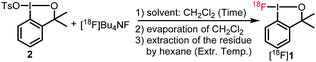Open Access Article
Open Access Article[18F]fluoro-benziodoxole: a no-carrier-added electrophilic fluorinating reagent. Rapid, simple radiosynthesis, purification and application for fluorine-18 labelling†
Miguel A.
Cortés González
 a,
Patrik
Nordeman
b,
Antonio
Bermejo Gómez
a,
Patrik
Nordeman
b,
Antonio
Bermejo Gómez
 ac,
Denise N.
Meyer
ac,
Denise N.
Meyer
 a,
Gunnar
Antoni
b,
Magnus
Schou
c and
Kálmán J.
Szabó
a,
Gunnar
Antoni
b,
Magnus
Schou
c and
Kálmán J.
Szabó
 *a
*a
aDepartment of Organic Chemistry, Stockholm University, SE-106 91, Sweden. E-mail: kalman.j.szabo@su.se; Web: http://www.organ.su.se/ks/
bDepartment of Medicinal Chemistry, Uppsala University, SE-751 23, Sweden
cAstraZeneca PET Centre at Karolinska Institutet, SE-171 76, Stockholm, Sweden
First published on 3rd April 2018
Abstract
Operationally simple radiosynthesis and purification of [18F]fluoro-benziodoxole was developed starting from a cyclotron produced [18F]F− precursor, [18F]TBAF, and tosyl-benziodoxole. The synthetic utility of [18F]fluoro-benziodoxole was demonstrated by electrophilic fluorocyclization of o-styrilamides proceeding with high RCC (typically 50–90%) and high molar activity (up to 396 GBq μmol−1).
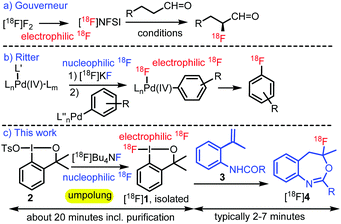
Organofluorine compounds1 are widely used in pharmaceutical2 and agrochemical3 industries as well as in medical diagnostics.4 The foremost radionuclide in Positron Emission Tomography (PET) is fluorine-18 because of its half-life of 109.8 minutes, the strong C–F bonding and the usually high metabolic stability of organofluorines.4 The optimal conditions for synthesis of organofluorines with the natural isotope fluorine-19 and those containing the radioactive isotope fluorine-18 are often fundamentally different. An important challenge in radiochemistry is to obtain 18F-labelled organofluorine species in high radiochemical conversion (RCC) and at high molar activity within the lifetime of fluorine-18. Traditionally, high molar activity could only be achieved by nucleophilic 18F-fluorination reactions.4 Electrophilic fluorination reactions target the realm of electron-rich functional groups, such as alkenes and aromatics with electron supplying substituents.1 A characteristic feature of an electrophilic fluorinating reagent is the ability to accept an electron pair to their low-lying LUMO orbital having a substantial AO contribution from the fluorine atom, i.e. the fluorine-18 functional group in the reagent is electron deficient. A trivial example for such an electrophilic fluorination reagent is [18F]F2. However, this reagent is difficult to handle in radiosynthesis, very reactive (thus unselective) and it cannot be obtained with high molar activity.5 Unfortunately, most of the selective electrophilic fluorination reagents, such as [18F]NFSI (Fig. 1a),6 [18F]Selectfluor7 and related species8 are also prepared from [18F]F2, which usually leads to unsatisfactory molar activities via rather cumbersome radiosynthetic procedures. On the other hand [18F]F− based reagents, such as[18F]KF or [18F]TBAF can be generated with high fluorine-18 molar activity.
However, these [18F]F− based reagents have an explicit nucleophilic reactivity, as the electron rich F− has an electron donating character. Construction of an electrophilic fluorinating reagent from [18F]F− requires the so-called “umpolung” of the reactivity. Ritter and co-workers9 have constructed an electrophilic fluorination reagent from a Pd(IV) complex and [18F]KF (Fig. 1b). In the applied ligand exchange process the K–[18F]F interaction was transformed into Pd(IV)–[18F]F bonding. Change of the potassium counterion to a palladium(IV) center rendered the electron-rich fluorine-18 to an electron deficient one. Thus, the subsequent reductive elimination from the Pd(IV) complex created a new [18F]F–carbon bond. A similar process was developed using Ni(III) complexes as well.10 However, the widespread use of these methods has been limited by the complexity of the synthesis of the Pd(IV) and Ni(III) complexes.
Safe, easy to handle and stable fluoro-benziodoxole reagent 1 was first reported by Prévost and Legault in 2012,11 and since then it has become a privileged fluorinating reagent.12 Recent DFT modelling studies13 discussed the mechanistic aspects of the electrophilic fluorination reactions by this hypervalent iodine reagent. Notably, fluoro-iodane 1 can easily be synthesized from anionic fluorine precursors.12c,14 Considering the importance of bridging the gap between the recent methodological advances in fluorine-19 chemistry and the high demand to access new types of fluorine-18 PET tracers for clinical diagnostics,15 we decided to develop a new methodology for rapid radiosynthesis of electrophilic [18F]fluoro-benziodoxole ([18F]1) reagent from standard [18F]F− precursors (Fig. 1c).
Our fluorine-19 experiments have shown that the synthesis of 1 from commonly used benziodoxole precursors, such as OH, OAc and halogenide derivatives requires relatively long reaction times (0.5–4 h). Therefore, the highly reactive (yet bench-stable) tosyl derivative 212c was selected as precursor for the radiosynthesis. Reagent 1 partially or completely decomposes under chromatography due to the high affinity of the iodine bound fluorine to Si, Al and alkali metal ions. Indeed, over 95% of [18F]1 was lost, when we attempted to purify it on silica or alumina adsorbents (ESI,† S12). Therefore, the analysis and purification of [18F]1 cannot be achieved by chromatographic methods, as fluoro-iodines 1 and [18F]1 decomposed when subjected to reverse-phase HPLC, TLC or paper chromatography. The alternative purification method was based on the relatively good solubility of 1 in hexane. According to our fluorine-19 experiments the starting materials tosyl-benziodoxole (2) and TBAF, as well as the inorganic compounds formed in the synthesis of 1 are insoluble in hexane. Thus, after the allotted times (Table 1) we evaporated the solvent (DCM) of the reaction mixture of 2 and [18F]Bu4NF, and then the solid residue was extracted with hexane. We assumed that the entire amount of the radioactivity of the hexane solution arose from [18F]1, and this radioactivity was the basis of the calculation of RCC (Table 1). To get further evidence for the formation of [18F]1 we studied the chemical composition of a decayed sample of [18F]1 by 19F-NMR and HRMS. Such analysis takes advantage of the inevitable co-production of unlabeled 1, which is formed by the reaction of 2 with the minute amounts of fluorine-19 that is present in the reaction mixture. Indeed, our analysis of the decayed sample of [18F]1 confirmed both by 19F-NMR and HRMS the presence of [19F]1 in the hexane extract (ESI,† S9–S12), which is diagnostic of the formation of [18F]1 and its extraction to hexane. Interestingly, TBAF was not detected in the 19F-NMR spectrum of the decayed sample indicating that the extracted sample did not contain significant contamination of [18F]Bu4NF.
| Entry | Temp. (°C) | Time (min) | 2 (mg) | Extr. temp. (°C) | RCC (%) |
|---|---|---|---|---|---|
| a 2 (12 μmol) and [18F]Bu4NF were mixed in DCM (500 μL) and stirred at the indicated temperature and time. The solvent was removed under a N2 stream. To the obtained solid residue was added hexane (500 μL) and stirred for 1 minute at the given temperatures (Extr. temp.). The extracted activity indicates the percentage of total activity extracted in the hexane phase as average of two experiments (n = 2). b 250 μL of DCM instead of 500 μL. c 1000 μL of DCM instead of 500 μL. DCM = dichloromethane. | |||||
| 1 | 70 | 40 | 5 | 70 | 47 ± 3 (n = 2) |
| 2 | 70 | 20 | 5 | 70 | 40 ± 7 (n = 2) |
| 3 | 70 | 20 | 20 | 70 | 42 ± 6 (n = 2) |
| 4b | 70 | 20 | 5 | 70 | 37 ± 1 (n = 2) |
| 5c | 70 | 20 | 5 | 70 | 56 ± 1 (n = 2) |
| 6 | RT | 20 | 5 | 70 | 40 ± 3 (n = 2) |
| 7 | RT | 5 | 5 | 70 | 41 ± 1 (n = 2) |
| 8 | RT | 5 | 5 | 40 | 23 ± 3 (n = 2) |
| 9 | RT | 5 | 5 | RT | 20 ± 2 (n = 2) |
| 10 | RT | 5 | 0 | 70 | 7 ± 1 (n = 2) |
| 11 | RT | 5 | 0 | 40 | 10 ± 4 (n = 2) |
| 12 | RT | 5 | 0 | RT | 5 ± 2 (n = 2) |
We have carefully optimized the reaction temperature, time and concentration of the reaction of 2 and [18F]TBAF and the temperature for the extraction with hexane (Table 1). Initially, we carried out the reactions in DCM (typically 500 μL) at 70 °C (entries 1–5). Using 5 mg of 2 the RCC of [18F]1 was similar (40–47%) after 40 and 20 minutes of reaction time (entries 1 and 2). Increasing the amount of 2 (entry 3) or increasing the concentration of the reactants (by reducing the amount of solvent, entry 4) did not change significantly the RCC (42% and 37% respectively). Contrarily, decreasing the concentration (entry 5) led to increasing of RCC (56%), albeit an elongated time for the evaporation of DCM (entry 5). Therefore, we kept the solvent volume at 500 μL in the subsequent experiments. It was found that the RCC was nearly unchanged when the reaction was carried out at room temperature instead of 70 °C (entry 6). Moreover, the reaction time could be shortened to five minutes without decreasing the RCC (41%, entry 7) thus reducing significantly the duration of the radiosynthesis of [18F]1.
In the above procedures (entries 1–7), we employed 70 °C for the extraction of the solid residue that remained after the evaporation of DCM. Decreasing the extraction temperature to 40 °C or room temperature (entries 8 and 9) led to significant decrease of the RCC (20–23%). The optimal procedure (entry 7) involves the reaction of 2 and [18F]TBAF in 500 μL DCM (5 minutes at room temperature); then evaporation of DCM and extraction of the solid residue by hexane for one minute at 70 °C.
As mentioned above, the fluorine-19 experiments showed that TBAF is insoluble in hexane. To confirm this observation, we made a further control experiment using [18F]TBAF. We carried out the above optimized three-step procedure without employing 2 (entry 10). The radioanalysis indicated 7% RCC for the extracted inorganic fluoride. The extracted amounts of [18F]fluoride were about the same by varying the extraction temperature (entries 11 and 12). These experiments (together with the fluorine-19 results) confirmed that the above three-step procedure results in high purity sample of [18F]1 with very low (if at all) contamination of [18F]TBAF. Notably, the chemical stability of 112c allows for centralized large-scale production and subsequent shipment of [18F]1 to PET centers that lack an on-site cyclotron.
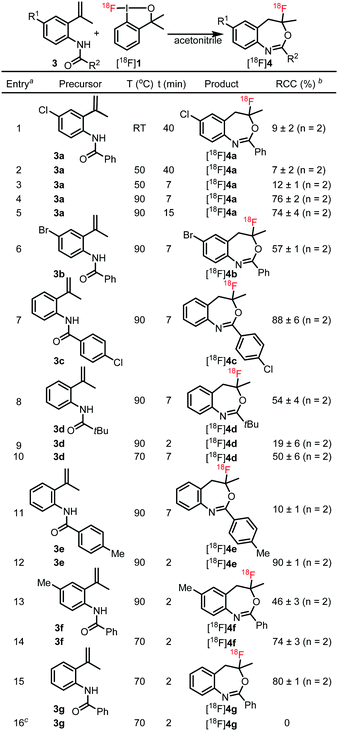
In order to demonstrate the radiosynthetic utility of purified [18F]1 for 18F-labelling we performed electrophilic13 fluorocyclization reactions of o-styrilamides 3. Gulder and co-workers12b,k have shown that using equimolar amounts of 3 and 1 a rapid fluorocyclization can be performed at room temperature resulting in fluoro-benzoxazepines bearing a quaternary fluoride.
Radiosynthesis of [18F]4 was performed by adding the hexane solution of [18F]1 to a solution of precursor 3 in acetonitrile followed by evaporation of the solvents. Subsequently, the solid residue was dissolved in acetonitrile (500 μL) and the fluorocyclization reaction was studied at different temperatures and reaction times (Table 2). Using the optimal fluorocyclization conditions reported by Gulder for the reaction with fluorine-1912b (using 3a and 1), we obtained low but encouraging (9% RCC) levels of the fluoro-benzoxazepines product [18F]4a (Table 2, entry 1). Increasing the temperature from room temperature to 90 °C and decreasing the reaction time from 40 minutes to 7 minutes led to a significant increase of the RCC to 76% (entries 2–4). Further elongation of the reaction time did not improve the RCC (74%, entry 5). The bromo analog 3b and the isomeric chloro o-styrilamide 3c reacted under identical conditions affording the corresponding fluoro-benzoxazepines [18F]4b and [18F]4c in 57 and 88% RCC (entries 6 and 7). Using these conditions, tBu amide [18F]4d was obtained in 54% RCC along with an unidentified 18F-labeled impurity (28%). This impurity was probably a decomposition product resulting from the chemical instability of [18F]4d. Decreasing the reaction temperature to 70 °C led to an RCC of 50% (entry 10) with reduction of the labeled impurity to 20%. When 3e, having a more electron rich nitrogen than 3c, was reacted with [18F]1 under identical conditions, the RCC of [18F]4e was much lower (10%, entry 11) than for [18F]4c (88%, entry 7). A possible reason can be the relatively low chemical stability of [18F]4e at elevated temperatures. When the reaction time was decreased from seven to two minutes, the RCC increased to 90% (entry 12). The electrophilic fluorination of the electron-rich alkene in 3f is probably faster than for its electron-poor counterparts in 3a–b (entries 4 and 6), but product [18F]4f has low thermostability compared to [18F]4a–b. Accordingly, the RCC for the formation of [18F]4f was relatively low at 90 °C (46%, entry 13). However, when the reaction temperature was decreased to 70 °C the RCC was increased to 74% (entry 14). The parent o-styrilamide 3g reacted also smoothly at 70 °C in 2 minutes affording [18F]4g with 80% of RCC. A final confirmation that the nucleophilic fluorination reagent [18F]TBAF is not able to perform fluorocyclization is demonstrated by entry 16. When 3g was reacted with [18F]TBAF instead of [18F]1 formation of [18F]4g was not observed. This control experiment also proved that even if small amounts of [18F]TBAF would have contaminated the purified sample of [18F]1, it was not able to perform fluorocyclization of o-styrilamides 3.
| a To a solution of 3 in MeCN (0.4 μmol, 25 μL) was added [18F]1 in hexane (50–100 μL). The mixture was evaporated and re-dissolved in MeCN (500 μL) before heating at the indicated temperatures and times. b Estimated by radio-HPLC of the crude reaction mixture starting from [18F]1. c Using [18F]Bu4NF instead of [18F]1. |
|---|
|
We also performed one-pot reactions without isolation and purification of [18F]1 (Fig. 2). When the one-pot reaction was performed in two sequential steps: (1) in situ formation of [18F]1 followed by (2) fluorocyclization of 3a the reaction time was shorter by about 5 minutes than for the version with purified [18F]1 (Table 2, entry 4, RCC = 76%). However, the RCC in the one-pot reaction was poor: 14% and 34% based on [18F]Bu4NF or [18F]1 as limiting reagent, respectively (Fig. 2). These results confirm that radiosynthetic attempts for using in situ generated [18F]1 proceed with inferior results compared to the above method with purified [18F]1 reagent.12j
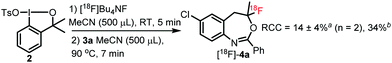 | ||
| Fig. 2 One-pot approach for the synthesis of [18F]4a. a RCC based on [18F]Bu4NF as limiting reagent; b RCC based on [18F]1 assuming that it formed with 41% RCC (Table 1, entry 7). | ||
The optimized protocol was employed for the determination of the isolated yield (RCY) and the molar activity. In this reaction 3.93 GBq of [18F]Bu4NF were reacted with 2 affording 904 MBq of [18F]1, which was used for the labelling of 3a in MeCN at 90 °C for 7 minutes. The reaction resulted in 92.4 MBq of [18F]4a (with >99% radiochemical purity) corresponding to 10% isolated activity yield (RCY) and a molar activity of 396 GBq μmol−1.4c This molar activity is in the range of the values obtained for tracers by nucleophilic fluorination in our laboratories and orders of magnitude higher than the corresponding values reported for the electrophilic fluorination methods based on application of [18F]F2.6–8 The overall time of the reaction from the bombardment to isolation of the [18F]fluoro-benzoxazepine product was about two hours, of which the radiosynthesis and purification of [18F]1 from [18F]TBAF was achieved in about 20 minutes. This method was not automatized, and thus proper automatization may further improve the efficiency of fluorine-18 labelling by [18F]1 in clinical applications.
In summary, we have presented a new radiosynthetic method for the preparation of the electrophilic fluorination reagent [18F]1 from a nucleophilic [18F]F− precursor, [18F]TBAF. The reaction and purification of [18F]1 could be performed by an operationally simple three-step process within about 20 minutes. The purified [18F]1 reagent was free from the excess of its precursor 2 and may contain only traces of [18F]TBAF. Since 1 is chemically stable for an indefinite time, the purified [18F]1 is even transportable in hexane solution to PET facilities that lack on-site cyclotrons. We have also demonstrated the synthetic utility of [18F]1 for electrophilic fluorocyclization of o-stryrilamides. The reactions could be performed in 2–7 minutes with typically high RCC. The preparative scale experiments led to a product with 396 GBq μmol−1 of molar activity, which is higher than the molar activity typically obtained in electrophilic 18F-labelling experiments. We will continue in our laboratories the studies of exploitation of [18F]1 in further 18F-labelling applications and development of further hypervalent iodine based 18F-fluorination/alkylfluorination reagents.
Support by the Stockholm Brain Institute, VINNOVA and the Swedish Research Council (VR) is gratefully acknowledged. We thank Mihály Szabó for his valuable help in some of the fluorine-19 experiments.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) T. Liang, C. N. Neumann and T. Ritter, Angew. Chem., Int. Ed., 2013, 52, 8214 CrossRef CAS PubMed; (b) J. Charpentier, N. Früh and A. Togni, Chem. Rev., 2015, 115, 650 CrossRef CAS PubMed.
- (a) J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432 CrossRef CAS PubMed; (b) Y. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Aceña, V. A. Soloshonok, K. Izawa and H. Liu, Chem. Rev., 2016, 116, 422 CrossRef CAS PubMed.
- P. Jeschke, ChemBioChem, 2004, 5, 570 CrossRef CAS PubMed.
- (a) P. W. Miller, N. J. Long, R. Vilar and A. D. Gee, Angew. Chem., Int. Ed., 2008, 47, 8998 CrossRef CAS PubMed; (b) S. Preshlock, M. Tredwell and V. Gouverneur, Chem. Rev., 2016, 116, 719 CrossRef CAS PubMed; (c) D. van der Born, A. Pees, A. J. Poot, R. V. A. Orru, A. D. Windhorst and D. J. Vugts, Chem. Soc. Rev., 2017, 46, 4709 RSC.
- E. L. Cole, M. N. Stewart, R. Littich, R. Hoareau and P. J. H. Scott, Curr. Top. Med. Chem., 2014, 14, 875 CrossRef CAS PubMed.
- (a) F. Buckingham, A. K. Kirjavainen, S. Forsback, A. Krzyczmonik, T. Keller, I. M. Newington, M. Glaser, S. K. Luthra, O. Solin and V. Gouverneur, Angew. Chem., Int. Ed., 2015, 54, 13366 CrossRef CAS PubMed; (b) H. Teare, E. G. Robins, E. Arstad, S. K. Luthra and V. Gouverneur, Chem. Commun., 2007, 2330 RSC.
- (a) I. S. R. Stenhagen, A. K. Kirjavainen, S. J. Forsback, C. G. Jorgensen, E. G. Robins, S. K. Luthra, O. Solin and V. Gouverneur, Chem. Commun., 2013, 49, 1386 RSC; (b) H. Teare, E. G. Robins, A. Kirjavainen, S. Forsback, G. Sandford, O. Solin, S. K. Luthra and V. Gouverneur, Angew. Chem., Int. Ed., 2010, 49, 6821 CrossRef CAS PubMed.
- (a) N. Satyamurthy, G. T. Bida, M. E. Phelps and J. R. Barrio, Int. J. Radiat. Appl. Instrum., Part A. Appl. Radiat. Isot., 1990, 41, 733 CrossRef CAS; (b) R. Chirakal, N. Vasdev, G. J. Schrobilgen and C. Nahmias, J. Fluorine Chem., 1999, 99, 87 CrossRef CAS.
- E. Lee, A. S. Kamlet, D. C. Powers, C. N. Neumann, G. B. Boursalian, T. Furuya, D. C. Choi, J. M. Hooker and T. Ritter, Science, 2011, 334, 639 CrossRef CAS PubMed.
- E. Lee, J. M. Hooker and T. Ritter, J. Am. Chem. Soc., 2012, 134, 17456 CrossRef CAS PubMed.
- C. Y. Legault and J. Prévost, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2012, 68, o1238 CAS.
- (a) S. V. Kohlhepp and T. Gulder, Chem. Soc. Rev., 2016, 45, 6270 RSC; (b) A. Ulmer, C. Brunner, A. M. Arnold, A. Pöthig and T. Gulder, Chem. – Eur. J., 2016, 22, 3660 CrossRef CAS PubMed; (c) G. C. Geary, E. G. Hope, K. Singh and A. M. Stuart, Chem. Commun., 2013, 49, 9263 RSC; (d) G. C. Geary, E. G. Hope and A. M. Stuart, Angew. Chem., Int. Ed., 2015, 54, 14911 CrossRef CAS PubMed; (e) N. O. Ilchenko, B. O. A. Tasch and K. J. Szabó, Angew. Chem., Int. Ed., 2014, 53, 12897 CrossRef CAS PubMed; (f) W. Yuan and K. J. Szabó, Angew. Chem., Int. Ed., 2015, 54, 8533 CrossRef CAS PubMed; (g) W. Yuan, L. Eriksson and K. J. Szabó, Angew. Chem., Int. Ed., 2016, 55, 8410 CrossRef CAS PubMed; (h) N. O. Ilchenko, M. A. Cortés and K. J. Szabo, ACS Catal., 2016, 6, 447 CrossRef CAS; (i) N. O. Ilchenko, M. Hedberg and K. J. Szabo, Chem. Sci., 2017, 8, 1056 RSC; (j) B. Yang, K. Chansaenpak, H. Wu, L. Zhu, M. Wang, Z. Li and H. Lu, Chem. Commun., 2017, 53, 3497 RSC; (k) C. Brunner, A. Andries-Ulmer, G. M. Kiefl and T. Gulder, Eur. J. Org. Chem., 2018 DOI:10.1002/ejoc.201800129.
- (a) J. Zhang, K. J. Szabo and F. Himo, ACS Catal., 2017, 7, 1093 CrossRef CAS; (b) B. Zhou, T. Yan, X.-S. Xue and J.-P. Cheng, Org. Lett., 2016, 18, 6128 CrossRef CAS PubMed; (c) T. Yan, B. Zhou, X.-S. Xue and J.-P. Cheng, J. Org. Chem., 2016, 81, 9006 CrossRef CAS PubMed.
- V. Matoušek, E. Pietrasiak, R. Schwenk and A. Togni, J. Org. Chem., 2013, 78, 6763 CrossRef PubMed.
- (a) M. G. Campbell, J. Mercier, C. Genicot, V. Gouverneur, J. M. Hooker and T. Ritter, Nat. Chem., 2017, 9, 1 CrossRef CAS PubMed; (b) M. S. Sanford and P. J. H. Scott, ACS Cent. Sci., 2016, 2, 128 CrossRef CAS PubMed; (c) Z. Yuan, R. Cheng, P. Chen, G. Liu and S. H. Liang, Angew. Chem., Int. Ed., 2016, 55, 11882 CrossRef CAS PubMed; (d) A. K. Podichetty, S. Wagner, S. Schröer, A. Faust, M. Schäfers, O. Schober, K. Kopka and G. Haufe, J. Med. Chem., 2009, 52, 3484 CrossRef CAS PubMed; (e) E. E. Gray, M. K. Nielsen, K. A. Choquette, J. A. Kalow, T. J. A. Graham and A. G. Doyle, J. Am. Chem. Soc., 2016, 138, 10802 CrossRef CAS PubMed; (f) X. Huang, W. Liu, H. Ren, R. Neelamegam, J. M. Hooker and J. T. Groves, J. Am. Chem. Soc., 2014, 136, 6842 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization, radiochemistry and spectral data. See DOI: 10.1039/c8cc00526e |
| This journal is © The Royal Society of Chemistry 2018 |