Carbodiimides as catalysts for the reduction of a cadmium hydride complex†
Abstract
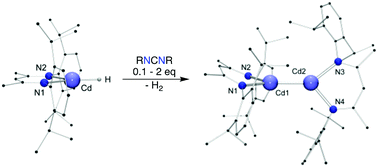
A rare terminal cadmium hydride complex [(BDI)CdH] (BDI = [{N(2,6-iPr2C6H3)C(Me)}2CH]) has been synthesised from [(BDI)CdCl] and LiEt3BH. The hydride can be reduced to the cadmium(I) dimer, [(BDI)CdCd(BDI)] upon treatment with a catalytic amount of diisopropyl- or dicyclohexylcarbodiimide.
- This article is part of the themed collection: Philip Power at 65: an icon of organometallic chemistry


 Please wait while we load your content...
Please wait while we load your content...