Open Access Article
Open Access ArticleMicrowave-induced covalent functionalization of few-layer graphene with arynes under solvent-free conditions†
M. V.
Sulleiro
a,
S.
Quiroga
b,
D.
Peña
b,
D.
Pérez
 b,
E.
Guitián
b,
A.
Criado
b,
E.
Guitián
b,
A.
Criado
 *c and
M.
Prato
*c and
M.
Prato
 *acd
*acd
aDepartment of Chemical and Pharmaceutical Sciences, University of Trieste, Piazzale Europa, 1, 34127 Trieste, Italy. E-mail: prato@units.it
bCentro de Investigación en Química Biolóxica e Materiais Moleculares (CIQUS) and Departamento de Química Orgánica, Universidade de Santiago de Compostela, 15782 Santiago de Compostela, Spain. E-mail: acriado@cicbiomagune.es
cCIC BiomaGUNE, Parque Tecnológico de San Sebastián, Paseo Miramón, 182, 20014 San Sebastián, Guipúzcoa, Spain. E-mail: alejandro.criado@gmail.com
dBasque Foundation for Science, Ikerbasque, 48013 Bilbao, Spain
First published on 4th January 2018
Abstract
A non-conventional modification of exfoliated few-layer graphene (FLG) with different arynes under microwave (MW) irradiation and solvent-free conditions is reported. The described approach allows reaching fast, efficient and mild covalent functionalization of FLG.
Graphene is an allotrope of carbon with a unique set of electronic, mechanical, and thermal properties,1 which make graphene-based materials (GBMs)2 potential candidates for applications in sensing, energy storage, optoelectronic devices, as catalyst supports, supercapacitors and composites.3
Chemical modification of GBMs is also a topic of great interest, as it allows tuning the chemical and physical properties of the materials,4 such as suspension stability, mechanical properties, and electrical conductivity. At the same time, functionalization permits the combination with other interesting moieties. However, chemical transformations of GBMs frequently require time consuming procedures and multiple additions of reagents.5 In addition, the lack of solubility of carbon-based materials and their subsequent unstable suspensions hinder their manipulation.6 Nevertheless, in many cases, the use of non-conventional condition reactions is able to save time, avoid unstable suspensions and improve reaction efficiency.7 Among the different chemical modifications, cycloaddition reactions are powerful tools in carbon-based materials.5a,8 For instance, a Diels–Alder reaction was reported to produce the exfoliation of graphite into functionalized graphene layers under solvent-free conditions in a one-step process.9 In particular, aryne cycloadditions have been reported for CNTs,10 carbon nanohorns11 and graphene derivatives via fluoride-induced decomposition of o-(trimethylsilyl)aryl triflates.12 However, in many cases the synthesis of these aryne precursors is not easily performed and their decomposition usually needs fluoride ions that can interfere in the reaction and alter the functionalized carbon-based material.13 Thus, establishment of fast and scalable protocols for GBM functionalization with accessible reagents is still needed.
Microwave-assisted (MW) chemistry is an important tool in the field of carbon-based nanomaterials.7a,b In particular, GBMs and in general carbon-based materials are extensively used as MW matrices because of their capacity to absorb MW with high efficiency, rapidly generating high surface temperatures.14 In addition, different methods for the preparation of graphene derivatives have been recently reported by using MW irradiation.15
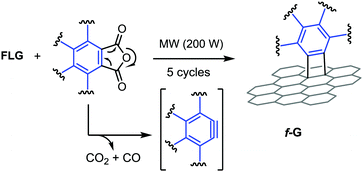
In this work we report a new, facile, rapid and non-conventional approach to efficiently and mildly functionalize GBMs under MW irradiation and solvent-free conditions. We focused on underexplored cycloaddition reactions of arynes with exfoliated few-layer graphene (FLG).12a,b In particular, different arynes were generated by the thermal decomposition of the corresponding arylene anhydrides at high temperatures. Due to the good MW absorption, GBMs played two roles in the reaction process: as a reagent and, at the same time, as a MW absorbing matrix which allows high temperatures to be reached in short times under solvent-free conditions.
The pristine graphene material used in this work was obtained through exfoliation from graphite following Coleman's protocol.16 Raman spectroscopy showed that the produced graphene consisted of a few layers.17 The 2D band of the pristine graphene materials can be deconvoluted into four Lorentzian-shaped peaks (Fig. S9, ESI†), which is a characteristic feature of few-layer (less than five) graphene as previously reported. In addition, the spectrum is also quite different from bulk graphite (Fig. S11, ESI†).
We initially investigated a controllable chemical functionalization of FLG with benzyne generated by the thermal decomposition of the commercially available phthalic anhydride (1).18 The benzyne precursor 1 and the FLG were thoroughly mixed in a mortar, then the homogeneous mixture was rapidly heated up under MW irradiation and solvent-free conditions (Fig. 1).
After considering different reaction parameters such as number of equivalents, temperature, reaction time, and power irradiation, we concluded that the irradiation power is the key to modulate the functionalization degree. Thus, different homogenous powder mixtures of FLG and 1 were carried out at different cycles of MW irradiation. In each cycle, 200 watts of MW power were applied until reaching 250 °C, in about 5 seconds (Fig. S12, ESI†).
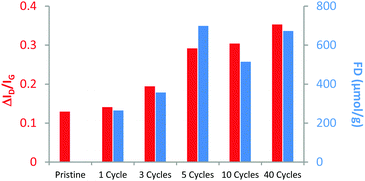
The reaction evolution on the microwave reactor was followed by thermogravimetric analysis (TGA) and Raman spectroscopy, clearly confirming the chemical modification of graphene with benzyne (Fig. 2). TGA allows the estimation of the functionalization degree (FD), defined as the number of micromole of functional groups per gram of sample (eqn (S1), ESI†). The TGA results show significant variations in the FD depending on the number of cycles, while a slight increase in the ID/IG ratio using Raman was observed. Both ID/IG ratios and FDs are in accordance up to 5 cycles of MW irradiation, where the corresponding functionalized graphene (f-G) presented a FD of 122 μmol g−1 with a ID/IG ratio of 0.29 (Table S1, ESI†). However, after 5 cycles, the functionalization degree observed using TGA decreases while the defects still mildly increase, observed using Raman. It is worth mentioning that this chemical process is performed at high temperatures. Probably, the functional groups are partially cleaved probably through the retro-cycloaddition of benzyne under the reaction conditions.10,19 In fact, theoretical studies support this possibility as they have reported energy barriers of 1.9 eV for the retro-cycloaddition reaction of the corresponding adduct of benzyne with graphene, which can be overcome at moderately high temperatures.19a Accordingly, functionalized graphene materials with more than 5 cycles showed smaller FD with a high ID/IG ratio. In addition, defects can be introduced in the graphene lattice after a large number of cycles, independently of the organic functionalization with benzyne. This last assessment is supported by TGA profiles of functionalized graphene with arynes, where a thermal decomposition is observed after around 200 °C (red square in Fig. S13, ESI†). Therefore, the 5 cycle modification was established as the standard method because it generates an adequate FD in line with the ID/IG ratio. The morphology of the functionalized materials was studied using transmission electron microscopy (TEM, Fig. S15–S18, ESI†). The graphene derivative f-G(7) (Fig. 3) keeps a similar structure and dimensions of the pristine material after the described treatment at high temperatures. Besides, it was not possible to observe organic layers on the graphene surface from these images, thus supporting our previous claim about the molecular functionalization.
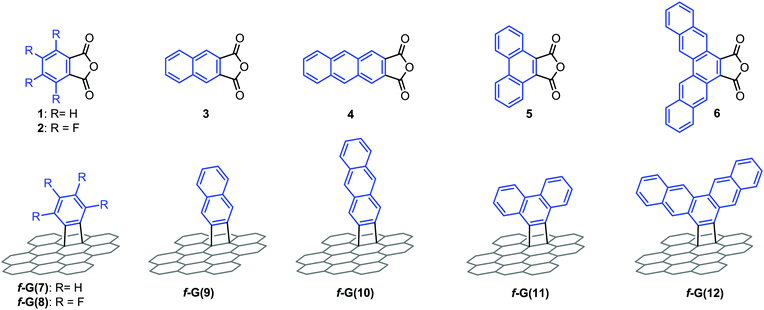 | ||
| Fig. 3 Chemical representation of the arylene anhydrides 1–6 to obtain f-G (7–12), under MW irradiation and solvent-free conditions. | ||
To confirm the generation of aryne under the established reaction conditions, the “capture” of the generated benzyne was tried by adding anthracene as a thermally stable diene into the solid mixture, because it can yield the easily characterized aromatic compound triptycene (15), derived from a [4+2] cycloaddition (Fig. 4). The NMR analysis of the reaction mixture led to 15 as the major product in approximately 33% conversion, and 60 and 27% of recovered 1 and 13, respectively (Fig. S8, ESI†). This result successfully proves the generation of 14 from 1 by thermal decomposition under the described conditions.
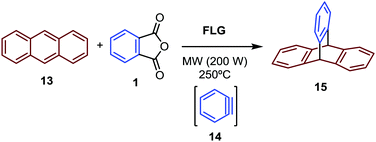 | ||
| Fig. 4 Trapping of benzyne (14) generated upon MW irradiation of 1 under solvent-free conditions with FLG as the MW absorbing matrix. | ||
To date, aryne cycloaddition reactions with diverse carbon nanomaterials have been reported. For example, benzyne cycloaddition with fullerene C60 leads to [2+2] adducts.20 Nevertheless, for reactions performed on carbon nanotubes,10 carbon nanohorns,11 and graphene derivatives,12 there is no experimental evidence as yet about the exact structure of the adducts obtained. Several theoretical studies suggest that the benzyne reaction with carbon nanotubes presents periselectivity (i.e., either [4+2] or [2+2]) depending on different parameters, such as the type of carbon nanotube (i.e., number of walls, chirality, etc.) and its diameter.10a,21 In the case of graphene, theoretical studies argued that the reaction with benzyne on a graphene fragment of 4 × 4, 6 × 6 and 8 × 8 unit cells is more energetically favorable through [2+2] cycloaddition, but both can occur.19b,21c Other theoretical calculations suggest that cycloaddition reactions occur at edges and defected areas.19a,22 However, cycloaddition reactions have been also reported on defect-free epitaxial graphene.8b
The described graphene functionalization was extended to structurally more complex arynes (Fig. 3) via thermal decomposition of the corresponding anhydrides (2–6) under the standard conditions described for phthalic anhydride. In particular, arylene groups with heteroatoms, such as a fluorine-substituted phenylene (f-G(8)), or aromatic moieties with a large number of fused benzene rings (f-G(9–12)) have been covalently linked to the graphene structure. In the latter case, aromatic groups were introduced with the same number of benzene rings but with different topologies (f-G(10–11)). The new modified graphene derivatives were characterized using Raman spectroscopy, TGA, TEM, as well as XPS techniques, showing consistent functionalization yields in all cases (Table S2, ESI†).
In addition, X-ray photoelectron spectroscopy (XPS) was used to provide evidence for the formation of functionalized graphene derivatives by identifying and quantifying the functional groups anchored to the surface. The binding energies and peak assignments for different cores are summarized in Tables S3 and S4 (ESI†). In particular, fluorine atoms from the aryne precursor 2 provided a diagnostic signal proving the functionalization of FLG. The atomic percentages of C, O and F atoms for the FLG, f-G(8), a FLG treated under optimized reaction conditions and a control sample graphene material in the absence of any aryne precursor (cs-G) are summarized in Table 1.
f-G(8) has 17.5% of F atoms due to the introduced tetrafluorophenylene moieties. The presence of oxygen in the modified graphene derivative is probably due to oxygenated groups, generated during the exfoliation and the irradiation process (see the compositions of FLG and cs-G, respectively). The deconvolution of C1s and F1s core levels of f-G(8) (Fig. 4) showed peaks at 286.11 and 687.15 eV, respectively, corresponding to the C–F bonds.23 The presence of fluorine and the energy of these peaks suggest the presence of tetrafluorophenylene moieties in the graphene structure, confirming the chemical modification.
The functionalization of graphene is expected to improve the dispersibility of the graphene material in organic solvents, because the introduced functional groups can preclude re-aggregation and subsequent precipitation.12b,24 In order to explore the dispersion stability of the functionalized few-layer graphene materials, different solvents were tested. The f-Gs exhibited significantly improved solubility in ethanol and DMF, after sonication for 5 min (Fig. S19, ESI†). The f-Gs showed an improved dispersibility in ethanol after 15 days (Fig. 5), especially f-G(8).
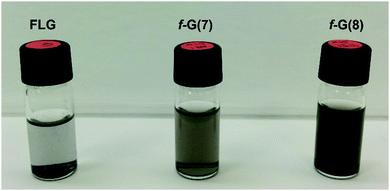 | ||
| Fig. 5 Suspensions of FLG, f-G(7) and f-G(8) (from left to right) in EtOH (0.2 mg mL−1) after 2 weeks. | ||
In conclusion, we have developed a novel, fast, scalable and simple entry to covalently modified graphene. This approach shows further advantages than classical functionalization reactions, since it allows avoiding unstable graphene suspensions at low concentrations by the introduction of moieties of different nature. It is worthy to note that such moieties must be thermally stable due to the necessary high temperatures employed in the method. The addition of a variety of functional groups will be considered in a further work to study their influence in exfoliation and suspension stability. Therefore, the described solvent-free conditions pave the way for green protocols, large-scale functionalization processes of graphene materials and new methods to modulate electronic properties of graphene derivatives. Besides, to the best of our knowledge, it is the first chemical modification where GBMs behave like unique MW absorbing matrixes and reagents at the same time in covalent modification.
M. P. is the recipient of the AXA Chair (2016-2023). This work was supported by the EU H2020-Adhoc-2014-20 Graphene Core1 (no. 696656).
Conflicts of interest
There are no conflicts to declare.Notes and references
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- P. Wick, A. E. Louw-Gaume, M. Kucki, H. F. Krug, K. Kostarelos, B. Fadeel, K. A. Dawson, A. Salvati, E. Vázquez, L. Ballerini, M. Tretiach, F. Benfenati, E. Flahaut, L. Gauthier, M. Prato and A. Bianco, Angew. Chem., Int. Ed., 2014, 53, 2–7 CrossRef PubMed.
- (a) A. C. Ferrari, F. Bonaccorso, V. Fal’ko and K. S. Novoselov, et al. , Nanoscale, 2015, 7, 4598–4810 RSC; (b) A. K. Geim, Science, 2009, 324, 1530–1534 CrossRef CAS PubMed.
- (a) A. Criado, M. Melchionna, S. Marchesan and M. Prato, Angew. Chem., Int. Ed., 2015, 54, 10734–10750 CrossRef CAS PubMed; (b) S. Eigler and A. Hirsch, Angew. Chem., Int. Ed., 2014, 53, 7720–7738 CrossRef CAS PubMed; (c) L. Rodríguez-Pérez, M. Á. Herranz and N. Martín, Chem. Commun., 2013, 49, 3721–3735 RSC.
- (a) M. Quintana, K. Spyrou, M. Grzelczak, W. R. Browne, P. Rudolf and M. Prato, ACS Nano, 2010, 4, 3527–3533 CrossRef CAS PubMed; (b) M. Quintana, A. Montellano, A. E. del Rio Castillo, G. Van Tendeloo, C. Bittencourt and M. Prato, Chem. Commun., 2011, 47, 9330–9332 RSC.
- (a) Y. Hernandez, V. Nicolosi, M. Lotya, F. M. Blighe, Z. Sun, S. De, I. T. McGovern, B. Holland, M. Byrne, Y. K. Gun’Ko, J. J. Boland, P. Niraj, G. Duesberg, S. Krishnamurthy, R. Goodhue, J. Hutchison, V. Scardaci, A. C. Ferrari and J. N. Coleman, Nat. Nanotechnol., 2008, 3, 563–568 CrossRef CAS PubMed; (b) J. N. Coleman, Acc. Chem. Res., 2013, 46, 14–22 CrossRef CAS PubMed.
- (a) E. Vázquez, F. Giacalone and M. Prato, Chem. Soc. Rev., 2014, 43, 58–69 RSC; (b) E. Vázquez and M. Prato, ACS Nano, 2009, 3, 3819–3824 CrossRef PubMed; (c) H. J. Salavagione, J. Mater. Chem. A, 2014, 2, 7138–7146 RSC.
- (a) K. Kostarelos, L. Lacerda, G. Pastorin, W. Wu, S. Wieckowski, J. Luangsivilay, S. Godefroy, D. Pantarotto, J.-P. Briand, S. Muller, M. Prato and A. Bianco, Nat. Nanotechnol., 2007, 2, 108–113 CrossRef CAS PubMed; (b) L. Daukiya, C. Mattioli, D. Aubel, S. Hajjar-Garreau, F. Vonau, E. Denys, G. Reiter, J. Fransson, E. Perrin, M.-L. Bocquet, C. Bena, A. Gourdon and L. Simon, ACS Nano, 2017, 11, 627–634 CrossRef CAS PubMed; (c) M. Barrejón, M. J. Gómez-Escalonilla, J. L. G. Fierro, P. Prieto, J. R. Carrillo, A. Rodriguez, G. Abellán, M. C. López-Escalante, M. Gabás, J. T. López Navarrete and F. Langa, Phys. Chem. Chem. Phys., 2016, 18, 29582–29590 RSC.
- J.-M. Seo and J.-B. Baeka, Chem. Commun., 2014, 50, 14651–14653 RSC.
- (a) A. Criado, M. Vizuete, M. J. Gómez-Escalonilla, S. García-Rodriguez, J. L. G. Fierro, A. Cobas, D. Peña, E. Guitián and F. Langa, Carbon, 2013, 63, 140–148 CrossRef CAS; (b) A. Criado, M. J. Gómez-Escalonilla, J. L. G. Fierro, A. Urbina, D. Peña, E. Guitián and F. Langa, Chem. Commun., 2010, 46, 7028–7030 RSC.
- D. Chronopoulos, N. Karousis, T. Ichihashi, M. Yudasaka, S. Iijima and N. Tagmatarchis, Nanoscale, 2013, 5, 6388–6394 RSC.
- (a) D. García, L. Rodríguez-Pérez, M. A. Herranz, D. Peña, E. Guitián, S. Bailey, Q. Al-Galiby, M. Noori, C. J. Lambert, D. Pérez and N. Martín, Chem. Commun., 2016, 52, 6677–6680 RSC; (b) X. Zhong, J. Jin, S. Li, Z. Niu, W. Hu, R. Li and J. Ma, Chem. Commun., 2010, 46, 7340–7342 RSC; (c) I. V. Magedov, L. V. Frolova, M. Ovezmyradov, D. Bethke, E. A. Shaner and N. G. Kalugin, Carbon, 2013, 54, 192–200 CrossRef CAS PubMed.
- C. Romero, D. Peña, D. Pérez and E. Guitián, Chem. – Eur. J., 2006, 12, 5677–5684 CrossRef CAS PubMed.
- (a) H. Y. Cho, A. Ajaz, D. Himali, P. A. Waske and R. P. Johnson, J. Org. Chem., 2009, 74, 4137–4142 CrossRef CAS PubMed; (b) N. D. Kim, A. Metzger, V. Hejazi, Y. Li, A. Kovalchuk, S. K. Lee, R. Ye, J. A. Mann, C. Kittrell, R. Shahsavari and J. M. Tour, ACS Appl. Mater. Interfaces, 2016, 8, 12985–12991 CrossRef CAS PubMed; (c) H. Naeimi and M. Golestanzadeh, New J. Chem., 2015, 39, 2697–2710 RSC.
- (a) M. Matsumoto, Y. Saito, C. Park, T. Fukushima and T. Aida, Nat. Chem., 2015, 7, 730–736 CrossRef CAS PubMed; (b) D. Ghosh, G. Halder, A. Sahasrabudhe and S. Bhattacharyya, Nanoscale, 2016, 8, 10632–10641 RSC; (c) H. Luo, R. Gong, X. Wang, K. Song, C. Zhu and L. Wang, New J. Chem., 2016, 40, 6238–6243 RSC; (d) A. Akın and N. Işıklan, Int. J. Biol. Macromol., 2016, 82, 530–540 CrossRef PubMed; (e) K. Cao, Y. Tian, Y. Zhang, X. Yang, C. Bai, Y. Luo, X. Zhao, L. Ma and S. Li, Nanoscale, 2014, 6, 13518–13526 RSC; (f) Y. M. Shulga, S. A. Baskakov, E. I. Knerelman, G. I. Davidova, E. R. Badamshina, N. Y. Shulga, E. A. Skryleva, A. L. Agapov, D. N. Voylov, A. P. Sokolov and V. M. Martynenko, RSC Adv., 2014, 4, 587–592 RSC.
- S. Barwich, U. Khan and J. N. Coleman, J. Phys. Chem. C, 2013, 117, 19212–19218 CAS.
- (a) A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K. S. Novoselov, S. Roth and A. K. Geim, Phys. Rev. Lett., 2006, 97, 187401 CrossRef CAS PubMed; (b) L. M. Malard, M. A. Pimenta, G. Dresselhaus and M. S. Dresselhaus, Phys. Rep., 2009, 473, 51–87 CrossRef CAS.
- E. K. Fields and S. Meyerson, J. Am. Chem. Soc., 1966, 31, 3307 CAS.
- (a) J. Zhao, H. Wang, B. Gao, X. Wang, Q. Cai and X. Wang, J. Mol. Model., 2012, 18, 2861–2868 CrossRef CAS PubMed; (b) P. A. Denis and F. Iribarne, J. Mater. Chem., 2012, 22, 5470–5477 RSC; (c) S. Sarkar, E. Bekyarova, S. Niyogi and R. C. Haddon, J. Am. Chem. Soc., 2011, 133, 3324–3327 CrossRef CAS PubMed.
- S. H. Hoke, J. Molstad, D. Dilettato, M. J. Jay, D. Carlson, B. Kahr and R. G. Cooks, J. Org. Chem., 1992, 57, 5069–5071 CrossRef CAS.
- (a) T. Yang, X. Zhao and S. Nagase, Org. Lett., 2013, 15, 5960–5963 CrossRef CAS PubMed; (b) J. P. Martínez, F. Langa, F. Matthias Bickelhaupt, S. Osuna and M. Solá, J. Phys. Chem. C, 2016, 120, 1716–1726 CrossRef; (c) M. Hammouri, S. K. Jha and I. Vasiliev, J. Phys. Chem. C, 2015, 119, 18719–18728 CrossRef CAS.
- (a) Y. Cao, S. Osuna, Y. Liang, R. C. Haddon and K. N. Houk, J. Am. Chem. Soc., 2013, 135, 17643–17649 CrossRef CAS PubMed; (b) P. A. Denis, Chem. – Eur. J., 2013, 19, 15719–15725 CrossRef CAS PubMed.
- J. F. Moulder, W. F. Stickle, P. E. Sobol and K. D. Bomben, Handbook of X-Ray Photoelectron Spectroscopy, ed. J. Chastain and R. King Jr., Physical Electronics, Inc., 3rd edn, 1995 Search PubMed.
- (a) A. Ciesielski and P. Samorì, Chem. Soc. Rev., 2014, 43, 381–398 RSC; (b) J. M. Englert, C. Dotzer, G. Yang, M. Schmid, C. Papp, E. Spiecker, F. Hauke, A. Hirsch, J. M. Gottfried and H. Steinru, Nat. Chem., 2011, 3, 279–286 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cc08676h |
| This journal is © The Royal Society of Chemistry 2018 |