Open Access Article
Open Access ArticleNegishi coupling reactions with [11C]CH3I: a versatile method for efficient 11C–C bond formation†
Luka
Rejc
*a,
Vanessa
Gómez-Vallejo
a,
Jesús
Alcázar
b,
Nerea
Alonso
b,
José Ignacio
Andrés
b,
Ana
Arrieta
c,
Fernando P.
Cossío
 c and
Jordi
Llop
c and
Jordi
Llop
 *a
*a
aRadiochemistry and Nuclear Imaging, CIC biomaGUNE, Paseo Miramón 182, 20014 San Sebastián, Guipúzcoa, Spain. E-mail: jllop@cicbiomagune.es; lrejc@cicbiomagune.es; Tel: +34 943 00 53 33
bLead Discovery Chemistry–Discovery Sciences, Janssen Research & Development, Jarama 75A, 45007 Toledo, Spain
cDepartment of Organic Chemistry I, ORFEO-CINQA, Universidad del País Vasco/Euskal Herriko Unibertsitatea UPV/EHU, Donostia International Physics Center (DIPC), Paseo Manuel Lardizabal 3, 20018 San Sebastián/Donostia, Spain
First published on 17th April 2018
Abstract
Herein, we present a fast, efficient and general one-pot method for the synthesis of 11C-labelled compounds via the Negishi cross-coupling reaction. Our approach, based on the in situ formation of [11C]CH3ZnI and subsequent reaction with aryl halides or triflates, has proven efficient to synthesize [11C]thymidine, a biologically relevant compound with potential applications as a proliferation marker. Theoretical calculations have shown irreversible formation of a tetracoordinated nucleophilic 11C–Zn(II) reagent and electronic requirements for an efficient Negishi coupling.
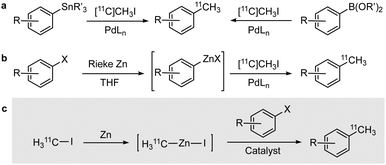
Emerging applications in positron emission tomography (PET)1 have resulted in a boost in the demand for new positron-emitter-labelled radiotracers. Carbon-11 (11C; t1/2 = 20.4 min) is one of the most attractive positron emitters because its stable isotopes form the main building blocks of all organic molecules, providing an opportunity to prepare a wide variety of radiolabelled organic compounds through 11C-methylation, 11C-cyanation, 11C-carbonylation, and 11C-carboxylation.2 Among these, the most frequently used method is 11C-methylation, which encompasses a fast and efficient SN2 nucleophilic substitution reaction using the easily produced labelling agent [11C]CH3I.3 However, it is limited to the production of [11C]methoxides, [11C]methylamines, and [11C]methylthio compounds. Recently reported possibilities for radiolabelling via palladium(0)-mediated cross-coupling reactions to form 11C–C bonds (Scheme 1a) do not offer a general alternative.4 The direct 11C–C coupling between [11C]CH3I and aryl stannanes or aryl boronic acids indeed results in [11C]methylaryl-based compounds; however, the preparation, isolation, and purification of the required precursors is sometimes challenging or even impossible.5 Additionally, the Stille reaction has been found to result in low molar radioactivity (MA) and is hampered by the biotoxicity of organotin reagents.6 The use of [11C]methyl-tin and [11C]methyl-lithium reagents enabled [11C]methylation reactions on more accessible substrates, but the formation and manipulation of the organo-tin and organo-lithium compounds remains challenging.7 Furthermore, strong polarisability of the C–Li bond makes methyllithium highly nucleophilic, diminishing its selectivity and group tolerance.
Highly specific and versatile Negishi cross-coupling reaction offers an alternative to 11C–C bond formation, as reported recently (Scheme 1b).8 However, the main challenge remains the preparation of a general [11C]methylating reagent that would omit the preparation of aryl zincates on a case-by-case basis. Within our tracer development program, we devised a unique way to explore the unprecedented reaction of zinc with [11C]CH3I to form the corresponding umpolung species 11CH3ZnI, which might enable direct [11C]methylation of aryl halides (Scheme 1c). The fact that radiochemical reactions proceed under pseudo-first order kinetics in the presence of a clear deficiency of the labelling agent led us to hypothesise that the reaction would proceed fast and efficiently.
Hoping to avoid notoriously long procedures of alkyl zincate formation9 that would impact the final radiochemical yield, we envisaged in situ [11C]CH3ZnI formation in a zinc-filled cartridge, followed by cross-coupling with an aryl halide.10 At the initial stage, the [11C]methylation of 4-bromoacetophenone to produce 4-[11C]methylacetophenone was explored as a model reaction. Due to high susceptibility of zinc to oxidation, the metal surface was activated with an iodine solution. Iodine rather than the traditionally used trimethylsilyl chloride was selected because of its compatibility with the subsequent cross-coupling reaction and the ability to form higher-order zincate complexes, which reportedly favoured the C–C bond formation.11N,N-Dimethylacetamide (DMA) has been used as a solvent as it promotes the formation/improves the stability of the methyl zincate complex and supports the transition metal-catalyzed cross-coupling reaction. Upon activation, [11C]CH3I was directly distilled into the cartridge. Promisingly, almost quantitative trapping of [11C]CH3I in the cartridge (>95%) could be achieved. After 1-minute reaction at 65 °C, the contents were eluted with anhydrous DMA into a vial pre-loaded with tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4) and an aryl halide (see ESI,† Fig. S1a). The reaction was carried out for 15 min at T = 65 °C, and the crude product was analyzed by high performance liquid chromatography equipped with a radioactivity detector (radio-HPLC). A radioactive peak with a retention time (rt) of 6.2 min co-eluted with 4-methylacetophenone in the UV chromatogram. The radiochemical conversion, calculated as the ratio between the area under the peak at rt = 6.2 min and the sum of the areas of all peaks in the chromatogram (radioactive detector), was close to 1%. Interestingly, the highest peak in the chromatogram corresponded to [11C]CH3I (rt = 4.8 min), suggesting that the reason for the low reaction yield was the lack of formation of the activated species [11C]CH3ZnI.
Based on a recent theoretical study on the beneficial effects of Pd–Zn bond formation in oxidative addition, transmetalation, and reductive elimination in the Negishi coupling reaction,12 the introduction of the palladium complex during [11C]methyl-zincate formation was taken into consideration. Indeed, when Pd(PPh3)4 (10 μmol) was added to the zinc-activating iodine solution, the chromatographic yield increased to 6% (Fig. S1b, ESI†).
To facilitate automatisation and further increase the reaction yield, a one-pot set up was finally designed (Fig. S1c, ESI†). Herein, [11C]CH3I gas was directly distilled into a zinc-filled cartridge, pre-loaded with a solution containing iodine, 4-bromoacetophenone, and Pd(PPh3)4 in anhydrous DMA, and the contents of the cartridge were eluted with anhydrous THF after 5 minutes. Moreover, two major radioactive peaks were observed in the chromatogram: the 11C-methylated product, which accounted for 83% of the total radioactivity (Table 1, entry 1) and a peak with an rt = 3.2 min identified as [11C]CH4, resulting from the immediate and quantitative hydrolysis of [11C]CH3ZnI in the water-containing chromatographic mobile phase. To confirm the origin of [11C]CH4, the reaction mixture was analyzed at 1 and 3 minutes. The relative concentration of the radioactive species [11C]CH4, [11C]CH3I, and 4-[11C]methylacetophenone followed the expected trend with reaction time: the relative amount of [11C]CH3I progressively decreased, whereas the 11C-methylated product followed an opposite trend (see ESI,† Fig. S2). The peak corresponding to [11C]CH4 slightly increased from 1 to 3 minutes, and almost disappeared at 5 minutes, completely disproving the theory of formation of [11C]CH4 during the reaction and confirming the formation of the active [11C]methyl-zincate complex. Importantly, the [11C]CH3I trapping was not compromised under these experimental conditions. The possibility of formation of aryl zincate, a possible by-product during the reaction, acting as a nucleophile in the Negishi reaction was rejected by a reverse activation experiment (Fig. S1d, ESI†). In this case, a zinc cartridge was preloaded with a solution of 4-bromoacetophenone and palladium catalyst, and the contents eluted to [11C]CH3I and Pd(PPh3)4-filled vial after 5 minutes at 65 °C. No product was observed in the reaction mixture.
| Entry | |||
|---|---|---|---|
| R | X | Producta (%) | |
| a Calculated as the ratio between the area of the peak corresponding to [11C]methylaryl and the sum of the areas of all the peaks in the radiochromatogram. b Reaction time = 5 min. c Reaction time of 10 min. d In brackets, the amount of [11C]CH3I left in the solution after reaction. | |||
| 1 | 4-Acetyl | Br | 83b |
| 2 | 3-Acetyl | Br | 22b/35c |
| 3 | 2-Acetyl | Br | 30b/53c |
| 4 | 4-Ethyl ester | Br | 69b |
| 5 | 1-Naphtyl | Br | 21b |
| 6 | 4-Amino | Br | 19b |
| 7 | 2-Amino | Br | 2b |
| 8 | 4-Methoxy | Br | 8b |
| 9 | 2,4-Dichloro | I | 60 (32)d |
| 10 | 2-Acetyl | I | 28 (21)b,d |
| 11 | 1-Naphtyl | I | 26 (41)d |
| 12 | 2-Amino | I | 12 (28)d |
| 13 | 4-Methoxy | I | 33 (22)d |
| 14 | 4-Acetyl | OTf | 73b |
| 15 | 4-Methoxy | OTf | 0b |
To prove the generality of our method and the tolerability to a variety of functional groups, we successfully formed 11C-labelled methyl aryls using different aryl bromides (Table 1, entries 1–8), iodides (entries 9–13), and triflates (entries 14 and 15). In general, electron-withdrawing groups (EWG) (Table 1, entries 1–4) promoted reaction yields, whereas electron-donating group (EDG)-bearing substrates exhibited low conversion values (Table 1, entries 6–8). In all the cases (entries 1–8), the relative amount of [11C]CH3I remaining in the reaction crude accounted for less than 5% of the total radioactivity. These results, together with the increasing conversion values at longer reaction times (Table 1, entries 2 and 3), further support the kinetic dependency of the reaction on the ability of a substrate-palladium complex formation/dissociation, rather than inactivation of zinc or deactivation of the palladium catalyst.
Surprisingly, parallel reactions with aryl iodides resulted in equivalent or only slightly increased chromatographic yields. Note that in these cases, the presence of a significant amount of unreacted [11C]CH3I was detected (in brackets in the table); this suggested hampered formation of the activated 11C-methyl zincate species. Further inspection of the UV chromatograms revealed the presence of additional peaks, identified by GC-MS as the de-iodinated precursor (see ESI,† Fig. S3). De-halogenation, although seen in some aryl bromides, had a bigger effect on aryl iodides, which readily de-halogenated in an activated zinc cartridge at 65 °C after 10 min probably via the formation of an arylzinc iodide complex. Similar to the detection of [11C]CH3ZnI, the formation of arylzincates was only indirectly confirmed by the detection of a de-halogenated product after hydrolysis. De-halogenation yields followed the trend observed for the oxidative addition to palladium; thus, higher de-halogenation was observed for EWG-bearing aryl iodides.
To better understand the origins of our results, we performed DFT calculations at the B3LYP(SCRF)-D3/6-31G(d)&LANL2DZ13 theoretical level. Both experimental and theoretical studies14 indicate that in the presence of coordinating solvents such as THF, solvated Zn(II)-containing species are the nucleophiles present in the Negishi coupling. In effect, our calculations show that when DMA is used as a solvent, the reaction of [11C]CH3I with solvated zinc yields the tetracoordinated species 1via a highly exergonic process (Fig. 1a). The nucleophilic intermediate 1 shows a tetrahedral coordination pattern, as expected for a d10 Zn(II) metallic centre, and enters into the catalytic cycle shown in Fig. 1b.12,15
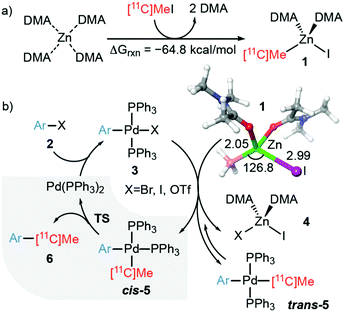 | ||
| Fig. 1 (a) The calculated free reaction energy (B3LYP-D3(SCRF=DMA)/6-31G(d)&LANL2DZ level of theory T = 333.15 K) associated with the formation of nucleophile 1 from solvated Zn0⋯(DMA)4 and [11C]CH3I. (b) Chief geometric features of tetrahedral species 1 and catalytic cycle associated with the formation of adducts 6. The rate-determining step leading to the reaction product via transition structure TS is highlighted in grey and presented in more detail in Fig. 2. DMA: N,N-dimethylacetamide. Bond distances and 11C–Zn–I bond angle are given in Å and deg., respectively. | ||
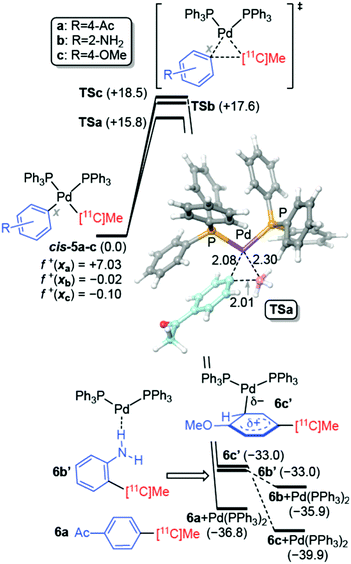
According to this mechanism, the catalyst Pd(PPh3)2 reacts with the alkyl halide 2 to give rise to adduct 3. This intermediate reacts with 1 to yield the isomeric products 5via a quite complex process in which heterobimetallic intermediates are involved. Many previous experimental and computational mechanistic studies16 have focused on these stages of the catalytic cycle. Formation of both cis-5 and trans-5 is a quite fast process, the latter being even faster. However, cis-5 intermediates are thermodynamically more stable than their trans congeners. For instance, in our case, cis-5b (R = 2-NH2, see Fig. 2) is calculated to be 4.1 kcal mol−1 more stable than trans-5b. The 11C–C bond forming step is slower and determines the reaction rate. This reductive elimination step yields adduct 6via the saddle point TS, with concomitant release of the catalyst Pd(PPh3)2 and completion of the catalytic cycle (Fig. 1b).
The energy profile associated with the cis-5 → 6 + Pd(PPh3)2 transformation was then computed. We selected three substitution patterns a, b, and c, in which the electrophilicity of the aryl moiety varied according to the electron-withdrawing or electron-releasing character of the R substituent. In the three cases, we located three-membered cyclic transition structures TSa–c (Fig. 2) that were quite synchronous. When we computed the reaction profiles associated with the cis-5a → 6a + Pd(PPh3)2 process for 12C and 13C isotopes at the methyl moiety arising from MeI, we obtained very similar relative Gibbs energies for the [11C]Me and [12C]Me groups. However, a noticeable increase in the activation free energy was computed while transitioning from [11C]Me to [13C]Me, with ΔΔG‡333 = ΔG‡333(13C) − ΔG‡333(11C) = +0.3 kcal mol−1, which corresponded to an estimated KIE of ca. 1.6. We found that the computed free activation energies at 333.15 K correlated with the electrophilicity of the reactants cis-5a–c estimated by means of the electrophilic local Fukui functions f+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 17 calculated at the carbon atom of the aryl moiety involved in the formation of the Cx–11C bond (Fig. 2). In the case of the formation of the adduct 6a, in which R = 4-Ac, the f+ value is highest, and the associated Gibbs activation energy was found to be lowest. This is in good agreement with the high yield obtained for this reaction (Table 1, entry 1). When we analyzed the reactivity of the adducts cis-5b and c, where R = 2-NH2 and R = 4-OMe, respectively (Fig. 2), we found that the local electrophilicities were negligible, and the activation energies were 2.0–2.7 kcal mol−1 higher than that calculated for TSa. These higher values and the corresponding lower reaction rates are in line with the low yields found in the Negishi couplings between [11C]CH3I and 2-bromoaniline (Table 1, entry 7) and 1-bromo-4-methoxybenzene (Table 1, entry 8). Moreover, in these latter two cases, the complexes 6b′ and 6c′ were found, in which the coupling products 6b and c were bound to the catalyst by means of a weak hydrogen bond and a Wheland-like interaction, respectively. Therefore, we conclude that in the cases involving electron-rich coupling products, release of the catalyst and completion of the catalytic cycle can be partially hampered by weak inhibition of the catalyst by the adduct generated after the reductive elimination step.
17 calculated at the carbon atom of the aryl moiety involved in the formation of the Cx–11C bond (Fig. 2). In the case of the formation of the adduct 6a, in which R = 4-Ac, the f+ value is highest, and the associated Gibbs activation energy was found to be lowest. This is in good agreement with the high yield obtained for this reaction (Table 1, entry 1). When we analyzed the reactivity of the adducts cis-5b and c, where R = 2-NH2 and R = 4-OMe, respectively (Fig. 2), we found that the local electrophilicities were negligible, and the activation energies were 2.0–2.7 kcal mol−1 higher than that calculated for TSa. These higher values and the corresponding lower reaction rates are in line with the low yields found in the Negishi couplings between [11C]CH3I and 2-bromoaniline (Table 1, entry 7) and 1-bromo-4-methoxybenzene (Table 1, entry 8). Moreover, in these latter two cases, the complexes 6b′ and 6c′ were found, in which the coupling products 6b and c were bound to the catalyst by means of a weak hydrogen bond and a Wheland-like interaction, respectively. Therefore, we conclude that in the cases involving electron-rich coupling products, release of the catalyst and completion of the catalytic cycle can be partially hampered by weak inhibition of the catalyst by the adduct generated after the reductive elimination step.
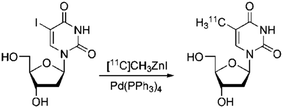
As a proof of the suitability of our method to prepare biologically relevant compounds, we tackled the synthesis of [11C]thymidine (proliferation marker), starting from 5-iodo-2′-deoxyuridine (Scheme 2). The synthesis resulted in a 53% radiochemical conversion in the 5 min reaction. Inclusion of a purification step (see ESI†) resulted in pure [11C]thymidine with a 6.1% radiochemical yield and MA > 50 GBq μmol−1.
Our study confirms that the Negishi cross-coupling reaction of in situ formed [11C]MeZnI can be performed with aryl halides and triflates commonly available in medicinal chemistry programs. Good yields and high group tolerability should enable the preparation of a wide range of 11C-labelled biologically relevant compounds, as demonstrated with the synthesis of [11C]thymidine. Together with the development of new metal complexes, our simple and general method provides a new alternative of 11C-radiolabelling and may change the way of how 11C-radiochemistry can be carried out in the future. Further applications of this approach will be matters of future research.
Financial support for this work was provided by the Spanish MINECO (CTQ2017-87637-R, CTQ2016-80375-P and CTQ2016-81797-REDC) and Eusko Jaurlaritza (GV/EJ, Grant IT673-13).
Conflicts of interest
The authors declare no conflict of interest.Notes and references
- J. S. Fowler and N. D. Volkow, Neuroscience in the 21st Century: From Basic to Clinical, 2nd edn, 2016, pp. 2929–2954 Search PubMed.
- (a) P. W. Miller, N. J. Long, R. Vilar and A. D. Gee, Angew. Chem., Int. Ed., 2008, 47, 8998 CrossRef CAS PubMed; (b) O. Itsenko, V. Goméz-Vallejo, J. Llop and J. Koziorowski, Curr. Org. Chem., 2013, 17, 2067 CrossRef CAS.
- R. Bolton, J. Labelled Compd. Radiopharm., 2001, 44, 701 CrossRef CAS.
- K. Dahl, C. Halldin and M. Schou, Clin. Transl. Imaging, 2017, 5, 275 CrossRef PubMed.
- T. Hosoya, K. Sumi, H. Doi, M. Wakao and M. Suzuki, Org. Biomol. Chem., 2006, 4, 410 CAS.
- (a) J. Madsen, P. Merachtsaki, P. Davoodpour, M. Bergström, B. Långström, K. Andersen, C. Thomsen, L. Martiny and G. M. Knudsen, Bioorg. Med. Chem., 2003, 11, 3447 CrossRef CAS PubMed; (b) L. Samuelsson and B. Långström, J. Labelled Compd. Radiopharm., 2003, 46, 263 CrossRef CAS; (c) M. Huiban, S. Pampols-Maso and J. Passchier, Appl. Radiat. Isot., 2011, 69, 1390 CrossRef CAS PubMed.
- (a) M. Huiban, A. Huet, L. Barré, F. Sobrio, E. Fouquet and C. Perrio, Chem. Commun., 2006, 97 RSC; (b) D. Heijnen, F. Tosi, C. Vila, M. C. A. Stuart, P. H. Elsinga, W. Szymanski and B. L. Feringa, Angew. Chem., Int. Ed., 2017, 56, 3354 CrossRef CAS PubMed.
- S. Kealey, J. Passchier and M. Huiban, Chem. Commun., 2013, 49, 11326 RSC.
- S. Huo, Org. Lett., 2003, 5, 423 CrossRef CAS PubMed.
- (a) L. Huck, M. Berton, A. De La Hoz, A. Díaz-Ortiz and J. Alcázar, Green Chem., 2017, 19, 1420 RSC; (b) N. Alonso, L. Z. Miller, J. De, M. Muñoz, J. Alcázar and D. T. McQuade, Adv. Synth. Catal., 2014, 356, 3737 CrossRef CAS; (c) M. Berton, L. Huck and J. Alcázar, Nat. Protoc., 2018, 13, 324 CrossRef CAS PubMed.
- L. C. McCann, H. N. Hunter, J. A. C. Clyburne and M. G. Organ, Angew. Chem., Int. Ed., 2012, 51, 7024 CrossRef CAS PubMed.
- B. Fuentes, M. García-Melchor, A. Lledós, F. Maseras, J. A. Casares, G. Ujaque and P. Espinet, Chem. – Eur. J., 2010, 16, 8596 CrossRef CAS PubMed.
- (a) C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS; (b) A. D. Becke, J. Chem. Phys., 1993, 98, 1372 CrossRef CAS; (c) A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS; (d) J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999 CrossRef CAS PubMed; (e) M. J. Frisch et al. , Gaussian 09, Revision B.01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed, see ESI† for the full reference.
- J. Del Pozo, M. Pérez-Iglesias, R. Álvarez, A. Lledós, J. A. Casares and P. Espinet, ACS Catal., 2017, 7, 3575 CrossRef CAS.
- M. García-Melchor, A. A. C. Braga, A. Lledós, G. Ujaque and F. Maseras, Acc. Chem. Res., 2013, 46, 2626 CrossRef PubMed.
- (a) M. García-Melchor, B. Fuentes, A. Lledós, J. A. Casares, G. Ujaque and P. Espinet, J. Am. Chem. Soc., 2011, 133, 13519 CrossRef PubMed; (b) Q. Liu, Y. Lan, J. Liu, G. Li, Y. D. Wu and A. Lei, J. Am. Chem. Soc., 2009, 131, 10201 CrossRef CAS PubMed.
- P. W. Ayers, W. Yang and L. J. Bartolotti, in Chemical Reactivity Theory: A Density Functional TheoryView, ed. P. Chattaraj, Taylor & Francis, Boca Raton, 2009, pp. 255–267 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental details, characterization of new compounds, and computational data. See DOI: 10.1039/c8cc01540f |
| This journal is © The Royal Society of Chemistry 2018 |