V) | |||
| – pros: absence of back relaxation, high force | |||
| – cons: slow switching response | |||
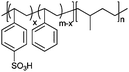
|
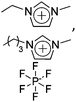
|
Bucky gel actuator | 73 |
| – operation voltage: 0.2–3 V | |||
| – actuation strain ∼ 4% (at ±3 V, 40 s), 0.2% (at 0.2 V, 1 s) | |||
| – well-defined morphology | |||
| – pros: absence of back relaxation, low operation voltage | |||
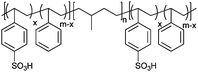
|
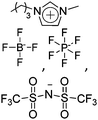
|
Bucky gel actuator | 74 |
| – operation voltage: 1–3 V | |||
| – actuation strain ∼ 1.5% (at ±3 V, 2 s) | |||
| – well-defined morphology | |||
| – pros: absence of back relaxation, high mechanical stability (modulus ∼ 700 MPa) | |||
Undoubtedly, self-assembled polymer electrolytes can significantly contribute to the progress in the era of low-voltage actuators. Unfortunately, achieving fast switching times on the order of tens of milliseconds from low-voltage-driven actuators seems far-fetched. However, as this was only feasible with the use of special carbon electrodes, does it imply that the polymer layer of actuators is not that important to accomplishing fast-moving actuators?
Recent strategies to develop fast-moving actuators
Among the many reasons, the depletion of cations and anions near the electrode surface at a given voltage is an important cause for the slow switching speed of actuators.76,77 The asymmetric diffusion of cations and anions is also a major cause for the back relaxation behaviour.34,40 Perhaps, these issues may be easily resolved if one of the ions is immobilized on the polymer matrices to form the so-called single-ion conductors.In light of this, Park et al.45 proposed a new platform of bucky gel actuators that can be operated with a small battery (<1.5 V) in air with an unprecedented fast switching time of tens of milliseconds based on the synthesis of single-ion conducting block copolymers. This work is particularly noteworthy given that the results were obtained with the widely used SWCNT electrode without the need to use specially designed carbon electrodes. The key strategy for this actuator was the design of new ionic additives beyond ionic liquids, i.e., the zwitterion.
The role of the zwitterion was two-fold. First, the permanent dipole in the zwitterion increased the ion dissociation degree by providing high dielectric constant environments near the ion.46,78,79 Second, while the zwitterion is electroneutral, the covalently bonded cation and anion can still contribute to intermolecular interactions in ionic domains, controlling the binding energy of ions and polymer chains.80
The single-ion conducting block copolymer containing an optimized zwitterion showed an exceptionally high dielectric constant of 76 and a 300-fold enhancement in ionic conductivity compared with that without a zwitterion. Such properties were directly associated with the marked improvements in actuation performance, as shown in Fig. 8.
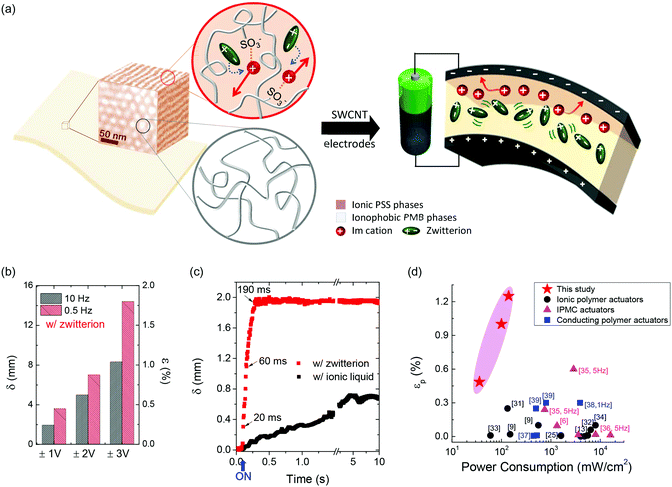 | ||
| Fig. 8 (a) Schematic illustration of the actuators comprising a cation-conducting block copolymer sandwiched between SWCNT electrodes. (b) Displacement (δ) and bending strain (ε) of the actuator containing a zwitterion at voltages of ±1, ±2 and ±3 V and frequencies of 0.5 and 10 Hz. (c) Laser-displacement measurements of the actuator with a zwitterion, compared with that containing ionic liquid, by applying 1 V. (d) Strain–power consumption dependency of the actuator, compared with other types of soft actuators reported in literature, which clearly represents the impact of this work on the structure of widely studied actuators fabricated with polymeric materials. Figure is adapted from ref. 46 with the permission of the Nature publishing group. | ||
After applying a voltage of 1 V to the actuator, the initial response time to the actuation field was as short as 20 ms, and the actuator readily moved 1 mm within 60 ms. These correspond to several times larger bending strain and over 100 times faster response time than those of the actuators based on ionic liquid-containing polymers. The actuators further demonstrated negligible changes in the actuator stroke over 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles in air when ±1 V square-wave input signals were applied with a cycle time of 50 ms. This clearly implies that rational molecular and structural designs of polymer layers are crucial for the development of next-generation soft actuators.
000 cycles in air when ±1 V square-wave input signals were applied with a cycle time of 50 ms. This clearly implies that rational molecular and structural designs of polymer layers are crucial for the development of next-generation soft actuators.
A comparison of power consumption and generated strain of the actuator with those of other types of actuators reported in the literature revealed that the single-ion conducting actuators consume only 1/10th of the power consumed by others, which highlights its massive potential in the field of soft robotics, artificial muscles, and biomedical microdevices.
Summary and outlook: strong actuators capable of various motions
We conclude this article by commenting on the unexplored challenges of low-voltage polymer actuators that can be summarized as a large blocking force and various actuation motions beyond the bending motion.The current level of actuation force of low-voltage EAP actuators is a few tens of mN, which is far below the requirement of artificial muscles in the order of N.43,81 Chen et al. recently demonstrated a force of 1.5 N, the largest among the reported EAP actuators at an applied voltage of 6 V.82 This was attributed to the fabrication of bucky paper-type electrodes by combining porous SWCNTs, Nafion™, and ionic liquids. Jho et al. also demonstrated an enhancement in the blocking force of actuators by using multi-stacked Nafion™ membranes combined with nanodispersed metal electrodes.83 Nevertheless, the most desirable approach should be the enhancement of Young's moduli of actuator ingredients by seeking new materials.
The achievement of various motions from EAP actuators needs to be urgently considered. The most favourable approach is the complex design of actuators, which has been quite common for hydraulically controlled actuators with 3D-printed elastomers.84,85 This has also become an attractive topic for thermo-sensitive actuators86 and photo-sensitive actuators87,88 to achieve complex deformation such as twisting and oscillating motions. If dry electrochemical actuators can show linear or oscillating motions under low-voltage operation in a muscle-like fashion, this would be a true breakthrough in future wearable soft robot technology; however, this has received less interest so far.
Very recently, Park et al. developed new actuators combined with light-active polymer (LAP) and EAP mimicking natural double-layered structures observed in living organisms (Fig. 9).89 While the focus of the study was reduction of power consumption by achieving self-locking motion in the absence of power supply, the double-layered actuators suggested a new avenue towards unprecedented performance of EAP actuators beyond the bending motions with the aid of another type of stimuli-responsive material.
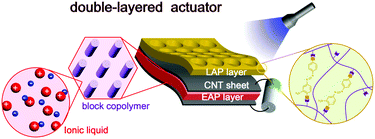 | ||
| Fig. 9 Schematic illustration of light- and electro-active polymer actuators based on the double-layered structures. Figure is reproduced from ref. 89 with the permission of Wiley-VCH. | ||
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. NRF-2017R1A2B3004763) and by the Korea government (MSIT) (No. NRF-2017R1A5A1015365). We also acknowledge the Global Frontier R&D program on Center for Multiscale Energy System funded by the NRF of Korea under MEST.References
- S. Bauer, S. Bauer-Gogonea, I. Graz, M. Kaltenbrunner, C. Keplinger and R. Schwödiauer, Adv. Mater., 2014, 26, 149–162 CrossRef CAS PubMed.
- D. Rus and M. T. Tolley, Nature, 2015, 521, 467–475 CrossRef CAS PubMed.
- N. W. Bartlett, M. T. Tolley, J. T. B. Overvelde, J. C. Weaver, B. Mosadegh, K. Bertoldi, G. M. Whitesides and R. J. Wood, Science, 2015, 349, 161–165 CrossRef CAS PubMed.
- I. A. Anderson, T. A. Gisby, T. G. McKay, B. M. O’Brien and E. P. Calius, J. Appl. Phys., 2012, 112, 041101 CrossRef.
- S. Kurumaya, K. Suzumori, H. Nabae and S. Wakimoto, Robomech J., 2016, 3, 18 CrossRef.
- P. Polygerinos, Z. Wang, K. C. Galloway, R. J. Wood and C. J. Walsh, Robot. Auton. Syst., 2015, 73, 135–143 CrossRef.
- P. Moseley, J. M. Florez, H. A. Sonar, G. Agarwal, W. Curtin and J. Paik, Adv. Eng. Mater., 2016, 18, 978–988 CrossRef CAS.
- R. Pelrine, R. Kornbluh, Q. Pei and J. Joseph, Science, 2015, 287, 836–839 CrossRef.
- F. Carpi, R. Kornbluh, P. Sommer-Larsen and G. Alici, Bioinspiration Biomimetics, 2011, 6, 045006 CrossRef PubMed.
- B. Ni, H.-L. Xie, J. Tang, H.-L. Zhang and E.-Q. Chen, Chem. Commun., 2016, 52, 10257–10260 RSC.
- Q. Shi, J. Li, C. Hou, Y. Shao, Q. Zhang, Y. Li and H. Wang, Chem. Commun., 2017, 53, 11118–11121 RSC.
- L. Zhang, I. Desta and P. Naumov, Chem. Commun., 2016, 52, 5920–5923 RSC.
- H. Shahsavan, S. M. Salili, A. Jákli and B. Zhao, Adv. Mater., 2017, 29, 1604021 CrossRef PubMed.
- L. Wang, Y. Jian, X. Le, W. Lu, C. Ma, J. Zhang, Y. Huang, C.-F. Huang and T. Chen, Chem. Commun., 2018, 54, 1229–1232 RSC.
- F. Ilievski, A. D. Mazzeo, R. F. Shepherd, X. Chen and G. M. Whitesides, Angew. Chem., Int. Ed., 2011, 50, 1890–1895 CrossRef CAS PubMed.
- Q. Zhao, J. W. C. Dunlop, X. Qiu, F. Huang, Z. Zhang, J. Heyda, J. Dzubiella, M. Antonietti and J. Yuan, Nat. Commun., 2014, 5, 4293 Search PubMed.
- S. Naficya and G. M. Spinks, J. Polym. Sci., Part B: Polym. Phys., 2015, 53, 218–225 CrossRef.
- Y. Bar-Cohen, Electroactive Polymer Actuators as Artificial Muscles: Reality, Potential, and Challenges, SPIE Press, Bellingham, 2004 Search PubMed.
- R. H. Baughman, Synth. Met., 1996, 78, 339–353 CrossRef CAS.
- K. Kanto, M. Kaneko, Y. Min and A. G. MacDiarmid, Synth. Met., 1995, 71, 2211–2212 CrossRef.
- E. Smela, Adv. Mater., 2003, 15, 481–494 CrossRef CAS.
- C. Plesse, F. Vidal, D. Teyssié and C. Chevrot, Chem. Commun., 2010, 46, 2910–2912 RSC.
- K. Asaka, K. Oguro, Y. Nishimura, M. Mizuhata and H. Takenaka, Polym. J., 1995, 27, 436–440 CrossRef CAS.
- S. Nemat-Nasser and J. Y. Li, J. Appl. Phys., 2000, 87, 3321–3331 CrossRef CAS.
- R. Tiwari and E. Garcia, Smart Mater. Struct., 2011, 20, 083001 CrossRef.
- T. Fukushima, K. Asaka, A. Kosaka and T. Aida, Angew. Chem., 2005, 117, 2462–2465 CrossRef.
- J. Lee and T. Aida, Chem. Commun., 2011, 47, 6757–6762 RSC.
- K. Mukai, K. Asaka, T. Sugino, K. Kiyohara, I. Takeuchi, N. Terasawa, D. N. Futaba, K. Hata, T. Fukushima and T. Aida, Adv. Mater., 2009, 21, 1582–1585 CrossRef CAS.
- S. Imaizumi, Y. Kato, H. Kokubo and M. Watanabe, J. Phys. Chem. B, 2012, 116, 5080–5089 CrossRef CAS PubMed.
- F. Vidal, C. Plesse, D. Teyssié and C. Chevrot, Synth. Met., 2004, 142, 287–291 CrossRef CAS.
- A. Khaldi, C. Plesse, C. Soyer, E. Cattan, F. Vidal, C. Legrand and D. Teyssié, Appl. Phys. Lett., 2011, 98, 164101 CrossRef.
- A. Khaldi, C. Plesse, F. Vidal and S. K. Smoukov, Adv. Mater., 2015, 27, 4418–4422 CrossRef CAS PubMed.
- A. Maziz, C. Plesse, C. Soyer, E. Cattan and F. Vidal, ACS Appl. Mater. Interfaces, 2016, 8, 1559–1564 CAS.
- J. D. Madden, R. A. Cush, T. S. Kanigan and I. W. Hunter, Synth. Met., 2000, 113, 185–192 CrossRef CAS.
- J.-M. Sansinena, J. Gao and H.-L. Wang, Adv. Funct. Mater., 2003, 13, 703–709 CrossRef CAS.
- B. J. Akle, M. D. Bennett, D. J. Leo, K. B. Wiles and J. E. McGrath, J. Mater. Sci., 2007, 42, 7031–7041 CrossRef CAS.
- M. D. Bennett and D. J. Leo, Sens. Actuators, A, 2004, 115, 79–90 CrossRef CAS.
- M. Shahinpoor and K. J. Kim, Smart Mater. Struct., 2001, 10, 819–833 CrossRef CAS.
- Y. Liu, M. Ghaffari, R. Zhao, J. H. Lin, M. Lin and Q. M. Zhang, Macromolecules, 2012, 45, 5128–5133 CrossRef CAS.
- J. H. Park, M. J. Han, D. S. Song and J. Y. Jho, ACS Appl. Mater. Interfaces, 2014, 6, 22847–22854 CAS.
- M. D. Green, D. Wang, S. T. Hemp, J.-H. Choi, K. I. Winey, J. R. Heflin and T. E. Long, Polymer, 2012, 53, 3677–3686 CrossRef CAS.
- J. Kim, J.-H. Jeon, H.-J. Kim, H. Lim and I.-K. Oh, ACS Nano, 2014, 8, 2986–2997 CrossRef CAS PubMed.
- D. Wang, C. Lu, J. Zhao, S. Han, M. Wu and W. Chen, RSC Adv., 2017, 7, 31264–31271 RSC.
- T. Patino, R. Mestre and S. Sánchez, Lab Chip, 2016, 16, 3626–3630 RSC.
- S. Imaizumi, Y. Ohtsuki, T. Yasuda, H. Kokubo and M. Watanabe, ACS Appl. Mater. Interfaces, 2013, 5, 6307–6315 CAS.
- O. Kim, H. Kim, U. H. Choi and M. J. Park, Nat. Commun., 2016, 7, 13576 CrossRef CAS PubMed.
- W. Lu, A. G. Fadeev, B. Qi, E. Smela, B. R. Mattes, J. Ding, G. M. Spinks, J. Mazurkiewicz, D. Zhou, G. G. Wallace, D. R. MacFarlane, S. A. Forsyth and M. Forsyth, Science, 2002, 297, 983–987 CrossRef CAS PubMed.
- T. F. Otero and M. T. Cortes, Chem. Commun., 2004, 284–285 RSC.
- G. Alici and N. N. Huynha, Sens. Actuators, A, 2006, 132, 616–625 CrossRef CAS.
- C. H. Nguyen, G. Alici and G. G. Wallace, Sens. Actuators, A, 2012, 185, 82–91 CrossRef CAS.
- J. Liu, Z. Wang, X. Xie, H. Cheng, Y. Zhao and L. Qu, J. Mater. Chem., 2012, 22, 4015–4020 RSC.
- H. Yan, K. Tomizawa, H. Ohno and N. Toshima, Macromol. Mater. Eng., 2003, 288, 578–584 CrossRef CAS.
- M. Kotal, J. Kim, K. J. Kim and I.-K. Oh, Adv. Mater., 2016, 28, 1610–1615 CrossRef CAS PubMed.
- R. K. Cheedarala, J.-H. Jeon, C.-D. Kee and I.-K. Oh, Adv. Funct. Mater., 2014, 24, 6005–6051 CrossRef CAS.
- S.-S. Kim, J.-H. Jeon, C.-D. Kee and I.-K. Oh, Smart Mater. Struct., 2013, 22, 085026 CrossRef.
- S. Naficy, N. Stoboi, P. G. Whitten, G. M. Spinks and G. G. Wallace, Smart Mater. Struct., 2013, 22, 075005 CrossRef.
- U. L. Zainudeen, M. A. Careem and S. Skaarup, Sens. Actuators, B, 2008, 134, 467–470 CrossRef CAS.
- S. Liu, W. Liu, Y. Liu, J. H. Lin, X. Zhou, M. J. Janik, R. H. Colby and Q. M. Zhang, Polym. Int., 2010, 59, 321–328 CrossRef CAS.
- Y. Liu, C. Lu, S. Twigg, M. Ghaffari, J. Lin, N. Winograd and Q. M. Zhang, Sci. Rep., 2013, 3, 973 CrossRef PubMed.
- F. W. Richey and Y. A. Elabd, J. Phys. Chem. Lett., 2012, 3, 3297–3301 CrossRef CAS.
- I. Takeuchi, K. Asaka, K. Kiyohara, T. Sugino, N. Terasawa, K. Mukai, T. Fukushima and T. Aida, Electrochim. Acta, 2009, 54, 1762–1768 CrossRef CAS.
- J. Torop, V. Palmre, M. Arulepp, T. Sugino, K. Asaka and A. Aabloo, Carbon, 2011, 49, 3113–3119 CrossRef CAS.
- N. Terasawa, N. Ono, K. Mukai, T. Koga, N. Higashi and K. Asaka, Carbon, 2012, 141, 311–320 CrossRef.
- J. Li, W. Ma, L. Song, Z. Niu, Q. Zeng, X. Zhang, H. Dong, W. Zhou and S. Xie, Nano Lett., 2011, 11, 4636–4641 CrossRef CAS PubMed.
- G. Wu, G. H. Li, T. Lan, Y. Hu, Q. W. Li, T. Zhang and W. Chen, J. Mater. Chem. A, 2014, 2, 16836–16841 CAS.
- N. Terasawa, I. Takeuchi and H. Matsumoto, Sens. Actuators, B, 2009, 139, 624–630 CrossRef CAS.
- N. Terasawa, I. Takeuchi, H. Matsumoto, K. Mukai and K. Asaka, Sens. Actuators, B, 2011, 156, 539–545 CrossRef CAS.
- S. Imaizumi, H. Kokubo and M. Watanabe, Macromolecules, 2012, 45, 401–409 CrossRef CAS.
- R. Gao, D. Wang, J. R. Heflin and T. E. Long, J. Mater. Chem., 2012, 22, 13473–13476 RSC.
- T. Wu, D. Wang, M. Zhang, J. R. Heflin, R. B. Moore and T. E. Long, ACS Appl. Mater. Interfaces, 2012, 4, 6552–6559 CAS.
- C. Jangu, J.-H. H. Wang, D. Wang, G. Fahs, J. R. Heflin, R. B. Moore, R. H. Colby and T. E. Long, J. Mater. Chem. C, 2015, 3, 3891–3901 RSC.
- J. W. Lee, S. Yu, S. M. Hong, J. Kim and C. M. Koo, J. Mater. Chem. C, 2013, 1, 3784–3793 RSC.
- O. Kim, T.-J. Shin and M. J. Park, Nat. Commun., 2013, 4, 2208 Search PubMed.
- O. Kim, S. Y. Kim, B. Park, W. Hwang and M. J. Park, Macromolecules, 2014, 47, 4357–4368 CrossRef CAS.
- E. Margaretta, G. B. Fahs, D. L. Inglefield, Jr., C. Jangu, D. Wang, J. R. Heflin, R. B. Moore and T. E. Long, ACS Appl. Mater. Interfaces, 2016, 8, 1280–1288 CAS.
- S. Nemat-Nasser and Y. Wu, Smart Mater. Struct., 2006, 15, 909–923 CrossRef CAS.
- M. Armand, F. Endres, D. R. MacFarlane, H. Ohno and B. Scrosati, Nat. Mater., 2009, 8, 621–629 CrossRef CAS PubMed.
- C. Tiyapiboonchaiya, J. M. Pringle, J. Sun, N. Byrne, P. C. Howlett, D. R. Macfarlane and M. Forsyth, Nat. Mater., 2004, 3, 29–32 CrossRef CAS PubMed.
- F. Wohde, R. Bhandary, J. M. Moldrickx, J. Sundermeyer, M. Schönhoff and B. Roling, Solid State Ionics, 2016, 284, 37–44 CrossRef CAS.
- Q. Shao and S. Jiang, Adv. Mater., 2015, 27, 15–26 CrossRef CAS PubMed.
- V. Palmre, D. Pugal, K. J. Kim, K. K. Leang, K. Asaka and A. Aabloo, Sci. Rep., 2014, 4, 6176 CrossRef CAS PubMed.
- I.-W. P. Chen, M.-C. Yang, C.-H. Yang, D.-X. Zhong, M.-C. Hsu and Y. W. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 5550–5555 CAS.
- H. S. Wang, J. Cho, D. S. Song, J. H. Jang, J. Y. Jho and J. H. Park, ACS Appl. Mater. Interfaces, 2017, 9, 21998–22005 CAS.
- E. Acome, S. K. Mitchell, T. G. Morrissey, M. B. Emmett, C. Benjamin, M. King, M. Radakovitz and C. Keplinger, Science, 2018, 359, 61–65 CrossRef CAS PubMed.
- N. Kellaris, V. G. Venkata, G. M. Smith, S. K. Mitchell and C. Keplinger, Sci. Rob., 2018, 3, eaar3276 CrossRef.
- J. A. Lee, N. Li, C. S. Haines, K. J. Kim, X. Lepró, R. Ovalle-Robles, S. J. Kim and R. H. Baughman, Adv. Mater., 2017, 29, 1700870 CrossRef PubMed.
- Y. Hu, J. Liu, L. Chang, L. Yang, A. Xu, K. Qi, P. Lu, G. Wu, W. Chen and Y. Wu, Adv. Funct. Mater., 2017, 27, 1704388 CrossRef.
- Q. Li, C. Liu, Y.-H. Lin, L. Liu, K. Jiang and S. Fan, ACS Nano, 2012, 9, 409–418 CrossRef PubMed.
- S. J. Kim, O. Kim and M. J. Park, Adv. Mater., 2018, 1706547, DOI:10.1002/adma.201706547.
| This journal is © The Royal Society of Chemistry 2018 |



